Abstract
This study aimed to conduct a comprehensive analysis of the national glaucoma screening program in Kazakhstan from 2000 to 2022. A thorough search was conducted to identify policy documents regulating the provision of glaucoma screening from its inception to the present day. Official data on reimbursement for tonometry, glaucoma incidence, and the population aged ≥ 50 years were retrieved. Forecast modeling was utilized to project disease incidences up to 2030. The current program largely inherits the framework of the Soviet glaucoma screening, with tonometry serving as the primary screening tool. The budget allocated for glaucoma screening is not specified in official documents, as glaucoma screening is integrated into the routine activities of healthcare facilities. The highest costs are incurred when Maklakov tonometry is applied alone, reaching 7,557,679,411 Kazakhstani Tenge in 2023. The incidence of glaucoma increased from 59.1 per 100,000 in 2000 to 135.5 in 2022, reflecting the aging population. Disparities in incidence rates were observed between residents of urban and rural areas, as well as among residents of different provinces. It is anticipated that the incidence of glaucoma will continue to rise, reaching 154.5 per 100,000 in 2030. There is a need to initiate a discussion on potential amendments to the existing screening program.
Keywords: Glaucoma screening, Incidence, Budget impact analysis, Time series, Kazakhstan
Subject terms: Disease prevention, Population screening
Introduction
Being defined as progressive optic neuropathy with associated visual field defects, glaucoma is a chronic condition that has the potential to lead to irreversible blindness if not treated appropriately. According to the Glaucoma Research Foundation, approximately 80 million people worldwide are living with glaucoma. It is estimated that for every diagnosed case, there is at least one additional case that remains undiagnosed. Globally, glaucoma is the leading cause of irreversible blindness and the second most common cause of blindness overall, following cataract1. The incidence of glaucoma increases with aging1, and in individuals aged 40 years and older, the diagnosis of primary glaucoma is made in the absence of predisposing ocular conditions. Primary open-angle glaucoma (POAG) constitutes the most common type of primary glaucoma, responsible for 60–70% of all glaucoma cases. The primary cause of POAG is decreased permeability of anterior chamber angle structures to aqueous humor, resulting in elevated intraocular pressure (IOP)2. Primary angle closure glaucoma (PACG) develops due to blockage of the anterior chamber angle with iris, which could be both acute and chronic3. Both types of glaucoma are more common in certain ethnicities, with POAG being more common in people of African descent2, while PACG is more common in certain Asian ethnic subgroups3.
Based on the Wilson and Jenner criteria for screening, glaucoma meets many requirements. This condition constitutes a major health problem, and its natural history is well-understood. Glaucoma has an asymptomatic early stage, during which visual loss is absent, and individuals are commonly unaware of having a problem. This stage may last for a considerable period, possibly several years2. The disease has an accepted treatment directed at lowering IOP, and if this treatment is initiated early, it can help prevent irreversible visual loss. Currently, there is international consensus on who should be treated and how. Additionally, both the treatment and diagnosis of glaucoma are cost-effective4. A major challenge in glaucoma screening is the lack of a single test that is simple, accurate, and both sensitive and specific. Combining multiple tests improves diagnostic accuracy but reduces cost-effectiveness—especially given the relatively low prevalence of glaucoma5.
The Republic of Kazakhstan (hereafter referred to as Kazakhstan) is a Central Asian country and a former member of the Soviet bloc. The country gained independence in 1991 when the Union of Soviet Socialist Republics (USSR) dissolved, and its healthcare system shares many features with the Semashko model to the present day6. Kazakhstan inherited a glaucoma screening program from Soviet times, which has largely remained unchanged7. However, there is a lack of studies investigating the performance of this screening program over a considerable period of time. Therefore, this study aims to analyze the health policies related to screening provision with a historical perspective. The study will analyze glaucoma incidences as they reflect the screening performance and provide estimates of the impact made by glaucoma screening on the healthcare budget. This analysis is supplemented by forecasts of incidence rates until 2030. The findings of this study have practical implications as they are necessary for tailoring the glaucoma screening program to the needs of the Kazakhstani population.
Materials and methods
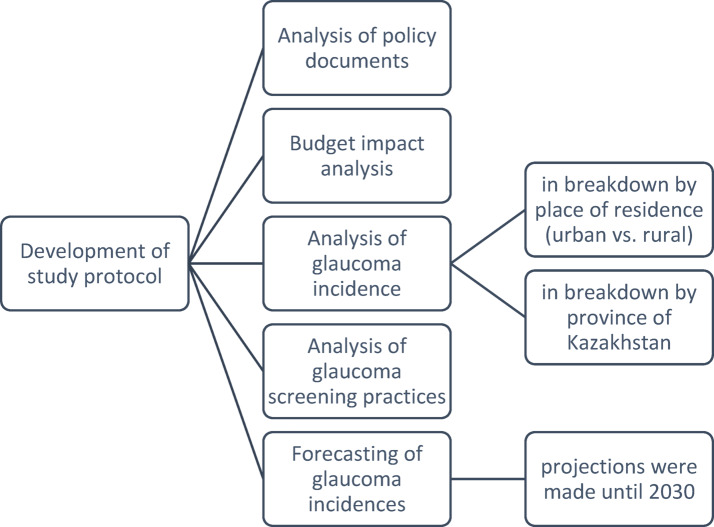
This study consisted of several stages outlined in Fig. 1. The first stage involved the analysis of policy documents governing the provision of glaucoma screening. This analysis facilitated the subsequent budget impact analysis conducted in the following stage. Glaucoma incidences, as reported by official statistics, were then analyzed in the subsequent stage, alongside the demographic data of the population aged over 50 years. Following this, the projections of glaucoma incidence until 2030 were generated.
Fig. 1.
Flow chart of study activities.
Analysis of policy documents regulating the provision of glaucoma screening
As a first step of our study, we performed an analysis of policy documents regarding the provision of glaucoma screening in Kazakhstan from inception to the present day. For this purpose, a series of searches were conducted to identify official documents commonly contained in the database maintained by the Ministry of Justice of Kazakhstan (“Adilet” database). This helped identify two orders issued by the Ministry of Health (MoH) of Kazakhstan in 2009 and 20208,9. Additionally, the database created by “GARANT-SERVICE” (a Russian company operating in the field of information technologies) was consulted as it contains historical documents issued during the times of the USSR. This helped identify the MoH USSR order issued in 19767. Finally, a Google search was conducted to identify any remaining policy documents pertaining to national glaucoma screening in Kazakhstan.
All searches were conducted in two languages (Kazakh and Russian), which have official language status in Kazakhstan. For searches in the Russian language, the following search queries were used: (i) “глаукома” (meaning “glaucoma”), (ii) “скрининг” (meaning “screening”), (iii) “раннее выявление” (meaning “early identification”), (iv) “целевые группы населения” (meaning “target population groups”), and (v) “Казахстан” (meaning “Kazakhstan”). For searches in the Kazakh language, the following keywords were used: (i) “суқараңғы” (meaning “glaucoma”), (ii) “скрининг” (meaning “screening”), (iii) “ерте анықтау” (meaning “early identification”), (iv) “мақсатты популяциялар” (meaning “target population groups”), and (v) “Қазақстан” (meaning “Kazakhstan”). In the first stage, the titles of all identified policy documents were reviewed, and lists of all relevant documents in Kazakh and Russian languages were compiled. After comparing the two lists, duplicates were removed, and the full-text versions of the remaining documents were retrieved. Next, data extraction forms were constructed to extract data on stages of the glaucoma screening, involved medical personnel, target population groups, screening procedures, and sources of funding.
Budget impact analysis of the national glaucoma screening program
As the first stage of our study failed to identify official information on the budgets specifically allocated for glaucoma screening in Kazakhstan and the number of individuals screened every year, we estimated the possible impact of the glaucoma screening program on the national healthcare budget. For this purpose, we retrieved information on the amount of money reimbursed from the state budget for the performance of tonometry, which is the only glaucoma screening test utilized in Kazakhstan7–9. This amount of money is referred to as “tariffs for medical services”, which are reconsidered annually following the devaluation of the national currency, the Kazakhstani Tenge (KZT), and are regulated by the Orders of the MoH. These tariffs have been available in open access since 2018 and are contained in the Adilet database10. Our previous stage of the study showed that Maklakov tonometry and air-puff tonometry are utilized on an equal basis for glaucoma screening in Kazakhstan8, but they are not reimbursed on equal grounds10. Therefore, the costs of these screening tests were considered separately and in combination with each other (50%/50%). All cost estimations were conducted from a societal perspective. Since glaucoma screening is offered free of charge to the target population in Kazakhstan, the cost analysis included expenses related to equipment acquisition and operational implementation10.
To estimate the number of the target population eligible for screening in accordance with the acting MoH order, the demographic yearbooks issued by the Bureau of National Statistics of Kazakhstan for the period 2018–2023 were consulted11. Thus, the biannual number of individuals aged 40–70 years was retrieved for 2018–2020, while for 2021–2023, the biannual number of individuals aged 30–70 years was retrieved, along with the annual number of the rural population aged 18–29 years. The cost of tonometry was evaluated for both eyes, broken down by the type of tonometry test employed. Since the number of the population actually undergoing screening is not known, different scenarios were evaluated, with screening coverage constituting 100%, 75%, 50%, and 25%. For international comparisons, the KZT was converted to United States Dollar (USD), and the official conversion rate for each corresponding year was obtained from the website of the National Bank of Kazakhstan12. All calculations were performed using Excel software (Microsoft Office 2021).
Analysis of glaucoma incidence: national and regional breakdown
The data on glaucoma incidence were obtained from the statistical compilation titled “Public Health in the Republic of Kazakhstan and Activities of Healthcare Facilities,” which is issued annually by the MoH and is freely accessible on the website of the National Research Center for Health Development named after Salidat Kairbekova13. The earliest available statistics on glaucoma incidence rates in urban-rural breakdown are from 2000, with the latest data available for 2022. The MoH calculates glaucoma incidences based on annual reports of newly diagnosed cases submitted by the country’s healthcare facilities. These data are presented in the subsection of the statistical compilation titled “Health Indicators” and are expressed per 100,000 population. The analysis of glaucoma incidence rates from 2000 to 2022 was complemented by analyzing the annual population numbers of individuals aged 50 years and older, sourced from the Demographic Yearbooks11. This age group was chosen for analysis due to the observed trend of elevated glaucoma rates in populations aged 50 and older14,15. The extracted data were entered into an Excel spreadsheet, and graphs depicting variations in glaucoma incidence were constructed.
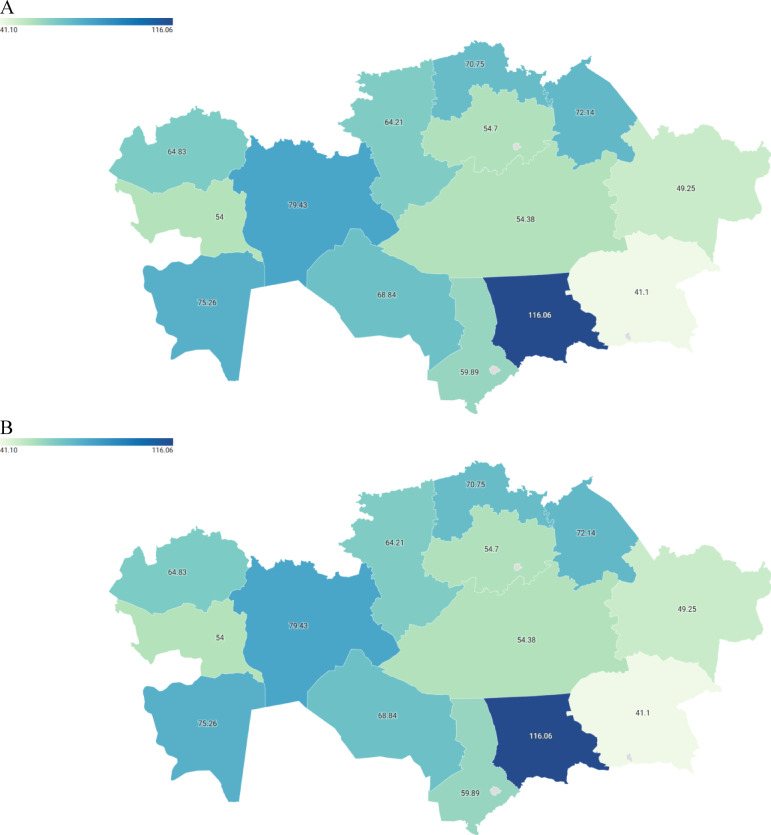
In addition to disaggregation by the principle of “urban-rural,” the statistical compilations provide data on glaucoma incidence disaggregated by Kazakhstan’s provinces. Over the study period (2000–2022), Kazakhstan comprised 14 administrative subdivisions known as “oblasts.” Data on glaucoma incidences were extracted for each of these provinces (Akmola, Aktobe, Almaty, Atyrau, East Kazakhstan, Karaganda, Kostanay, Kyzylorda, Mangystau, North Kazakhstan, Pavlodar, South Kazakhstan, West Kazakhstan, and Zhambyl) from 2000 to 2022. Average incidence rates were calculated using Excel software for the periods 2000–2009 and 2010–2022 (before and after the MoH began introducing amendments to the national glaucoma screening program). To visualize regional variations in glaucoma incidence, maps of Kazakhstan’s provinces for the respective time periods were created using Datawrapper (https://www.datawrapper.de/), with darker shades representing elevated incidences and lighter shades indicating lower incidences.
Time series analysis
The data on glaucoma incidence rates per 100,000 population and the population numbers in Kazakhstan aged 50 years and older for the period 2000–2022 were organized in an Excel spreadsheet. The Expert Modeler function of the Social Package for Statistical Sciences (SPSS), version 24.0 was employed to automatically identify the best-fit epidemiological models for projecting trends until 2030. All projections were reported as point estimates along with their corresponding 95% confidence intervals (CI). Graphs depicting both historical and projected values were generated for visualization purposes. Additionally, the average annual change, along with its 95% CI, was computed to assess trends during the historical period. The significance level for all statistical tests was pre-set at 0.05.
Ethics statement
The study adhered to the principles outlined in the Helsinki Declaration and in the Belmont Report, and an informed consent have been obtained from participants approval from the ethics committee was secured prior to the commencement of the study. (Minutes of the meeting of the Local Ethics Committee of Semey Medical University #2a dated 14.11.2022).
Results
At the time of Kazakhstan’s independence in 1991, a screening order issued by the MoH of the USSR was in effect. This order outlined a three-stage glaucoma screening process, starting with the measurement of IOP using Maklakov tonometry, followed by consultation with a community ophthalmologist. Final decisions regarding the presence, staging, and treatment of glaucoma were made either during hospitalization in the inpatient ophthalmology department or through examination at the out-patient glaucoma clinic. Screening was targeted at adults aged 40 and older, with examinations conducted every three years7. In November 2009, the Kazakhstan MoH issued an order largely consistent with the previous one. The target population was redefined as adults aged 40–70 years, with screening examinations conducted every two years. Air-puff tonometry was introduced alongside Maklakov tonometry, and screening was divided into two stages, with final decisions made by community ophthalmologists8. Starting in 2020, a new screening order came into effect, largely similar to the previous one but with a special focus on the rural population aged 18–29 years, who are to be screened annually. The rationale behind this provision is not explained9. The summary of glaucoma screening programs in Kazakhstan up to the present time is presented in Table 1.
Table 1.
Evolution of glaucoma screening program in Kazakhstan.
| Stages of screening program | Medical personnel involved | Target population | Screening procedures | Source of funding | |
|---|---|---|---|---|---|
|
Before 2010 (The USSR MoH* order issued in 19767) | |||||
| First stage, | Nurse | All individuals older than 40 years are advised to undergo screenings every three years. | IOP** measurement using Maklakov tonometry. | State budget | |
| Second stage | Community ophthalmologist | Consultation of an ophthalmologist in case of detected IOP elevation. | |||
| Third stage | Hospital ophthalmologist | The final decision regarding the presence, staging, and treatment of glaucoma was made either during hospitalization in the inpatient ophthalmology department or through examination at the glaucoma clinic. | |||
|
2010–2020 (The Kazakhstan MoH order issued in 20098) | |||||
| First stage | Nurse | All individuals between the ages of 40 and 70 are advised to undergo biennial screenings. |
1. Interviewing a patient. 2. IOP measurement using Maklakov tonometry or an air-puff tonometry. 3. Referral of a patient to an ophthalmologist in case of elevated IOP (over 25 mm Hg with Maklakov tonometry and over 20 mm Hg with air-puff tonometry). This referral is also necessary if there is a difference in IOP between the right and left eyes exceeding 5 mm Hg. |
State budget | |
| Second stage | Community ophthalmologist | Examination by an ophthalmologist in case of detected IOP elevation to determine the presence, staging, and treatment of glaucoma. | |||
|
2020-present (The Kazakhstan MoH order issued in 20209) | |||||
| First stage | Nurse |
1. All individuals between the ages of 30 and 70 are advised to undergo biennial screenings. 2. Rural dwellers between the ages of 18 and 29 are advised to undergo annual screening. |
IOP measurement using Maklakov tonometry or an air-puff tonometry. | State budget /Health Insurance Fund | |
| Second stage | Community ophthalmologist | Examination by an ophthalmologist in case of detected IOP elevation to determine the presence, staging, and treatment of glaucoma. | |||
*USSR MoH—Union Soviet Socialist Republics Ministry of Health.
**IOP—intraocular pressure.
Table 2 presents estimates of costs incurred by Kazakhstan’s budget on glaucoma screening for the period of six years (2018–2023). Since the actual number of individuals screened for glaucoma, along with the contribution of different tonometry tests to glaucoma screening, is not known, different scenarios were employed. These scenarios varied the screening coverage between 25% and 100%, with different tonometry techniques contributing differently to overall screening. In general, the cost of screening increased substantially after 2020 due to the adoption of the new MoH order, which envisages the screening of rural people aged 18–29 years. The highest costs were incurred if Maklakov tonometry is applied alone, reaching the level of 7,557,679,411 KZT (16,562,598.7 USD) in 2023, assuming 100% coverage of the eligible population. Since air-puff tonometry is reimbursed at a lower rate, the application of air-puff tonometry alone results in lower costs. However, it would be more realistic to propose that Maklakov tonometry is applied along with air-puff tonometry, with not more than 50% of the eligible population screened.
Table 2.
Budget impact analysis of glaucoma screening program, 2018–2023.
| Item | Year | ||||||
|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| Cost of Maklakov tonometry, KZT* | One eye | 517.17 | 562.07 | 675.91 | 733.48 | 813.08 | 902.4 |
| Two eyes | 1034.34 | 1124.14 | 1351.82 | 1466.96 | 1626.16 | 1804.8 | |
| Cost of air-puff tonometry, KZT | One eye | 281.8 | 326.7 | 414.15 | 471.72 | 551.32 | 640.64 |
| Two eyes | 563.6 | 653.4 | 828.3 | 943.44 | 1102.64 | 1281.28 | |
| The number of individuals to be screened, as determined by the acting MoH∞ order | 1,607,087 | 1,636,849 | 1,673,552 | 4,195,843 | 4,190,824 | 4,187,544 | |
| USD⁰ to KZT conversion rate | 344.71 | 382.75 | 412.95 | 426.03 | 460.48 | 456.31 | |
| Annual screening costs if Maklakov tonometry is performed alone, KZT (USD) | 100% coverage |
1,662,273,850 (4,822,238.55) |
1,840,046,873 (4,807,437.94) |
2,262,340,389 (5,478,485.02) |
6,155,133,847 (14,447,653.56) |
6,814,949,543 (14,799,664.57) |
7,557,679,411 (16,562,598.7) |
| 75% coverage |
1,246,705,387.81 (3,616,678.91) |
1,380,035,154.59 (3,605,578.46) |
1,696,755,291.55 (4,108,863.76) |
4,616,350,385.46 (10,835,740.17) |
5,111,212,157.07 (11,099,748.43) |
5,668,259,558.40 (12,421,949.02) |
|
| 50% coverage |
831,136,925.21 (2,411,119.27) |
920,023,436.40 (2,403,718.97) |
1,131,170,194.37 (2,739,242.51) |
3,077,566,923.64 (7,223,826.78) |
3,407,474,771.38 (7,399,832.29) |
3,778,839,705.60 (8,281,299.35) |
|
| 25% coverage |
415,568,462.60 (1,205,559.64) |
460,011,718.20 (1,201,859.49) |
565,585,097.18 (1,369,621.25) |
1,538,783,461.82 (3,611,913.39) |
1,703,737,385.69 (3,699,916.14) |
1,889,419,852.80 (4,140,649.67) |
|
| Annual screening costs if air-puff tonometry is performed alone, KZT (USD) | 100% coverage |
905,753,951.4 (2,627,582.46) |
1,069,516,810 (2,794,296.04) |
1,386,202,707 (3,356,829.42) |
3,958,526,120 (9,291,660.49) |
4,620,969,624 (10,035,114.72) |
5,365,416,376 (11,758,270.42) |
| 75% coverage |
679,315,463.6 (1,970,686.85) |
802,137,607.4 (2,095,722.03) |
1,039,652,031 (2,517,622.06) |
2,968,894,590 (6,968,745.37) |
3,465,727,218 (7,526,336.04) |
4,024,062,282 (8,818,702.82) |
|
| 50% coverage |
452,876,975.7 (1,313,791.23) |
534,758,405 (1,397,148.02) |
693,101,353.7 (1,678,414.71) |
1,979,263,060 (4,645,830.25) |
2,310,484,812 (5,017,557.36) |
2,682,708,188 (5,879,135.21) |
|
| 25% coverage |
226,438,487.9 (656,895.62) |
267,379,202.5 (698,574.01) |
346,550,676.9 (839,207.35) |
989,631,530 (2,322,915.12) |
1,155,242,406 (2,508,778.68) |
1,341,354,094 (2,939,567.61) |
|
| Annual screening costs if Maklakov tonometry is performed in combination with air-puff tonometry (50%/50%), KZT (USD) | 100% coverage |
1,284,013,901 (3,724,910.507) |
1,454,781,841 (3,800,866.992) |
1,824,271,548 (4,417,657.218) |
5,056,829,984 (11,869,657.03) |
5,717,959,583 (12,417,389.64) |
6,461,547,894 (14,160,434.56) |
| 75% coverage |
963,010,425.7 (2,793,682.88) |
1,091,086,381 (2,850,650.24) |
1,368,203,661 (3,313,242.91) |
3,792,622,488 (8,902,242.77) |
4,288,469,688 (9,313,042.23) |
4,846,160,920 (10,620,325.92) |
|
| 50% coverage |
642,006,950.5 (1,862,455.25) |
727,390,920.7 (1,900,433.5) |
912,135,774 (2,208,828.61) |
2,528,414,992 (5,934,828.51) |
2,858,979,792 (6,208,694.82) |
3,230,773,947 (7,080,217.28) |
|
| 25% coverage |
321,003,475.2 (931,227.63) |
363,695,460.3 (950,216.75) |
456,067,887 (1,104,414.30) |
1,264,207,496 (2,967,414.26) |
1,429,489,896 (3,104,347.41) |
1,615,386,973 (3,540,108.64) |
|
*KZT—Kazakhstani Tenge.
∞MoH—Ministry of Health.
⁰USD— United States Dollar.
The incidence of glaucoma more than doubled during the period 2000–2022. The overall annual incidence of glaucoma was 59.1 per 100,000 in 2000 and increased to 135.5 per 100,000 in 2022, with an average annual growth rate of 4.89% (95% CI: 4.03–5.76). The incidence rate of glaucoma in urban populations exceeded that in rural populations, ranging between 73.6 per 100,000 population in 2000 and 150.7 in 2022, with an average annual growth rate of 4.75% (95% CI: 3.82–5.70). The rising incidence of glaucoma reflects the increasing number of people aged 50 years and older. The overall number of people ≥ 50 years of age increased from 2,706,337 in 2000 to 4,538,904 in 2022, with an average annual growth rate of 2.46% (95% CI: 2.34–2.57). The rural population experienced a steadier increase than the urban population, with average annual growth rates of 2.75% (95% CI: 2.50-3.00) and 2.26% (95% CI: 1.98–2.54), respectively (Fig. 2).
Fig. 2.
Glaucoma incidence per 100,000 population (A) and the number of individuals aged 50 years and older (in thousands) (B) from 2000 to 2022.
Figure 3 illustrates the average incidence rates of glaucoma across different provinces of Kazakhstan for the periods 2000–2009 and 2010–2022. The pattern of glaucoma incidence was consistent across different provinces. For instance, Aktobe province had one of the highest average glaucoma incidences among all provinces, with rates of 79.43 per 100,000 population during 2000–2009 and 119.71 during 2010–2022. Similarly, Kyzylorda province exhibited one of the highest average incidences of glaucoma, with rates of 68.34 per 100,000 during 2000–2009 and the highest rate of 205.38 during 2010–2022. Conversely, the lowest average incidence rate of glaucoma was observed in Almaty province, with rates of 41.1 per 100,000 population during 2000–2009 and 69.45 during 2010–2022.
Fig. 3.
Average incidence rates of glaucoma per 100,000 population in the provinces of Kazakhstan for the periods 2000–2009 (A) and 2010–2022 (B).
Glaucoma will not lose its significance in the next few years, as forecast modeling has shown that its incidence will continue to grow. It is expected to reach 144.3 (95% CI: 106.2–182.3) per 100,000 in 2026 and 154.5 (95% CI: 80.0–229.0) in 2030. The projected growth of the population aged 50 years and older will be even more consistent, with the number of such individuals estimated to be 5,160,906 people (95% CI: 4,874,569–5,447,243) in 2026 and 5,792,905 people (95% CI: 5,120,533–6,465,277) in 2030 (Table 3). Figure 4 serves as an illustration of Table 3 and depicts the observed and projected incidence rates of glaucoma and the population ≥ 50 years by 2030.
Table 3.
The projected incidence rates of glaucoma and number of individuals aged 50 years and older in Kazakhstan for the years 2026 and 2030, accompanied by 95% confidence intervals.
| Parameter | Year | Model parameters | |||
|---|---|---|---|---|---|
| 2026 Rate (95% CI*) |
2030 Rate (95% CI*) |
Type of model | Alpha (level) | ||
| t | P-value | ||||
| Glaucoma incidence per 100,000 population |
144.3 (106.2; 182.3) |
154.5 (80.0; 229.0) |
Brown | 5.081 | < 0.001 |
| Number of population aged 50 years and older |
5,160,906 (4,874,569; 5,447,243) |
5,792,905 (5,120,533; 6,465,277) |
Brown | 5.151 | < 0.001 |
*95% CI—95% Confidence Interval
Fig. 4.
The observed and projected incidence rates of glaucoma (A) and the number of individuals aged 50 years and older (B) in Kazakhstan until 2030.
Discussion
This study aimed to analyze the national glaucoma screening program in Kazakhstan from 2000 to 2022. The current program is largely a legacy of the Soviet glaucoma screening, with tonometry serving as the main screening instrument. The latest amendments introduced in 2020 expanded the age range of the target population from 40 to 30 years for the general population and included screening for individuals aged 18 to 29 years in rural areas. The budget allocated for glaucoma screening is not specified in official documents, as glaucoma screening is embedded in the routine activities of healthcare facilities, which perform tonometry tests along with other ophthalmic procedures, applying for reimbursement from the state budget or health insurance fund. The incidence of glaucoma increased during the study period, reflecting the aging population, particularly those aged 50 years and older. There was a disparity in incidence rates between residents of urban and rural areas, as well as among residents of different provinces. It is anticipated that the incidence of glaucoma will continue to rise along with the increasing number of individuals aged 50 years and older. Therefore, the findings of this study warrant further discussion and examination.
Opportunistic glaucoma screening is widespread globally, while systematic screening is conducted in a limited number of countries5. The US Preventive Services Task Force discourages systematic glaucoma screening due to its low prevalence in the general population and the high associated costs of screening tests in primary care settings, which often yield false positives requiring further examinations and treatment16,17. However, opportunistic screening is recommended for at-risk populations, including individuals with diabetes mellitus (DM), a family history of glaucoma, and those of Black or Hispanic descent over 50 or 65 years old, respectively4. At the same time, the American Academy of Ophthalmology recommends screening for glaucoma and other eye diseases for individuals aged 40 years and older, with those who have DM, arterial hypertension, or a family history of eye disease needing to be screened earlier18. In the United Kingdom, systematic glaucoma screening is not implemented. Similar to the US, opportunistic screening is offered to individuals with a family history of glaucoma, diagnosed DM, and those over 60 years old19.
Traditionally, screening tests for POAG rely on one of three approaches: IOP measurement, visual field assessment, optic disc imaging, or a combination of these methods. Perimetry-based technologies have been evaluated in community settings, resulting in varying sensitivity and specificity. In a study by Dabasia et al., Frequency Doubling Technology for glaucoma suspects resulted in 62.1% sensitivity and 80.5% specificity, while for definitive POAG, sensitivity was 88.5% and specificity was 79.1%. Another perimetry technique, the Moorfield Motion Displacement Test, resulted in 51.1% sensitivity and 82.8% specificity for glaucoma suspects, and 65.4% sensitivity and 81.2% specificity for definitive glaucoma20.
The study by Dabasia et al. also showed that IOP measurement resulted in lower sensitivity (26.9%) but higher specificity (87.9%) for definitive glaucoma, with a cut-off range of referral > 21.0 mm Hg scale20. Another IOP-based screening study reported similar sensitivity and specificity for cut-off points of > 21 mmHg, with values of 24.1% and 88.6%, respectively, for combined glaucoma suspects and definitive glaucomas21. Air-puff tonometry, which is used in Kazakhstan along with Maklakov tonometry (Maklakov tonometry belongs to applanation tonometry, while air-puff tonometry is not), shows controversy when compared with applanation tonometry. Certain studies demonstrated good agreement between these methods22, while other studies demonstrated discordance23. Therefore, the value of air-puff tonometry as a standalone method of glaucoma screening requires further investigation before it is broadly implemented as a screening instrument.
Regarding the potential of optic disc imaging as a screening test, the study by Dabasia et al. also evaluated various optical coherence tomography (OCT)-based approaches to detect glaucoma in a community setting. OCT of the ganglion cell complex (GCC), focusing on focal loss of volume, demonstrated 73.1% sensitivity and 90.3% specificity for definitive glaucoma. OCT GCC focusing on global loss of volume resulted in 46.2% sensitivity and 97.9% specificity. Additionally, OCT of retinal nerve fiber layer thickness in the inferior quadrant yielded 76.9% sensitivity and 95% specificity for definitive glaucoma20. However, there is currently no consensus on the most effective combination of tests for POAG screening, nor on the appropriate cut-off levels for these screening tests. Given the variation in performance statistics across different studies, there is no international agreement on which screening test or combination of tests could be considered simple, safe, and precise for POAG19.
Another promising approach to screening for POAG involves the use of artificial intelligence (AI)-based optic disc imaging technologies, which have recently emerged as transformative tools in glaucoma detection. Sharma et al. reported the development of an AI-based glaucoma screening (AI-GS) system comprising six lightweight deep learning models capable of analyzing fundus images for early glaucomatous changes, such as optic disc cupping, hemorrhages, and retinal nerve fiber layer defects. In controlled settings, the AI-GS system demonstrated a sensitivity of 93.52% at a specificity of 95%. However, its performance in real-world clinical environments declined, with sensitivity dropping to 80.53% and specificity to 91.12%24. Despite its potential, the implementation of AI-assisted screening faces several challenges, including variability in image quality, differences in patient demographics, and the requirement for large, diverse datasets to ensure the generalizability and robustness of AI algorithms.
In the case of PACG, limbal anterior chamber depth (LACD) measurement (also referred to as the Van Herick method) is often considered an appropriate screening test. The proposed cutoff is an LACD of ≤ 25% corneal thickness, and gonioscopy is the gold standard to confirm angle closure25. The Cochrane review calculated the pooled sensitivity of LACD ≤ 25%, which equaled 83%, and the pooled specificity, which equaled 88%26. As for alternative screening tests, the oblique flashlight test (OFT), Scheimpflug photography (measurement of ACD in the central zone), scanning peripheral anterior chamber depth (SPACD) analyzer, and anterior segment OCT could be used for identifying individuals with occludable angles27. The same Cochrane review provided the pooled sensitivity and specificity for all of these screening tests, which equaled 51% and 92%, respectively, for OFT; 83% and 78%, respectively, for SPACD; 92% and 86%, respectively, for Scheimpflug photography; and 85% and 71%, respectively, for anterior segment OCT26.
The epidemiology of glaucoma in Kazakhstan is understudied, with a limited number of studies available. A study reporting the results of systematic glaucoma screening of individuals aged 40 years and older found that the prevalence of definitive glaucoma constituted 2.63% (452 cases out of 17,183 screened)28. Unfortunately, the study did not provide disaggregation by glaucoma type, i.e., POAG vs. PACG. Meanwhile, the population of Kazakhstan may have a higher prevalence of PACG, as the bulk of the population is composed of Kazakhs, who belong to a Turkic ethnic group, and there is an indication that the prevalence of PACG in this population is higher than in other ethnic groups15. Another study on the performance of the glaucoma screening program in Kazakhstan for the period 2009–2016 confirmed its suboptimal performance, as the incidence rates of definitive glaucoma were at least two times lower than the pooled global incidence rates for each of the age groups under study29.
This study illustrates the transition in glaucoma screening approaches in Kazakhstan from 2000 to 2022, including changes in screening indications, frequency, diagnostic methods, and the role of various ophthalmology workforce members. A major shift observed was the move from a historically Soviet-style (Semashko) model to a more decentralized approach. Under the early post-independence healthcare structure, Kazakhstan retained many features of the Semashko model, including an over-reliance on hospital-based services6, which directly impacted glaucoma management practices. At that time, community ophthalmologists primarily identified suspected glaucoma cases, while final decisions regarding diagnosis, staging, and treatment were made following admission to inpatient ophthalmology departments30. The transition away from the Semashko model was marked by a reduction in inpatient ophthalmology beds and a decrease in the number of hospital-based ophthalmologists. Consequently, many hospital ophthalmologists shifted to community practice. This change was institutionalized in the 2009 MoH order, which reassigned the responsibility for glaucoma diagnosis and treatment decisions to community ophthalmologists, while hospital-based specialists primarily focused on performing glaucoma surgeries8.
Although data on the preferred mode of tonometry for glaucoma screening in Kazakhstan are lacking, the widespread availability of air-puff tonometers in ophthalmology clinics30 suggests that screening is likely predominantly conducted using air-puff tonometry. Similarly, no official data exist regarding the actual screening coverage of the target population. However, given that a significant proportion of glaucoma patients are diagnosed at advanced or far-advanced stages29, it is reasonable to infer that current screening coverage is suboptimal and is likely to constitute 50% or even 25% from the target population.
The evaluation of the budget associated with the provision of glaucoma screening highlighted several issues. Since line-item budgeting is not implemented within the Kazakhstani healthcare system, control and accountability over expenditures are limited, and the transparency of fund distribution is not assured31. Consequently, the true costs associated with glaucoma screening remain unknown, and the issue of non-transparent allocation of public healthcare funds has become a topic of debate32. Additionally, as glaucoma screening is provided free of charge and considering the limited healthcare resources, there is potential for policy changes.
Recommendations to improve glaucoma screening practices in Kazakhstan
Based on the findings of this study, several recommendations can be made to improve the national glaucoma screening program in Kazakhstan. First, existing screening policies should be critically revised. For example, the 2020 policy mandates annual screenings for rural adults aged 18–29 years, despite a lack of evidence-based justification. Given the low prevalence of glaucoma in this age group and the constraints of limited healthcare resources, this provision appears inefficient. A more cost-effective and clinically appropriate strategy may involve screening high-risk populations every 2–3 years33.
Second, the current reliance on tonometry as the sole screening method should be reconsidered. Normal-tension primary open-angle glaucoma constitutes up to 80% of POAG cases in certain populations34, making tonometry alone insufficient for reliable detection. In addition, considering that the majority of Kazakhstan’s population consists of ethnic Kazakhs—who are at higher risk for PACG—the inclusion of anterior chamber depth assessments, such as LACD measurement, may represent a more suitable screening approach25. For earlier detection of POAG, incorporating optic disc imaging into screening protocols should also be considered18. Moreover, transitioning from systematic to opportunistic screening for individuals at increased risk may provide a more pragmatic and resource-sensitive alternative35,36.
To ensure that screening policies are evidence-based and aligned with available resources, national or regional population-based prevalence studies are crucial to accurately assess the burden of glaucoma, with disaggregation by age group. This will facilitate a more comprehensive understanding of glaucoma incidence across different age strata. Also, further research is needed to evaluate the effectiveness of the national screening program, including assessments of its clinical outcomes, economic efficiency, and overall value to the population.
Study limitations
This study has both strengths and limitations. To the best of our knowledge, it is the first to provide an in-depth analysis of the national glaucoma screening program in Kazakhstan from its inception to the present. A major limitation lies in the aggregated nature of the available data, which is disaggregated only by place of residence (urban vs. rural). Unfortunately, data on glaucoma incidence across different age strata are lacking due to the limitations inherent in official statistical compilations. Furthermore, the exact costs associated with glaucoma screening could not be determined, as specific budget allocations for this purpose are absent. The number of individuals screened annually is also not reported, which limits the precision of cost estimates and necessitates the use of broad approximations. Additionally, the forecasting analysis is subject to its own limitations, as such models depend on historical data and assume that past trends will persist under similar conditions. These assumptions may not hold in dynamic healthcare systems, where changes in policy, demographic shifts, or technological advancements can significantly affect disease incidence and screening practices. Therefore, further research is needed to more accurately assess the effectiveness and cost-efficiency of the national glaucoma screening program in Kazakhstan.
Author contributions
Study Conception and design: M.U. Acquisition of data: Y.S. Analysis and interpretation of data: E.K. Drafting of the work: Z.D. Critical Revision of the manuscript: E.K., Z.D. Statistical Analysis: M.U. Study supervision: Y.S. Final approval of the manuscript: M.U.
Funding
This research received no funding.
Data availability
The data used and analyzed during the current study are available from the corresponding author upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glaucoma Research Foundation. Glaucoma worldwide: A growing concern. Available online: https://glaucoma.org/glaucoma-worldwide-a-growing-concern/ (Accessed 17 Feb 2024).
- 2.American Academy of Ophthalmology. EyeWiki. Primary open-angle glaucoma. Available online: https://eyewiki.aao.org/Primary_Open-Angle_Glaucoma (Accessed 17 Feb 2024).
- 3.Dave, S. D. & Meyer, J. J. Chronic Closed Angle Glaucoma. [Updated 2024 Jan 25]. In StatPearls [Internet] (StatPearls Publishing, Treasure Island (FL), 2024). Available from: https://www.ncbi.nlm.nih.gov/books/NBK559098/. [PubMed]
- 4.Stein, J. D., Khawaja, A. P. & Weizer, J. S. Glaucoma in adults-screening, diagnosis, and management: A review. JAMA325(2), 164–174. 10.1001/jama.2020.21899 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Gunzenhauser, R. & Coleman, A. L. Glaucoma screening guidelines worldwide. J. Glaucoma9 S9–S12. 10.1097/IJG.0000000000002362 (2024). [DOI] [PMC free article] [PubMed]
- 6.Semenova, Y., Lim, L., Salpynov, Z., Gaipov, A. & Jakovljevic, M. Historical evolution of healthcare systems ofpost-soviet Russia, Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, Armenia, andAzerbaijan: A scoping review. Heliyon10(8), e29550. 10.1016/j.heliyon.2024.e29550. (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garant. Order of the Union Soviet Socialist Republics Ministry of Health No. 925 (dated September 22,1976) On measures for early detection and active monitoring of patients with glaucoma. Available online: https://base.garant.ru/4184302/ (Accessed 12 Feb 2024).
- 8.Adilet. Order of the MoH Kazakhstan No. 685 (dated November 10, 2009) On approval of the regulationsfor conducting preventive medical examinations among specific target population groups. Available online: https://adilet.zan.kz/rus/docs/V090005918_#z1_ (Accessed 12 Feb 2024).
- 9.Adilet dated October 30,. Order of the MoH No. No ҚР ДСМ-174/2020 On approval of the regulations for conducting preventive medical examinations among specific target population groups. Available online. https://adilet.zan.kz/rus/docs/V2000021820#z99 (Accessed 12 Feb 2024).
- 10.Adilet Orders of the MoH Kazakhstan On approval of tariffs for medical services provided within the framework of the guaranteed volume of free medical care and in the system of compulsory social health insurance. Available online: https://adilet.zan.kz/rus/docs/V1800017353/history (Accessed 18 Feb 2024).
- 11.Bureau of the national statistics. Demographic Yearbook of Kazakhstan. Available online https://stat.gov.kz/ru/publication/collections/?year=&name=16809.= (Accessed 18 Feb 2024).
- 12.National Bank of Kazakhstan. Official exchange rates of the national currency. Available online: https://www.nationalbank.kz/ru/news/oficialnye-kursy (Accessed 16 Feb 2024).
- 13.Salidat Kairbekova National Research Center for Health Development. Statistical yearbooks Public health in the Republic of Kazakhstan and activities of healthcare facilities. Available online: www.nrchd.kz/index.php/ru/?option=com_content&view=article&id=973 (Accessed 19 Feb 2024).
- 14.Zhang, N., Wang, J., Li, Y. & Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review. Sci. Rep.11(1), 13762. 10.1038/s41598-021-92971-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, N., Wang, J., Chen, B., Li, Y. & Jiang, B. Prevalence of primary angle closure glaucoma in the last 20 years: A meta-analysis and systematic review. Front. Med. (Lausanne)7, 624179. 10.3389/fmed.2020.624179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou, R. et al. Screening for glaucoma in adults: Updated evidence report and systematic review for the US preventive services task force. JAMA327(20), 1998–2012. 10.1001/jama.2022.6290 (2022). [DOI] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force. Final recommendation statement. Primary open angle glaucoma: screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/primary-open-angle-glaucoma-screening (Accessed 13 Feb 2024).
- 18.American Academy of Ophthalmology. Primary open-angle glaucoma suspect PPP 2020. Available online: https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-suspect-ppp (Accessed 17 Feb 2024).
- 19.Hamid, S., Desai, P., Hysi, P., Burr, J. M. & Khawaja, A. P. Population screening for glaucoma in UK: Current recommendations and future directions. Eye (Lond). 36(3), 504–509. 10.1038/s41433-021-01687-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabasia, P. L., Fidalgo, B. R., Edgar, D. F., Garway-Heath, D. F. & Lawrenson, J. G. Diagnostic accuracy of technologies for glaucoma case-finding in a community setting. Ophthalmology122, 2407–2415. 10.1016/j.ophtha.2015.08.019 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Wahl, J. et al. The Evonik-Mainz eye care-study (EMECS): Development of an expert system for glaucoma risk detection in a working population. PLoS ONE11, 1–24. 10.1371/journal.pone.0158824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilavuzoglu, A. E. B., Cosar, C. B., Celebi, A. R. & Al Parmak, U. E. Intraocular pressure based on dynamic bidirectional applanation and air-puff tonometry: A comparative study. J. Curr. glaucoma Pract.13(2), 68–73. 10.5005/jp-journals-10078-1251 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demirci, G., Erdur, S. K., Tanriverdi, C., Gulkilik, G. & Ozsutçu, M. Comparison of rebound tonometry and non-contact airpuff tonometry to Goldmann applanation tonometry. Therapeutic Adv. Ophthalmol.11, 2515841419835731. 10.1177/2515841419835731 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma, P. et al. A hybrid multi model artificial intelligence approach for glaucoma screening using fundus images. NPJ Digit. Med.8(1), 130. 10.1038/s41746-025-01473-w (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Glaucoma Association. 5th Consensus Meeting: Glaucoma Screening. Available online: https://wga.one/consensus-5/ (Accessed 13 Feb 2024).
- 26.Jindal, A., Ctori, I., Virgili, G., Lucenteforte, E. & Lawrenson, J. G. Non-contact tests for identifying people at risk of primary angle closure glaucoma. Cochrane Database Syst. Rev.5(5), CD012947. 10.1002/14651858.CD012947.pub2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riva, I. et al. Anterior chamber angle assessment techniques: A review. J. Clin. Med.9(12), 3814. 10.3390/jcm9123814 (2020). Published 2020 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashtitova, L. & Aldasheva, N. Study of the prevalence of glaucoma in Kazakhstan. Studie Zur Prävalenz des Glaukoms in Kasachstan. Klin. Monbl Augenheilkd. 239(2), 202–207. 10.1055/a-1327-3999 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Tashtitova, L., Aldasheva, N., Auyezova, A. & Kulmaganbetov, M. Population-based screening leads to improved glaucoma diagnosis in Kazakhstan. Cogent Public. Health10(1). 10.1080/27707571.2023.2191377 (2023).
- 30.Aldasheva, N., Tashtitova, L. & Kulmaganbetov, M. Organizational issues of glaucoma diagnosis in Kazakhstan, 15 July 2019, PREPRINT (Version 1) available at Research Square. 10.21203/rs.2.11404/v1
- 31.Shaltynov, A. et al. An analysis of financial protection and financing incidence of out-of-pocket health expenditures in Kazakhstan from 2018 to 2021. Sci. Rep.14(1), 8869. 10.1038/s41598-024-59742-9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Times of Central Asia. Criticism of Kazakhstan’s Health Insurance System Reaches Parliament. Available online: https://timesca.com/criticism-of-kazakhstans-health-insurance-system-reaches-parliament/?utm_source=chatgpt.com (Accessed 5 Apr 2025).
- 33.U.S. Preventive Services Task Force. Primary Open-Angle Glaucoma: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/primary-open-angle-glaucoma-screening/?utm_source=chatgpt.com (Accessed 5 Apr 2025).
- 34.He, M. et al. Prevalence and clinical characteristics of glaucoma in adult chinese: A population-based study in Liwan district, Guangzhou. Investig. Ophthalmol. Vis. Sci.47(7), 2782–2788. 10.1167/iovs.06-0051 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Mayor, S. Targeted screening may be a cost effective way to detect glaucoma. BMJ335(7630), 1114. 10.1136/bmj.39412.508993.94 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chelvaraj, R. et al. Opportunistic eye screening among first-degree relatives of glaucoma patients at a suburban tertiary center in Malaysia. Cureus14(6), e25772. 10.7759/cureus.25772 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analyzed during the current study are available from the corresponding author upon reasonable request from the corresponding author.