Abstract
Background
Serum uric acid (SUA) and renal status are associated with the Cardiovascular-Kidney-Metabolic (CKM) syndrome. However, the causal association among them along with drug therapy need to be explored.
Methods
We employed univariable, multivariate, mediation and drug-target mendelian randomization. Inverse variance weighting was the primary result, with extensive sensitivity analyses conducted to ensure robustness and reliability.
Results
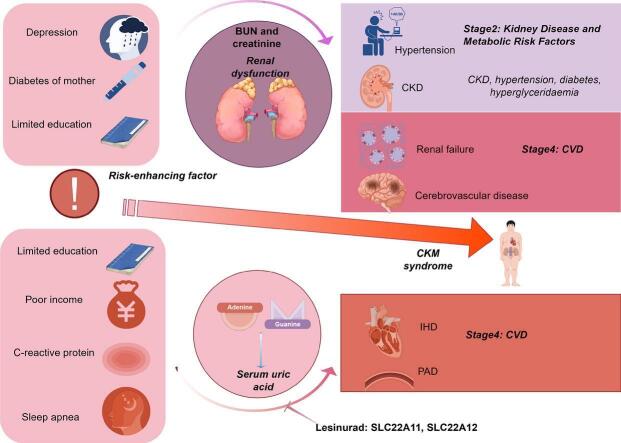
Regarding SUA, genetically predicted SUA demonstrated a potential risk effect on stage 4 of CKM syndrome (ischemic heart disease (IHD), OR = 1.090, 95 %CI: 1.003–1.184; peripheral artery disease, OR = 1.174, 95 %CI: 1.058–1.303). SUA remained a significant risk factor after excluding the confounding of eGFR and proteinuria (IHD: OR = 1.137, 95 %CI: 1.043–1.238; peripheral artery disease: OR = 1.224, 95 %CI: 1.107–1.354). SUA mediated the following causal effect: sleep apnea (2.37 %), income (1.92 %) and education (1.79 %) on IHD; C-reactive protein (11.65 %) and education (4.29 %) on peripheral artery disease. Regarding renal status, renal dysfunction led to a wider phenotype of CKM syndrome including hypertension, cerebrovascular disease, chronic kidney disease and renal failure. Similarly, renal status mediated the causal effect of education on hypertension (1.84 %), depression on cerebrovascular (0.46 %) and family history of diabetes on chronic kidney disease (3.49 %) and renal failure (2.81 %). Lesinurad targeting SLC22A11 and SLC22A12 was validated for treating IHD.
Conclusion
Our study clarified the complex relationship among SUA, renal status and CKM syndrome. Simultaneously providing innovative drug and social interventions for CKM syndrome.
Keywords: Serum uric acid, Renal status, Cardiovascular-kidney-metabolic syndrome, Mendelian randomization
Graphical abstract
Lay summaries
We identified elevated serum uric acid (SUA) is the culprit behind ischemic heart disease (IHD) and peripheral arterial disease, while renal dysfunction leads to a wider impaired, including hypertension, cerebrovascular disease, chronic kidney disease and renal failure. Additionally, SUA and renal status mediate the pathological process of cardiovascular-kidney-metabolic syndrome caused by its risk factors. More importantly, we clarified the therapeutic role of lesinurad in IHD. Besides, we identified SUA as a key biologic marker associated with social determinants of health and proposed: lower social status will cause high SUA levels and further result in cardiovascular disease.
Highlights
-
•
Elevated serum uric acid (SUA) is the culprit behind ischemic heart disease and peripheral arterial disease.
-
•
Renal dysfunction leads to a wider impaired, including hypertension, cerebrovascular disease, chronic kidney disease and renal failure.
-
•
SUA and renal status mediate the pathological process of cardiovascular-kidney-metabolic syndrome caused by its risk factors.
-
•
Lesinurad is a potential therapy for ischemic heart disease.
-
•
Lower social status will cause high SUA levels and further result in cardiovascular disease.
1. Introduction
Cardiovascular-kidney-metabolic (CKM) syndrome is an innovative health disorder concept attributable to the interplay among metabolic risk factors (obesity, diabetes, and hyperlipidemia), chronic kidney disease (CKD), and cardiovascular disease (CVD), including heart failure, arrhythmias, ischemic heart disease (IHD) and peripheral artery disease (PAD) [1]. CKM syndrome was divided into five stages based on pathophysiology according to the latest expert consensus: stage 0: no CKM risk factors; stage 1: excess or dysfunctional adiposity; stage 2: metabolic risk factors (hypertriglyceridemia, hypertension, diabetes, metabolic syndrome) or moderate-to-high risk CKD; stage 3: subclinical CVD in CKM syndrome or risk equivalents (high predicted CVD risk or very high-risk CKD); and stage 4: clinical CVD in CKM syndrome [2]. It is worth noting that the staging path can be bidirectional, which means that individuals with CKM syndrome can get relief through risk intervention and appropriate targeted treatment [3]. The high prevalence of CKM syndrome brings a heavy load to public health, thus there is an urgent need to find a risk prediction approach that reflects exposures and outcomes most relevant to CKM health so that we can better prevent and manage CKM-related adverse outcomes.
Uric acid (UA) is the end-product of purine catabolism, mainly present in serum and urine [4]. Many mammalian species possess the ability to convert UA to allantoin through uricase activity. In contrast, humans are believed to have lost uricase as an evolutionary adaptation, likely providing an advantage in regulating blood pressure under conditions of low-salt diets and offering protection against oxidative stress [5]. Thus, the serum uric acid (SUA) levels in humans are 3 to 10 times higher compared to other mammals that possess the uricase enzyme [6]. Hyperuricemia refers to elevated SUA concentration, potentially resulting in the deposition of urate crystals within joints, tendons, and other tissues. It also increases the risk of developing gout or other associated comorbidities [7]. As early as the 19th century, it was recognized that there are associations between hyperuricemia, kidney disease, and CVD [8]. Hence, SUA has the prospect of being a risk predictor for CKM syndrome. However, there is still a lack of conclusive evidence to explain the complex causal relationship between SUA, kidney disease, and CVD. Creatinine and urea are commonly used biomarkers in clinical practice to reflect renal function. The creatinine serum level reflects not only renal excretion, but also the generation, intake, and metabolism of creatinine [9]. Urea, another classical biomarker of kidney injury, is the maximum toxin among uremic retention solutes [10].
We explored the causal relationship among SUA, renal function (replaced with creatinine and urea), CKM syndrome, and its risk factors through extensive mendelian randomization (MR) analysis. MR is a novel study design that offers a genetic epidemiological approach to exploring the causal relationship between exposure and outcome, utilizing single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) [11]. Compared to conventional clinical studies, MR significantly mitigates susceptibility to reverse causation and confounding [12]. MVMR is an extension to MR that uses genetic variants associated with multiple potentially correlated exposures to estimate the impact of each exposure on a single outcome [13].
The purpose of our study is to evaluate the causal relationship among SUA, CKM syndrome, and CKM syndrome risk factors and contribute to the risk prediction and target therapy of CKM syndrome, meanwhile clarifying the complex interplay of SUA, renal status, and CVD through MR, mediation MR and MVMR analyses.
2. Methods
2.1. Research design
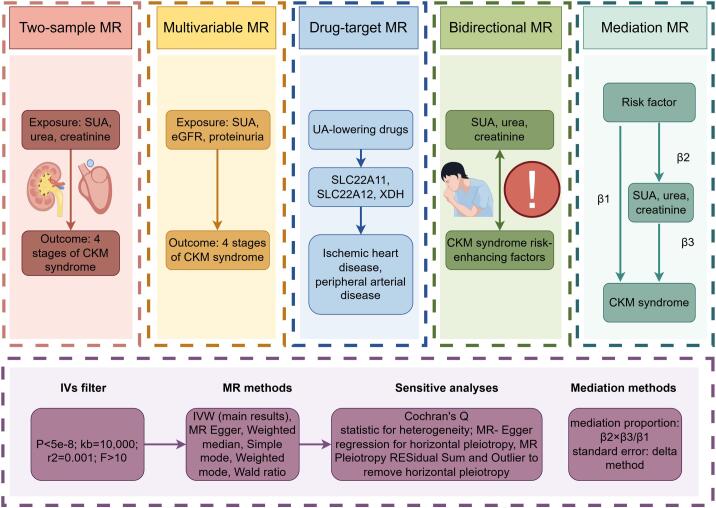
To better clarify the causal relationship among SUA, renal status (reflected by phenotypes like urea, creatinine, eGFR and proteinuria), different stages of CKM syndrome and risk-enhancing factors for CKM syndrome, we conducted four parts of MR analyses. Firstly, we identified the causal effect of SUA and renal status on 4 stages of CKM syndrome through two-sample MR. Then we investigated whether SUA is still a risk factor for CKM syndrome after adjusting the confounding of renal status by MVMR. Thirdly, we conducted bidirectional MR analyses to explore the causal association of SUA and renal status with the risk factors of CKM syndrome. Lastly, we conducted two-step MR to investigate the mediation effect of SUA and renal status between CKM syndrome and CKM risk factors. The overall research design is plotted as a chart (Fig. 1).
Fig. 1.
The overall research design. CKM: Cardiovascular-kidney-metabolic, MR: Mendelian randomization, MVMR: Multivariable mendelian randomization, SUA: Serum uric acid, UA: Uric acid.
2.2. Genome-wide association study (GWAS) summary statistics
The GWAS summary statistics of SUA (sample size: 343836), urea (sample size: 389608), creatinine (sample size: 389678), estimated glomerular filtration rate (sample size: 1,004,040), isolated proteinuria (sample size 218,591, 392cases), obesity (sample size: 463010, 4688cases), BMI (sample size: 461460), waist circumference (sample size: 407661), chronic kidney disease (sample size: 216743, 3902cases), type 2 diabetes (adjusted for BMI) (sample size: 298,957, 48286cases), hypertension (sample size: 361194, 1237 cases), hyperglyceridaemia (sample size: 197488, 229cases), NT proBNP plasma levels (sample size: 400), cardiac muscle troponin I (sample size: 3301), atrial fibrillation (sample size: 1030836, 60620cases), ischemic heart diseases (IHD) (sample size: 218792, cases: 30952), cerebrovascular diseases (sample size: 218792, cases: 15724), peripheral artery disease (sample size: 213639, cases: 7098), heart failure and BMI 25plus (sample size: 218792, cases: 23701), renal failure (sample size: 218792, cases: 5951), guttate psoriasis (sample size: 212407, cases: 165), rheumatoid arthritis (sample size: 153657, cases: 6236), systemic lupus erythematosus (sample size: 213683, cases: 538), AIDS (sample size: 218792, cases: 357), persons with potential health hazards related to socioeconomic and psychosocial circumstances (sample size: 218792, cases: 5137), depression (sample size: 462933, cases: 26595), anxiety disorder (sample size: 463010, cases: 1523), sleep apnea (sample size: 217955, cases: 16761), C-reactive protein (sample size: 575531), illnesses of mother: Diabetes (sample size: 423892, cases: 40091), illnesses of father: Diabetes (sample size:, cases: 40091), average total household income before tax (sample size: 400687, cases: 8850), educational attainment (years of education) (sample size: 461457), problems related to employment and unemployment (sample size: 213966, cases: 311), problems related to medical facilities and other health care (sample size: 192152, cases: 790) were collected from the Integrative Epidemiology Unit (IEU) Open GWAS database. Detailed information has been included in the supplementary materials (Table S1).
2.3. IVs selection
A qualified IV should satisfy three following requirements (i) the relevance assumption—the genetic variables are significantly associated with the exposure; (ii) the independence assumption—IV should not be related to any confounding factors; (iii) the exclusion restriction assumption—genetic variation only affects outcome through its impact on exposure variables, excluding any direct or indirect effects via other pathways [12]. We filter qualified IVs by setting strict thresholds (P < 5 × 10^-8, window size = 10,000 kb, r2 = 0.001). However, for some phenotypes, including AIDS, socioeconomic, depression, anxiety disorder, employment, and medical facilities, to obtain sufficient IV for MR, we adjusted the significant genome-wide association threshold (P < 1 × 10^-5). We used F-statistic (F = β2/se2, F > 10) to exclude weak IVs. F statistics greater than 10 indicate a lower likelihood of bias in weak instrumental variables [14].
2.4. Two sample MR analyses
We adopted five MR methods to make our results more credible and comprehensive, including MR Egger, weighted median, inverse variance weighted (IVW), simple mode, and weighted mode. IVW method was presented as the main result in our article, for its comprehensive evaluation of the causal link [15]. We conducted a detailed set of sensitivity analyses to examine the MR assumptions, the Cochran Q test to inspect heterogeneity among SNPs included in each analysis, the MR–Egger regression intercept alongside a more recent and robust method, “MR pleiotropy residual sum and outlier” to check horizontal pleiotropy and outliers [16].
2.5. MVMR analyses
Multivariable MR (MVMR) is a recent extension to MR that uses genetic variants associated with multiple potentially correlated exposures to estimate the impact of each exposure on a single outcome [13]. American Heart Association scientific statement pointed out that people with poor renal status have a higher risk for CVD [17]. We used MVMR to test whether the risk of SUA on CKM syndrome still exists after correcting for renal dysfunction (lower eGFR and proteinuria).
2.6. Mediation MR analyses
For mediating MR analyses, we employed a two-step MR approach to explore the potential mediate role of SUA and renal status in the association between CKM risk factor and CKM syndrome. Firstly, we calculated the genetic predicted effect (β2) of risk factor on SUA and renal status. Secondly, we estimated the causal effect (β3) of SUA and renal status on CKM syndrome. The mediation proportion was calculated by dividing the mediation effect (β2*β3) by the total, delta method was used to estimate the standard errors of the mediate effect [18].
2.7. Drug target MR analyses
Target information of uric acid lowering drugs was accessed from DrugBank [19]. 500 kb upstream and downstream of the drug target meanwhile significant related with SUA were used as IVs for further drug target MR analyses. A detailed set of sensitivity analyses to examine heterogeneity and pleiotropy.
2.8. Correction for multiple testing
Statistical significance was adjusted using the Holm–Bonferroni correction method to avoid type I error [20], according to the number of exposures tested at each stage of analysis. If the P-value is between 0.05 and the corrected p-value, we consider it suggestive of evidence for a potential association [21].
3. Results
3.1. Causal effect of SUA and renal status on 4 stages of CKM syndrome
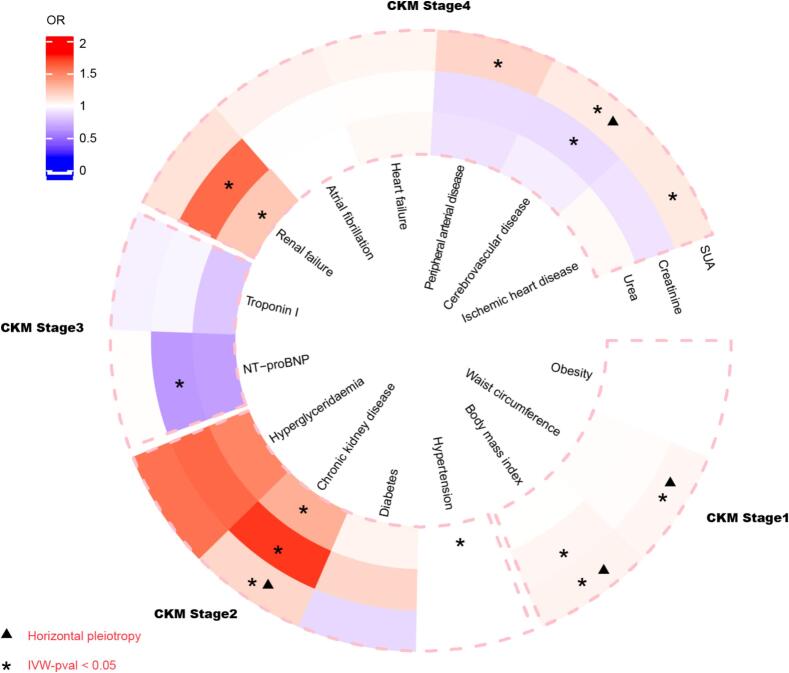
According to our MR results (Fig. 2), SUA has a potential risk effect on almost all stages of CKM syndrome (Stage1: BMI, OR = 1.049, 95 %CI: 1.002–1.098; Waist circumference, OR = 1.033, 95 %CI: 1.001–1.065; Stage2: CKD, OR = 1.169, 95 %CI: 1.010–1.351; Stage4: Cerebrovascular disease, OR = 1.083, 95 %CI: 1.011–1.159; IHD, OR = 1.090, 95 %CI: 1.003–1.184; PAD, OR = 1.174, 95 %CI: 1.058–1.303). It should be noted that there was a level of pleiotropy in the risk effect of SUA on BMI (P = 1.77 × 10−4), waist circumference (P = 1.99 × 10−4), CKD (P = 0.027) and cerebrovascular disease (P = 0.035). Renal status mainly manifested as being related to kidney disease while not related to CVD and metabolic disorders (Urea to CKD, OR = 1.332, 95 %CI: 1.057–1.680; Urea to renal failure, OR = 1.231, 95 %CI: 1.031–1.469; Creatinine to CKD, OR = 1.734, 95 %CI: 1.451–2.072; Creatinine to renal failure, OR = 1.591, 95 %CI: 1.383–1.831).
Fig. 2.
Causal effect of SUA and renal status on 4 stages of CKM syndrome. CKMS: Cardiovascular-kidney-metabolic syndrome, IVW: Inverse variance weighted, NT-proBNP: N-terminal B-type natriuretic peptide, SUA: Serum uric acid, OR: odd ratio.
The remaining four methods yielded results similar to the main results (Table S2). The results of sensitivity testing regarding heterogeneity and pleiotropy are presented in the supplementary table (Tables S3 and S4).
In conclusion, SUA is a qualified risk biomarker for CKM syndrome compared to creatinine and urea which only display significant risk roles in renal failure and CKD.
3.2. The causal effect of the SUA on CKM syndrome adjusted renal status through MVMR analyses
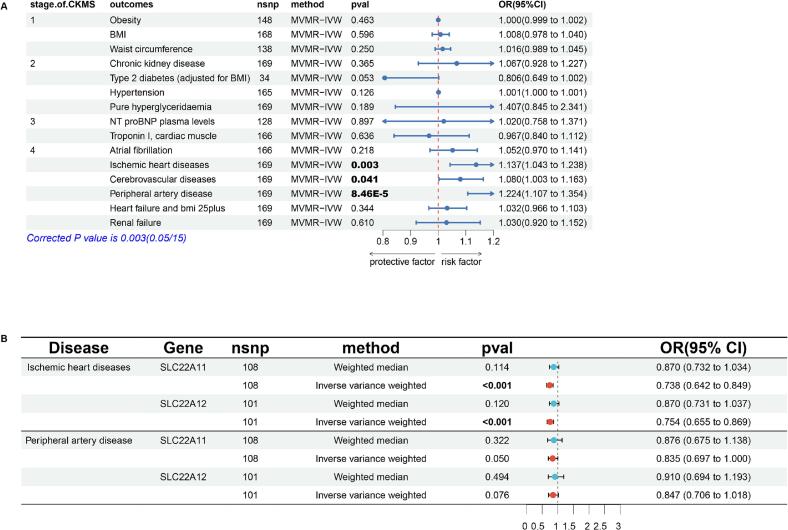
Given that the complex relationship of renal status (eGFR and proteinuria) in the effect of SUA on CVD [22], we conducted MVMR analyses to estimate the respective impact of them on CKM syndrome. SUA maintained a significant link with the incidence of stage 4 of CKM syndrome after excluding the confounding of eGFR and proteinuria (IHD: OR = 1.137, 95 %CI: 1.043–1.238; Cerebrovascular diseases: OR = 1.080, 1.003–1.163; Peripheral artery disease: OR = 1.224, 95 %CI: 1.107–1.354) (Fig. 3A). This result well explained the phenomenon of horizontal pleiotropy in the positive results of SUA for stage 1–3 of CKM syndrome in previous two-sample MR analyses (Fig. 2).
Fig. 3.
The results of MVMR and drug-target MR. (3A): The Causal effect of SUA on 4 stages of CKM syndrome after adjusting renal status. (3B): MR for UA-lowering drug targets on IHD and PAD. BMI: Body mass index, CI: Confidence interval, CKMS: Cardiovascular-kidney-metabolic syndrome, IVW: Inverse variance weighted, MVMR: Multivariable mendelian randomization, OR: odd ratio, SNP: Single nucleotide polymorphism.
Combining previous MR analyses, the increased of SUA is the culprit of IHD and PAD without the involvement of renal dysfunction. Meanwhile, renal dysfunction led to other phenotype like hypertension, cerebrovascular disease, CKD and renal failure.
3.3. MR for UA-lowering drug targets on IHD and PAD
We obtained target information of allopurinol, febuxostat, lesinurad, benzbromarone and probenecid (Table S5). Drug targets MR showed that lesinurad which targeted at SLC22A11 and SLC22A12 was benefit for IHD (SLC22A11: OR = 0.738, 95 %CI: 0.642–0.849; SLC22A12: OR = 0.754, 95 %CI: 0.655–0.869) (Fig. 3B). Lesinurad, an oral urate-anion exchanger transporter 1 inhibitor, which was approved for treatment of hyperuricemia in America [23]. Our research suggested that patients with IHD could benefit from lesinurad, especially those with concomitant hyperuricemia.
3.4. Bidirectional MR results between CKM syndrome risk-enhancing factor, SUA and renal status
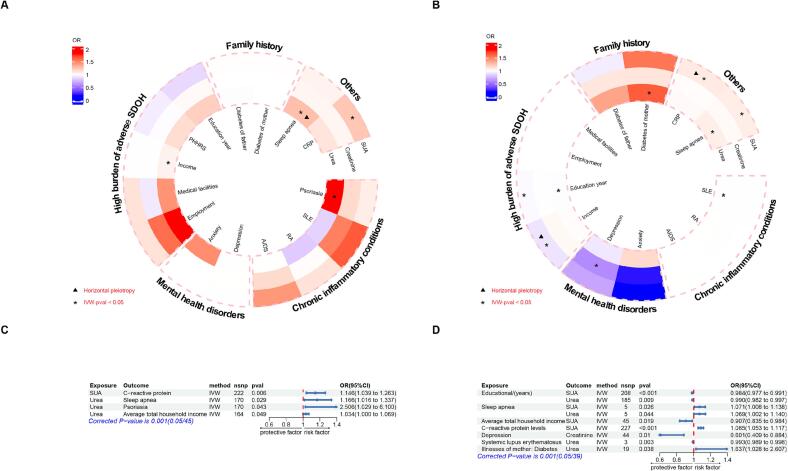
In terms of causal effect of SUA and renal status on risk factors (Fig. 4A and C), SUA caused an increase in C-reactive protein (P = 0.006, OR = 1.146) and urea was a risk factor for sleep apnea (P = 0.029, OR = 1.166) and psoriasis (P = 0.043, OR = 2.506). The results of other methods were similar to those of the IVW method (Table S6) and the above positive results did not exhibit pleiotropy or heterogeneity (Tables S7 and S8).
Fig. 4.
Bidirectional causal effect between CKM syndrome risk-enhancing factors, SUA and renal status. (4A): The circular heatmap depicting the causal effect of SUA and renal status on CKM syndrome risk-enhancing factors. (4B): The circular heatmap depicting the causal effect of CKM syndrome risk-enhancing factors on SUA and renal status. (4C): The forest plot describing the causal effect of SUA and renal status on CKM syndrome risk-enhancing factors. (4D): The forest plot describing the causal effect of CKM syndrome risk-enhancing factors on SUA and renal status. AIDS: Acquired Immune Deficiency Syndrome, CRP: C-reactive protein, CI: Confidence interval, IVW: Inverse variance weighted, OR: odd ratio, RA: Rheumatoid arthritis, SLE: Systemic lupus erythematosus, SNP: Single nucleotide polymorphism, SUA: Serum uric acid.
In terms of risk factors on SUA and renal status (Fig. 4B and D), MR analyses showed that average total household income before tax (OR = 0.907, 95 %CI: 0.835–0.984) and educational attainment (OR = 0.984, 0.977–0.991) are inversely proportional to SUA. Sleep apnea (OR = 1.071, 95 %CI: 1.008–1.138) and C-reactive protein (OR = 1.085, 95 %CI: 1.053–1.117) are positively correlated with SUA. It should be pointed out that there was horizontal pleiotropy in the causal effect of average total household income before tax (P = 0.024) and C-reactive protein (P = 0.042) on SUA. Remaining results, heterogeneity and pleiotropy were summarized into supplementary tables (Tables S9–S11).
In conclusion, part of the CKM syndrome risk-enhancing factor significantly contributed to the increase of SUA. Besides, systemic lupus erythematosus, sleep apnea, and diabetes of mother were associated with urea level.
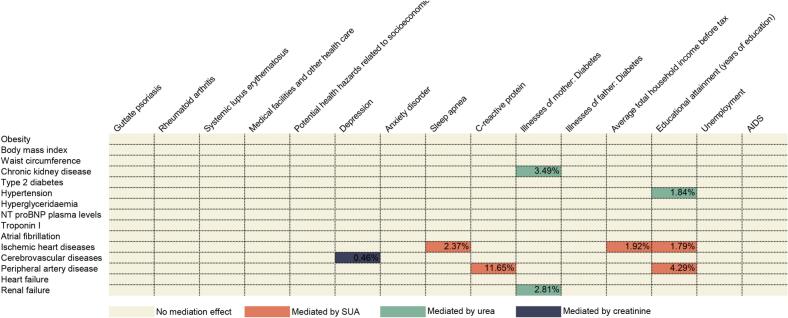
3.5. Mediation effect of SUA and renal status in CKM syndrome and its risk factor
Through two-step MR, we identified the mediating role of three biomarkers in CKM syndrome caused by its risk factors. As depicted in Fig. 5, SUA mediated the following sets of causal relationships: Income on IHD (1.3 %), C-reactive protein on PAD (11.65 %), education attainment on IHD (1.79 %), education attainment on PAD (4.29 %); Creatinine mediated the causal effect of depression on cerebrovascular disease (0.46 %); Urea mediated diabetes of mother on CKD (3.49 %), diabetes of mother on renal failure (2.81 %) and education attainment on hypertension (1.84 %).
Fig. 5.
The mediation effect of SUA and renal status in CKM syndrome and its risk factors. AIDS: Acquired Immune Deficiency Syndrome, NT-proBNP: N-terminal B-type natriuretic peptide, SUA: Serum uric acid.
4. Discussion
Our main findings contained the following three parts: (i) In terms of CKM syndrome, elevated SUA is the culprit behind IHD and PAD, while impaired renal function leads to a wider phenotype of CKM syndrome including hypertension, cerebrovascular disease, CKD and renal failure. (ii) Regarding risk factors of CKM syndrome, SUA and renal status mediate the pathological process of CKM syndrome caused by its risk factors. (iii) Lesinurad, a urate-lowering drug, could be a potential drug for IHD treatment.
UA, a product of purine protein metabolism in humans, can be found in the blood and urine and trace amounts in different organs of the body. Hyperuricemia is considered a key metabolic syndrome, alongside hypertension, hyperglycemia, and hyperlipidemia, with cutoffs determined by gout risk. However, recent studies indicate that lower levels of UA are also associated with cardiovascular diseases and mortality [24]. While the recognition of an association between UA, kidney disease, and CVD dates back to the late 19th century, the causal link between them has been subject to ongoing debate. Findings from physiological experiments and observational research hint at the possible yet uncertain presence of causal links interwoven within the relationships among UA, CKD, and CVD. Nevertheless, obtaining convincing evidence remains challenging due to methodological and statistical complexities inherent in studying uric acid, its determinants, and confounding factors [22].
SUA was demonstrated to be associated with obesity, diabetes, hypertension, hypertriglyceridemia, heart failure, atrial fibrillation, and renal failure (different stages of CKM) [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. However, the causal role of SUA in these diseases remains unclear due to the limited studies that could reveal the internal causality. In the current study, we utilized the MR analysis to integrate the causal relationships among CKM and found that SUA might exhibit a causal role in IHD (one type of coronary heart disease) and PAD. It means that to some extent SUA is a vital predicted risk factor of CKM. Additionally, further drug targets MR indicated that lesinurad was benefit for IHD by lowering SUA.
Kidney, is the main excretory organ of SUA, whose status is considered to be involved in the intermediary of CVD caused by SUA. Previous studies proposed three assumption models of the relationships among SUA, renal status, and cardiovascular events. They are as below: (i) Kidney disease mediates the relationship between uric acid and CVD; (ii) Kidney disease confounds the relationship between uric acid and CVD; (iii) Kidney disease, in varying degrees, is both a mediator and a confounder in a relationship where hyperuricemia, CKD, and CVD each impact the other [22]. Our MVMR analyses indicated that kidney dysfunction did not confound the relationship between SUA and CVD. Our study presented and proved a different perspective compared with previous researches: The elevation of SUA directly leads to an increased risk of cardiovascular disease without the involvement of renal function.
Social determinants of health (SDOH), containing economic, social, environmental, and psychosocial factors that influence health, was considered to significantly endanger cardiovascular health globally and lead to the occurrence of CVD ultimately [35]. It is worth noting that there were at least two unresolved issues in this pathological process: (i) The biological mechanism of SDOH leading to CVD development [36,37]; (ii) Identify key biologic markers associated with SDOH that may help implement early intervention in cardiovascular health [36]. A recent review proposed a possible biological pathway: lower social status (poor income, limited education and so on) - chronic stress - proinflammatory state and atherogenesis [38]. Our MR results indicated that poor income and limited education can lead to an increase in SUA levels. Based on the result, we identified SUA as a key biologic marker associated with SDOH and proposed an innovative hypothesis: lower social status led to higher SUA levels finally resulted in stage 4 of CKM syndrome.
Regarding the potential cardiovascular benefits of lesinurad, a more robust interpretation may be warranted. Numerous studies link UA (including hyperuricemia [39], diuretic-induced hyperuricemia [40] and urate crystallization [41]) to CVD and metabolic disorders [[42], [43], [44]]. Meanwhile, prospective analyses based on PURSUIT-HFpEF [45] and factorial mendelian analyses conducted by Wang et al. [46]suggest potential cardiovascular benefits of urate-lowering therapy. However, it is important to note that large randomized clinical trials have not yet supported urate-lowering for cardiovascular health. For example, The AMETHYST conducted at 59 centers in 12 countries failed to demonstrate benefit [47]. In conclusion, while nearly all studies agree on the potential theoretical possibility of urate-lowering therapy for CVD, this has not yet been confirmed by large-scale randomized controlled trials.
Several limitations of the current study should be acknowledged. First, owing to the design of MR analysis, we could not confirm the results obtained from the real world, more randomized control trials and observational studies are needed to be conducted to verify the results. Second, although potential related risk factors were adjusted for, we still cannot exclude the possibility of residual or unmeasured confounding given the MR study design of the present analysis. Third, we could not collect all GWAS data for diseases classified as CKM due to the limited available GWAS source, so it is hard to reflect the broad patterns of the relationships between SUA and CKM.
5. Conclusion
Our research supported that elevated SUA and renal dysfunction respectively mediate the pathogenesis of various phenotypes of CKM syndrome. Meanwhile, lesinurad and social interventions (expanding the duration of education and enhancing income levels) could potentially treating IHD by improving SUA.
Abbreviations
- CKD
Chronic kidney disease
- CKM
Cardiovascular-kidney-metabolic
- CVD
Cardiovascular disease
- GWAS
Genome-wide association study
- IHD
Ischemic heart disease
- IV
Instrument variable
- IVW
Inverse variance weighted
- MR
Mendelian randomization
- MVMR
Multivariable mendelian randomization
- PAD
Peripheral artery disease
- SUA
Serum uric acid
- eGFR
Estimated glomerular filtration rate
- SDOH
Social determinants of health
- SNPs
Single nucleotide polymorphisms
- UA
Uric acid
CRediT authorship contribution statement
Lingyun Luo: Supervision, Software, Resources, Project administration, Methodology, Investigation. Xuelian Luo: Writing – review & editing, Writing – original draft, Visualization, Validation. Zhen He: Funding acquisition, Formal analysis, Data curation, Conceptualization.
Consent for publication
All authors agree to publish.
Ethics approval and consent to participate
All the data in this article is sourced from open databases. Thus, ethical approval from the institutional review committee and informed consent from the subjects were obtained in each original research.
Funding
No fund.
Declaration of competing interest
The authors declare that they have no relevant conflicts of interest.
Acknowledgements
Thanks to the ieu open GWAS project (https://gwas.mrcieu.ac.uk/) for providing us with GWAS data support. Thanks to Figdraw (https://www.figdraw.com/static/index.html#/) for providing us with image elements. We would like to thank the anonymous reviewers for their valuable and constructive feedback on our studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2025.100587.
Contributor Information
Xuelian Luo, Email: 652055@hospital.cqmu.edu.cn.
Zhen He, Email: hezhen@gdph.org.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
After the article is published, readers can send an email to the corresponding author to obtain data or materials.
References
- 1.Ndumele C.E., Neeland I.J., Tuttle K.R., et al. A synopsis of the evidence for the science and clinical Management of Cardiovascular-Kidney-Metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. 2023;148(20):1636–1664. doi: 10.1161/CIR.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 2.Ndumele C.E., Rangaswami J., Chow S.L., et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148(20):1606–1635. doi: 10.1161/CIR.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 3.Khan S.S., Coresh J., Pencina M.J., et al. Novel prediction equations for absolute risk assessment of Total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American Heart Association. Circulation. 2023;148(24):1982–2004. doi: 10.1161/CIR.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 4.Dawson J., Quinn T., Walters M. Uric acid reduction: a new paradigm in the management of cardiovascular risk? Curr. Med. Chem. 2007;14(17):1879–1886. doi: 10.2174/092986707781058797. [DOI] [PubMed] [Google Scholar]
- 5.Copur S., Demiray A., Kanbay M. Uric acid in metabolic syndrome: does uric acid have a definitive role? Eur. J. Intern. Med. 2022:103. doi: 10.1016/j.ejim.2022.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Demiray A., Afsar B., Covic A., et al. The role of uric acid in the acute myocardial infarction: a narrative review. Angiology. 2022;73(1) doi: 10.1177/00033197211012546. [DOI] [PubMed] [Google Scholar]
- 7.Piao W., Zhao L., Yang Y., et al. The prevalence of hyperuricemia and its correlates among adults in China: results from CNHS 2015-2017. Nutrients. 2022;14(19) doi: 10.3390/nu14194095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N., Weiner D.E. Uric acid, CKD, and cardiovascular disease: confounders, culprits, and circles. Am. J. Kidney Dis. Aug 2010;56(2):247–250. doi: 10.1053/j.ajkd.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey A.S., Perrone R.D., Madias N.E. Serum creatinine and renal function. Annu. Rev. Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 10.Vanholder R., De Smet R., Glorieux G., et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63(5):1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 12.Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362 doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb. Perspect. Med. 2021;11(2) doi: 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce B.L., Ahsan H., Vanderweele T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011;40(3):740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer T.M., Sterne J.A.C., Harbord R.M., et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am. J. Epidemiol. 2011;173(12):1392–1403. doi: 10.1093/aje/kwr026. [DOI] [PubMed] [Google Scholar]
- 16.Verbanck M., Chen C.-Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarnak M.J., Levey A.S., Schoolwerth A.C., et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 18.Carter A.R., Sanderson E., Hammerton G., et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur. J. Epidemiol. 2021;36(5):465–478. doi: 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wishart D.S., Feunang Y.D., Guo A.C., et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordi T., Khamis H. Simple solution to a common statistical problem: interpreting multiple tests. Clin. Ther. 2004;26(5):780–786. doi: 10.1016/s0149-2918(04)90078-1. [DOI] [PubMed] [Google Scholar]
- 21.Larsson S.C., Traylor M., Malik R., Dichgans M., Burgess S., Markus H.S. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ (Clin. Res. Ed). 2017;359 doi: 10.1136/bmj.j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangri N., Weiner D.E. Uric acid, CKD, and cardiovascular disease: confounders, culprits, and circles. Am. J. Kidney Dis. 2010;56(2):247–250. doi: 10.1053/j.ajkd.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoy S.M. Lesinurad: first global approval. Drugs. 2016;76(4):509–516. doi: 10.1007/s40265-016-0550-y. [DOI] [PubMed] [Google Scholar]
- 24.Sosa F., Shaban M., Lopez J., et al. Impact of hyperuricemia and urate-lowering agents on cardiovascular diseases. Clin. Med. Insights Cardiol. 2024;18 doi: 10.1177/11795468241239542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng R., Chen C., Yang T., Chen Q., Lu R., Mao Y. Serum uric acid levels and the risk of obesity: a longitudinal population-based epidemiological study. Clin. Lab. Oct 1 2017;63(10):1581–1587. doi: 10.7754/Clin.Lab.2017.170311. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Q., Liu J., Xu Y. Effects of uric acid on diabetes mellitus and its chronic complications. Int. J. Endocrinol. 2019;2019:9691345. doi: 10.1155/2019/9691345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Lozada L.G., Rodriguez-Iturbe B., Kelley E.E., et al. Uric acid and hypertension: an update with recommendations. Am. J. Hypertens. 2020;33(7):583–594. doi: 10.1093/ajh/hpaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borghi C., Agnoletti D., Cicero A.F.G., Lurbe E., Virdis A. Uric acid and hypertension: a review of evidence and future perspectives for the Management of Cardiovascular Risk. Hypertension. Sep 2022;79(9):1927–1936. doi: 10.1161/hypertensionaha.122.17956. [DOI] [PubMed] [Google Scholar]
- 29.Tan M.Y., Mo C.Y., Li F., Zhao Q. The association between serum uric acid and hypertriglyceridemia: evidence from the national health and nutrition examination survey (2007-2018) Front. Endocrinol. (Lausanne) 2023;14:1215521. doi: 10.3389/fendo.2023.1215521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng R., Ren P., Chen Q., Yang T., Chen C., Mao Y. Serum uric acid levels and risk of incident hypertriglyceridemia: a longitudinal population-based epidemiological study. Ann. Clin. Lab. Sci. Sep 2017;47(5):586–591. [PubMed] [Google Scholar]
- 31.Huang H., Huang B., Li Y., et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur. J. Heart Fail. Jan 2014;16(1):15–24. doi: 10.1093/eurjhf/hft132. [DOI] [PubMed] [Google Scholar]
- 32.Qin S., Xiang M., Gao L., Cheng X., Zhang D. Uric acid is a biomarker for heart failure, but not therapeutic target: result from a comprehensive meta-analysis. ESC Heart Fail. Feb 2024;11(1):78–90. doi: 10.1002/ehf2.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding M., Viet N.N., Gigante B., Lind V., Hammar N., Modig K. Elevated uric acid is associated with new-onset atrial fibrillation: results from the Swedish AMORIS Cohort. J. Am. Heart Assoc. Feb 7 2023;12(3) doi: 10.1161/jaha.122.027089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Dov I.Z., Kark J.D. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem lipid research clinic cohort study. Nephrol. Dial. Transplant. Aug 2011;26(8):2558–2566. doi: 10.1093/ndt/gfq740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinge J.M., Modalsli J.H., Øverland S., et al. Association of household income with life expectancy and cause-specific mortality in Norway, 2005-2015. JAMA. 2019;321(19):1916–1925. doi: 10.1001/jama.2019.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumer Y., Powell-Wiley T.M. Interdisciplinary approaches are fundamental to decode the biology of adversity. Cell. 2021;184(11):2797–2801. doi: 10.1016/j.cell.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller G.E., Chen E., Shimbo D. Mechanistic understanding of socioeconomic disparities in cardiovascular disease. J. Am. Coll. Cardiol. 2019;73(25):3256–3258. doi: 10.1016/j.jacc.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 38.Powell-Wiley T.M., Baumer Y., Baah F.O., et al. Social determinants of cardiovascular disease. Circ. Res. 2022;130(5):782–799. doi: 10.1161/CIRCRESAHA.121.319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du L., Zong Y., Li H., et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024;9(1):212. doi: 10.1038/s41392-024-01916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloberti A., Bombelli M., Facchetti R., et al. Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid right for heArt health study. J. Hypertens. 2021;39(2):333–340. doi: 10.1097/HJH.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 41.Luo L., Zuo Y., Dai L. Metabolic rewiring and inter-organ crosstalk in diabetic HFpEF. Cardiovasc. Diabetol. 2025;24(1):155. doi: 10.1186/s12933-025-02707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang T., Luo L., Luo X., Liu X. Metabolic crosstalk and therapeutic interplay between diabetes and hyperuricemia. Diabetes Res. Clin. Pract. 2025;224 doi: 10.1016/j.diabres.2025.112204. [DOI] [PubMed] [Google Scholar]
- 43.Maloberti A., Tognola C., Garofani I., et al. Uric acid and metabolic syndrome: importance of hyperuricemia cut-off. Int. J. Cardiol. 2024;417 doi: 10.1016/j.ijcard.2024.132527. [DOI] [PubMed] [Google Scholar]
- 44.Maloberti A., Colombo V., Daus F., et al. Two still unanswered questions about uric acid and cardiovascular prevention: is a specific uric acid cut-off needed? Is hypouricemic treatment able to reduce cardiovascular risk? Nutr. Metab. Cardiovasc. Dis. 2025;35(3) doi: 10.1016/j.numecd.2024.103792. [DOI] [PubMed] [Google Scholar]
- 45.Nishino M., Egami Y., Kawanami S., et al. Lowering uric acid may improve prognosis in patients with hyperuricemia and heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2022;11(19) doi: 10.1161/JAHA.122.026301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Mesa-Eguiagaray I., Campbell H., et al. A phenome-wide association and factorial Mendelian randomization study on the repurposing of uric acid-lowering drugs for cardiovascular outcomes. Eur. J. Epidemiol. 2024;39(8):869–880. doi: 10.1007/s10654-024-01138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitzman D.W., Voors A.A., Mentz R.J., et al. Verinurad plus allopurinol for heart failure with preserved ejection fraction: the AMETHYST randomized clinical trial. JAMA Cardiol. 2024;9(10):892–900. doi: 10.1001/jamacardio.2024.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
After the article is published, readers can send an email to the corresponding author to obtain data or materials.