Abstract
Background
Some individuals with migraine fail to respond adequately to preventive treatments, bearing most of migraine burden. The European Headache Federation (EHF) classifies these individuals into resistant migraine (ResM) or refractory migraine (RefM) according to treatment failures, debilitating headache days, and disease duration. We investigated the evolution of these categories over six months in patients treated at tertiary headache centers and whether they accurately reflect disability and burden.
Methods
Participants from the multicenter, prospective REFINE study were classified into three categories of treatment responsiveness, namely RefM, ResM, and non-refractory non-resistant migraine (NRNRM). The primary objective was to determine the trajectories of category changes over six months. Secondary outcomes included changes in the 6-item Headache Impact Test (HIT-6), Headache-Attributed Lost Time (HALT), and Hospital Anxiety and Depression Scale (HADS-A and HADS-D) scores.
Results
Overall, 489 participants were included with a median age of 45 years (IQR = 36–53); 389 participants (79.7%) were female; 256 (52.4%) had NRNRM, 178 (36.4%) ResM, and 55 (11.2%) RefM. At follow-up, 200/256 (78.1%) NRNRM remained stable, while 56/256 (21.9%) progressed to ResM. Among those with ResM, 98/178 (55.1%) remained stable, 72/178 (40.5%) improved to NRNRM, and 8/178 (4.5%) worsened to RefM. Among participants with RefM, 37/55 (67.3%) remained stable, while 18/55 (32.7%) improved to NRNRM. Participants with RefM and ResM presented significantly higher scores at baseline than those with NRNRM. Over time, HIT-6, HALT, and HADS-A scores improved substantially in the overall cohort (p < 0.001, p < 0.001, and p = 0.006, respectively). Improvements were observed in participants with ResM across all scores and HIT-6 and HALT for NRNRM, but no improvement was noted in participants with RefM.
Conclusions
Over six months, ~ 40% of ResM and ~ 30% of RefM individuals improved to NRNRM, while ~ 20% of NRNRM developed treatment resistance after receiving care in tertiary headache centers. Participants with ResM had a better prognosis than those with RefM. While both ResM and RefM reflect high migraine disability burden, they might present relevant differences in their management and prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-025-02126-9.
Keywords: Headache, Chronic migraine, Intractable, Anti-CGRP, Monoclonal antibodies, Gepants, Disability, Validation
Introduction
Migraine is one of the leading causes of disability globally [1–3]. Over the past decade, the introduction of new migraine-specific medications has changed the management of migraine, significantly improving the overall quality of life of migraineurs [4, 5]. Nonetheless, a subgroup of individuals with migraine still fails to respond adequately to preventive migraine treatments, arguably enduring most of the burdensome effects of migraine [6]. Treatment failure can occur due to several factors, including ineffectiveness, intolerance, or contraindication [6]. These individuals may have distinctive underlying pathogenic mechanisms or may represent a different stage of migraine progression, therefore requiring tailored management strategies [6–11].
Various definitions of difficult-to-treat migraine have been proposed, yet none has gained wide acceptance and has been widely adopted in clinical practice [12–14]. In 2020, the European Headache Federation (EHF) proposed updated definitions of resistant (ResM) and refractory migraine (RefM) [15] which identified two different degrees of difficult-to-treat migraine. Notably, EHF criteria account for the recent introduction of calcitonin gene-related peptide (CGRPs) antagonists to define treatment resistance/refractoriness.4 Despite the large consensus and the potential clinical novelties of the EHF definitions, their criteria still require validation in real-world studies, and no longitudinal assessment has been conducted to assess the evolution from one category of treatment responsiveness to another (e.g., how many patients progressed from ResM to RefM over a specific interval). We aimed to investigate the evolution of these categories over six months in patients who received care at tertiary headache centers and determine whether they accurately reflect the individuals’ disability burden.
Methods
Standard protocol approvals, registrations, and patient consents
Ethics approval was obtained from the Institutional Review Board and/or relevant Ethics Committees at all participating centers (protocol number from coordinating center: 45/2020-21). Participants or their authorized representatives provided written informed consent.
Study cohort and study design
REFINE is a prospective, multicenter, observational study conducted from 2020 to 2022 across 15 European tertiary headache centers in Austria, Estonia, Germany, Greece, Italy, Lithuania, Poland, Portugal, Spain, and Turkey [16]. The study included consecutive adult individuals with a diagnosis of episodic or chronic migraine according to the International Classification of Headache Disorders (ICHD)-III diagnostic criteria [17]. Individuals unable to reliably collect information for the study or included in interventional research were excluded. Participants were classified into three categories of treatment responsiveness according to the EHF criteria [15]: (i) RefM (failure and/or contraindications of all classes of migraine preventives and ≥ eight debilitating headache days/month for at least 6 months), (ii) ResM (failure and/or contraindications of ≥ 3 classes of migraine preventives and ≥ eight debilitating headache days/month for at least 3 months), and (iii) non-refractory non-resistant migraine (NRNRM) which included participants not meeting the definitions of either ResM or RefM (Fig. 1). A debilitating headache was defined as a headache causing serious impairment in daily living activities despite the use of pain-relief medications with established efficacy. Drug failure was defined as a lack of effectiveness due to persistent headache despite an appropriate dose and duration or a lack of tolerability due to side effects. As participants fulfilling the criteria for RefM also satisfy those for ResM, participants were included in the ResM category only if they also did not meet the criteria for RefM. Recruitment was stratified according to those three categories to balance the final cohort and allow comparisons. Nevertheless, participants were recruited consecutively within each of the RefM, ResM, and NRNRM categories.
Fig. 1.
Diagnostic flowchart to classify treatment responsiveness of migraine individuals
Participants received treatment at the discretion of the treating physicians and local clinical protocols. Clinical status and variables of interest were collected for each participant at two time points, i.e., at baseline and after 6 months. Data collected included demographics, medical history, lifestyle, headache characteristics, and migraine-specific treatments. Headache diaries were used to assess the severity and frequency of headache attacks. The following migraine-related disability scales and anxiety/depressive scales were collected systematically at each time point: the 6-item Headache Impact Test (HIT-6), Headache-Attributed Lost Time (HALT), and Hospital Anxiety and Depression Scale (HADS-A and HADS-B). At follow-up, participants were re-classified into one of the three categories of treatment responsiveness, according to their preventive treatment history during the previous six months. Specifically, participants reporting less than eight monthly disabling headache days after the prescription of preventive medication were re-classified into the NRNRM category. In contrast, those developing new treatment failures progressed from the NRNRM to the ResM or from the ResM to the RefM category (Fig. 2).
Fig. 2.
Study design and Flowchart. *Patient can revert from RefM to NRMRM as a novel class medication results in a drop of debilitating headache days per month below a certain threshold (8 days/month) RefM: refractory migraine. ResM: resistant migraine. NRNRM: non-refractory, non-resistant migraine. EHF: European Headache Federation. HIT-6: 6-item Headache Impact Test. HALT: Headache-Attributed Lost Time. HADS: Hospital Anxiety and Depression Scale
To evaluate how different medications were used in the three categories of NRNRM, ResM, and RefM, we assessed prescriptions for onabotulinumtoxinA and monoclonal antibodies targeting the CGRP pathway. These medications were specifically analyzed because they are injectable treatments typically prescribed to patients who do not respond to oral migraine medications. We did not include oral preventive migraine medications in this analysis, as at the time of the survey they were all non-specific – gepants were not yet in use during the recruitment period.
Objectives and outcomes
The primary objective of the study was to determine the number and proportions of participants with NRNRM, ResM, and RefM at baseline and follow-up. Secondarily, we assessed the changes in the 6-item Headache Impact Test (HIT-6), Headache-Attributed Lost Time (HALT), and Hospital Anxiety and Depression Scale (HADS-A and HADS-D) scores between baseline and after 6 months, stratified by baseline category of treatment responsiveness – NRNRM, ResM, or RefM. Moreover, we classified participants according to change in category into (i) improvement (i.e., participants changing from ResM or RefM to NRNRM), (ii) worsening (i.e., participants changing from NRNRM to ResM/RefM or from ResM to RefM), and (iii) no changes (i.e., participants who maintained their baseline status).
Statistical analysis
For the present study, we only included participants who had completed the follow-up.
Continuous variables were presented as medians and interquartile ranges (IQR), while categorical variables were reported as counts and percentages. Demographics, medical history, headache characteristics, and scale scores were reported as descriptive statistics. Comparisons across categories of treatment responsiveness were performed using the chi-squared test for categorical variables and the Kruskal-Wallis test for continuous variables. As all our analyses were exploratory, no adjustment was performed for multiple comparisons and no formal sample size calculation was performed for these analyses.
Participants’ characteristics potentially associated with category worsening or improvement with p-value < 0.1 in univariable comparisons were entered in two multivariable logistic regression models – one for characteristics associated with improvement and one for those associated with worsening – with the outcomes as a dependent variable. Effect size estimates derived from logistic regression models were reported as adjusted odds ratio (aOR) with 95% confidence intervals (95%CI). The aORs reported in this study represent the odds ratios for developing the outcomes (improvement or worsening) versus participants who had no change, with no change as the reference category. Participants with RefM at baseline, who could not further worsen their treatment responsivenesss category during follow-up, were excluded from the model on worsening, while those with NRNRM at baseline, who could not further improve during follow-up, were excluded from the model on improvement.
All calculated P-values were two-tailed. Statistical significance was assumed at p < 0.05. The statistical analysis was performed with R (Version 4.2.2).
Results
Baseline characteristics
Out of 689 initial participants, 489 (71.0%) completed the 6-month follow-up. Compared with participants who completed follow-up, those lost to follow-up had a higher proportion of females (90.5% vs. 79.7%; p = 0.003), were older (median age 49 years, IQR 39–57; p = 0.006), had a higher number of monthly migraine days during the previous three months (median 13, IQR 9–20, vs. 11, IQR 7–18; p < 0.001), lower scores of the HIT-6 (median 62, IQR 58–66, vs. 64, IQR 60–68; p = 0.002) and HALT questionnaire (median 24, IQR 14–48, vs. 40, IQR 13–78; p < 0.001; Supplementary Table 1).
Table 1.
Baseline sociodemographic and clinical characteristics of individuals included in the study
| Characteristic | Overall (n = 489) |
NRNRM (n = 256) |
ResM (n = 178) |
RefM (n = 55) |
p value |
|---|---|---|---|---|---|
| Female, n (%) | 389 (79.7) | 201 (78.5) | 149 (83.7) | 39 (70.9) | 0.208 |
| Age, years (median– IQR) | 45 (36–53) | 42 (34–51) | 48 (37–56) | 42 (34–51) | < 0.001 |
| Current smoking, n (%) | 82 (16.8) | 39 (15.2) | 27 (15.2) | 16 (29.1) | 0.090 |
| Alcohol use, n (%)* | 225 (46.1) | 132 (51.6) | 68 (38.2) | 25 (45.5) | 0.026 |
| Caffeine use, n (%) | 0.174 | ||||
| 1–2 cups/day | 290 (59.4) | 160 (62.5) | 99 (55.6) | 31 (56.4) | |
| 3–4 cups/day | 118 (24.2) | 62 (24.2) | 45 (25.3) | 11 (20.0) | |
| ≥5 cups/day | 10 (2.0) | 4 (1.6) | 6 (3.4) | - | |
| BMI, kg/m2 (median– IQR) | 24 (21–27) | 24 (21–28) | 24 (21–27) | 24 (23–26) | 0.646 |
| Age at migraine onset, years (median– IQR) | 17 (13–23) | 17 (13–23) | 16 (13–24) | 17 (15–22) | 0.212 |
| Migraine duration, years (median– IQR) | 26 (16–35) | 22 (14–32) | 28 (18–39) | 32 (24–36) | < 0.001 |
| Monthly migraine days (median – IQR) | 11 (7–18) | 8 (4–15) | 13 (10–20) | 15 (10–19) | < 0.001 |
| Chronic migraine, n (%) | 275 (57.2) | 98 (38.3) | 124 (69.7) | 53 (96.4) | < 0.001 |
| Medication overuse, n (%) | 172 (35.2) | 58 (22.7) | 87 (37.6) | 27 (49.1) | < 0.001 |
| HIT-6 score, median (IQR) | 64 (60–68) | 63 (57–66) | 66 (62–68) | 66 (65–68) | < 0.001 |
| HALT score, median (IQR) | 40 (13–78) | 19 (7–49) | 60 (33–87) | 98 (59–140) | < 0.001 |
| HADS-A score, median (IQR) | 8 (5–12) | 7 (4–10) | 9 (6–12) | 13 (9–15) | < 0.001 |
| HADS-D score, median (IQR) | 6 (3–10) | 5 (2–8) | 8 (5–11) | 12 (9–14) | < 0.001 |
*Either habitual or abuse
BMI: Body Mass Index. HIT-6: 6-item Headache Impact Test. HALT: Headache-Attributed Lost Time. HADS: Hospital Anxiety and Depression Scale
The median age of the 489 participants with complete follow-up was 45 years (IQR = 36–53), and 389 (79.7%) were female. At baseline, 256 participants (52.4%) were diagnosed with NRNRM, 178 (36.4%) with ResM, and 55 (11.2%) with RefM. Compared with participants with NRNRM, those with RefM and ResM were older, had a longer migraine duration and frequency, a higher prevalence of chronic migraine and medication overuse, and had higher scores of the HIT-6, HALT, HADS-A, and HADS-D scales, as shown in Table 1.
Longitudinal assessment and evolution of migraine treatment categories
After the six-month follow-up, 284/489 participants (58.1%) were diagnosed with NRNRM, 160/489 (32.7%) with ResM, and 45 (9.2%) with RefM. Among the 256 participants diagnosed with NRNRM at baseline, 200/256 (78.1%) remained in the same category, whereas 56/256 (21.9%) worsened to ResM at follow-up. Among the 178 participants diagnosed with ResM at baseline, 98/178 (55.1%) remained in the same category, 72/178 (40.4%) improved to NRNRM, and 8/178 (4.5%) worsened to RefM at follow-up. Among the 55 participants with RefM at baseline, 37/55 (67.3%) remained in the same category, while 18/55 (32.7%) improved to NRNRM. Figure 3 shows the participants’ change in categories over the follow-up period.
Fig. 3.
Alluvial plot showing the transition from one diagnosis to the other during follow-up in the present study. Data refer to participants with complete follow-up. NRNRM: Non-refractory, Non-resistant Migraine. ResM: Resistant Migraine. RefM: Refractory Migraine
Longitudinal assessment of migraine-related burden across categories
The HIT-6 score improved over time in the overall population (baseline: 64 [IQR = 60–68], follow-up: 61 [IQR = 55–65]; p < 0.001). Participants with RefM were the only group in which no improvement in the HIT-6 score was observed over time (baseline 66 [IQR = 65–68], follow-up: 65 [IQR = 58–68], p = 0.054). A statistically significant improvement in the HALT score was observed in the NRNRM (baseline: 19 [IQR = 7–49], follow-up: 15 [IQR = 5–40]; p = 0.038) and in the ResM group (baseline: 60 [IQR = 33–87], follow-up: 33 [IQR = 15–60]; p < 0.001), while an improvement in the HADS-A (baseline: 9 [IQR = 6–12], follow-up: 7 [IQR = 4–11]; p = 0.005) and HADS-D scores (baseline: 8 [IQR = 5–11], follow-up: 6 [IQR = 3–10]; p = 0.038) was present only in the ResM category. Changes in scores at the different time points for each category are reported in Fig. 4 and in Supplementary Table 2.
Fig. 4.
Baseline and follow-up outcomes according to treatment response categories following the European Headache Federation proposal [15].
Table 2.
Independent predictors of improvement of treatment responsiveness status from baseline characteristics
| Characteristic | Improvement (n = 90) |
No change (n = 145) |
OR (95% CI - univariate) |
p value (univariate) |
OR (95% CI - multivariate) | p value (multivariate) |
|---|---|---|---|---|---|---|
| Female, n (%) | 74 (82.2) | 118 (81.4) | 1.06 (0.53–2.10) | 0.871 | - | - |
| Age, years (median– IQR) | 46 (36–54) | 49 (40–56) | - | 0.223 | - | - |
| Contraceptive use, n (%)* | 11 (14.9) | 19 (16.1) | 0.91 (0.41–2.04) | 0.819 | - | - |
| Menopause, n (%)* | 26 (35.1) | 39 (33.1) | 1.10 (0.59–2.02) | 0.767 | - | - |
| Current smoking, n (%) | 21 (23.3) | 25 (17.2) | 1.46 (0.76–2.80) | 0.254 | - | - |
| Alcohol use, n (%)** | 40 (44.4) | 49 (33.8) | 1.57 (0.91–2.69) | 0.103 | - | - |
| Caffeine use, n (%) | 0.078 | |||||
| 1–2 cups/day | 53 (58.9) | 82 (56.6) | 1.09 (0.97–1.22) | 0.304 | ||
| 3–4 cups/day | 25 (27.8) | 28 (19.3) | 1.12 (0.99–1.27) | 0.141 | ||
| ≥5 cups/day | 3 (3.3) | 3 (2.1) | 1.13 (0.85–1.51) | 0.084 | ||
| Illicit drug use, n (%) | - | - | - | - | - | - |
| Comorbidities, n (%) | ||||||
| Cardiovascular disease | 1 (1.1) | 3 (2.1) | 0.53 (0.05–5.22) | 0.999 | - | - |
| Cerebrovascular disease | 3 (3.5) | 9 (6.3) | 0.52 (0.14–1.99) | 0.382 | - | - |
| Hypertension | 16 (17.8) | 31 (21.5) | 0.80 (0.41–1.56) | 0.512 | - | - |
| Fibromyalgia | 5 (5.6) | 16 (11.1) | 0.48 (0.17–1.35) | 0.238 | - | - |
| Neck/back pain | 55 (61.1) | 74 (51.4) | 1.31 (0.76–2.27) | 0.137 | - | - |
| Trigger points | 29 (32.2) | 29 (20.1) | 1.91 (1.05–3.50) | 0.041 | 1.17 (1.05–1.30) | 0.006 |
| Temporomandibular joint dysfunction | 19 (21.1) | 18 (12.5) | 1.90 (0.94–3.86) | 0.096 | 1.00 (0.88–1.13) | 0.057 |
| Rheumatological disease | 6 (6.7) | 10 (6.9) | 0.97 (0.34–2.76) | 0.731 | - | - |
| Asthma | 6 (6.7) | 9 (6.3) | 1.08 (0.37–3.16) | 0.437 | - | - |
| Rhinitis | 14 (15.6) | 25 (17.4) | 0.89 (0.43–1.82) | 0.857 | - | - |
| Depression | 39 (43.3) | 53 (36.8) | 1.31 (0.77–2.25) | 0.338 | - | - |
| Anxiety | 25 (27.8) | 44 (30.6) | 0.89 (0.50–1.59) | 0.480 | - | - |
| Bipolar disorder | 1 (1.1) | 3 (2.1) | 0.53 (0.05–5.22) | 0.999 | - | - |
| Other psychiatric disorders | 10 (11.1) | 11 (7.6) | 1.53 (0.62–3.77) | 0.357 | - | - |
| Thyroiditis | 21 (23.3) | 21 (14.6) | 1.53 (0.62–3.77) | 0.168 | - | - |
| Urticaria | 1 (1.1) | 3 (2.1) | 0.53 (0.05–5.22) | 0.999 | - | - |
| Gastrointestinal disease | 2 (2.2) | 2 (1.4) | 1.63 (0.23–11.80) | 0.638 | - | - |
| Celiac disease | 1 (1.1) | - | - | 0.382 | - | - |
| Other autoimmune diseases | 1 (1.1) | 6 (4.2) | 0.26 (0.03–2.19) | 0.302 | - | - |
| Sleep disturbances | 29 (32.2) | 59 (41.0) | 0.70 (0.40–1.22) | 0.425 | - | - |
| Previous preventive treatment failures (median – IQR) | 4 (3–6) | 5 (3–7) | - | 0.085 | 1.05 (1.03–1.07) | < 0.001 |
| BMI, kg/m2 (median – IQR) | 23 (21–27) | 24 (21–27) | - | 0.396 | - | - |
| Age at migraine onset, years (median – IQR) | 16 (13–21) | 16 (13–23) | - | 0.664 | - | - |
| Migraine duration, years (median – IQR) | 26 (18–36) | 29 (20–38) | - | 0.460 | - | - |
| Migraine days*** (median – IQR) | 11 (10–20) | 15 (10–22) | - | 0.012 | 1.00 (1.00-1.01) | 0.109 |
| Chronic migraine, n (%) | 58 (64.4) | 121 (82.9) | 0.37 (0.20–0.69) | 0.001 | 0.95 (0.87–1.04) | 0.256 |
| Medication overuse, n (%) | 38 (42.2) | 77 (53.1) | 0.65 (0.38–1.10) | 0.105 | - | - |
| HIT-6 score, median (IQR) | 65 (62–68) | 66 (64–68) | - | 0.193 | - | - |
| HALT score, median (IQR) | 67 (35–88) | 64 (37–111) | - | 0.313 | - | - |
| HADS-A score, median (IQR) | 9 (6–13) | 11 (8–13) | - | 0.361 | - | - |
| HADS-D score, median (IQR) | 8 (6–12) | 9 (5–12) | - | 0.827 | - | - |
*Proportions are reported on the total of women in each group
**Either habitual or abuse
***During the previous three months
BMI: Body Mass Index. CGRP-mAbs: monoclonal antibodies targeting the calcitonin gene-related peptide pathway. HIT-6: 6-item Headache Impact Test. HALT: Headache-Attributed Lost Time. HADS: Hospital Anxiety and Depression Scale
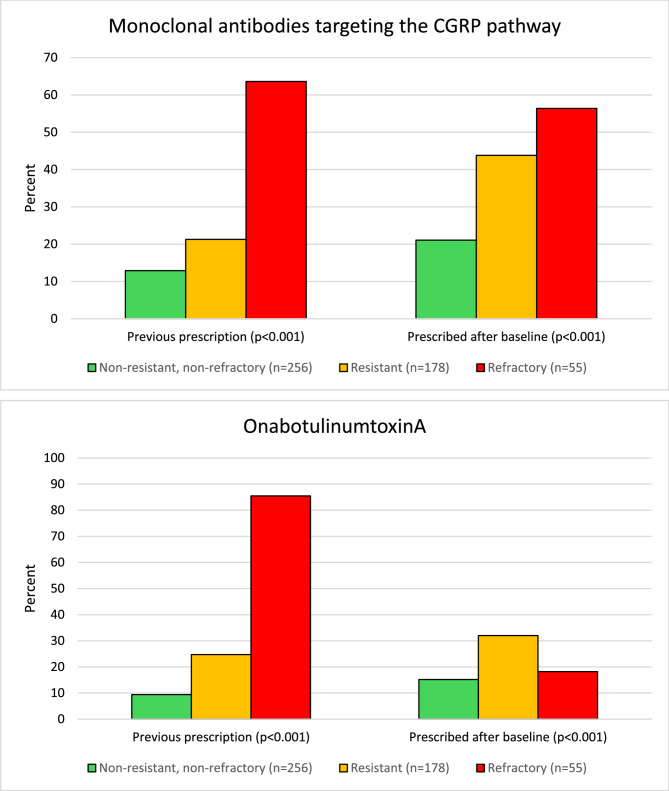
The changes in the prescription of injectable preventive medications are reported in Fig. 5. The proportion of participants with ResM who were prescribed monoclonal antibodies targeting the CGRP pathway increased from 21.3% before baseline to 43.8% after baseline. In comparison, those drugs had already been prescribed before baseline in 63.6% of participants with RefM. The proportion of participants with ResM who received a prescription for OnabotulinumtoxinA also increased between baseline and after six months (from 24.7 to 32.0%). In comparison, most participants with RefM (85.5%) had already been prescribed OnabotulinumtoxinA at baseline. In line with monoclonal antibodies targeting the CGRP pathway, OnabotulinumtoxinA prescriptions increased in participants with NRNRM and ResM while decreasing in those with RefM (Fig. 5).
Fig. 5.
Prescriptions of injectable treatments for migraine prevention in participants with non-resistant and non-refractory, resistant, and refractory migraine over the study period. Data refers to the 489 participants with complete follow-up. Participants’ categories are those attributed at baseline. The graphs reflect treatments that were prescribed at least once in their migraine history, rather than prescriptions at a specific time point. CGRP: calcitonin gene-related peptide
Factors associated with improvement and worsening of treatment responsiveness status
In the multivariable analysis, improvement in treatment responsiveness status was significantly associated with the presence of trigger points (adjusted OR 1.17 [95%CI = 1.05–1.30]) and a higher number of prior treatment failures (adjusted OR 1.05 [95%CI = 1.03–1.07] per each additional treatment failure) (Table 2). Conversely, worsening of treatment responsiveness status was significantly associated with fewer baseline monthly migraine days (adjusted OR 1.00 [95%CI = 1.00-1.01] per each additional day) and a lower number of prior treatment failures (adjusted OR 0.97 [95%CI = 0.96–0.99] per each additional treatment failure) (Table 3).
Table 3.
Independent predictors of worsening of treatment responsiveness status from baseline characteristics
| Characteristic | Worsening (n = 64) |
No change (n = 318) |
OR (95% CI - univariate) |
p value (univariate) |
OR (95% CI - multivariate) | p value (multivariate) |
|---|---|---|---|---|---|---|
| Female, n (%) | 52 (81.3) | 258 (81.1) | 1.02 (0.93–1.11) | 0.904 | - | - |
| Age, years (median – IQR) | 44 (36–53) | 44 (34–52) | - | 0.649 | - | - |
| Contraceptive use, n (%)* | 7 (13.4) | 48 (18.6) | 0.90 (0.78–1.04) | 0.534 | - | - |
| Menopause, n (%)* | 15 (28.8) | 54 (20.9) | 1.02 (0.93–1.12) | 0.102 | - | - |
| Current smoking, n (%) | 8 (12.5) | 48 (15.1) | 0.91 (0.80–1.04) | 0.224 | - | - |
| Alcohol use, n (%)* | 29 (45.3) | 138 (43.4) | 1.08 (0.63–1.85) | 0.002 | 0.94 (0.88-1.00) | 0.059 |
| Caffeine use, n (%) | 0.750 | - | - | |||
| 1–2 cups/day | 41 (64.1) | 188 (59.1) | - | - | ||
| 3–4 cups/day | 12 (18.8) | 80 (25.2) | - | - | ||
| ≥5 cups/day | 1 (1.6) | 6 (1.9) | - | - | ||
| Illicit drug use, n (%) | - | 3 (0.9) | - | - | - | - |
| Comorbidities, n (%) | ||||||
| Cardiovascular disease | 1 (1.6) | 5 (1.6) | 0.99 (0.11–8.65) | 0.999 | - | - |
| Cerebrovascular disease | 2 (3.1) | 8 (2.5) | 1.25 (0.26–6.03) | 0.732 | - | - |
| Hypertension | 15 (23.4) | 56 (17.7) | 1.38 (0.72–2.64) | 0.279 | - | - |
| Fibromyalgia | 6 (9.4) | 18 (5.7) | 1.72 (0.66–4.53) | 0.060 | 1.07 (0.93–1.22) | 0.358 |
| Neck/back pain | 29 (45.3) | 161 (50.8) | 0.81 (0.47–1.39) | 0.644 | - | - |
| Trigger points | 14 (21.9) | 43 (13.6) | 1.79 (0.91–3.51) | 0.216 | - | - |
| Temporomandibular joint dysfunction | 9 (14.1) | 34 (10.7) | 1.37 (0.62–3.01) | 0.328 | - | - |
| Rheumatological disease | 2 (3.1) | 24 (7.6) | 0.40 (0.09–1.72) | 0.229 | - | - |
| Asthma | 7 (10.9) | 26 (8.2) | 1.38 (0.57–3.33) | 0.706 | - | - |
| Rhinitis | 8 (12.5) | 60 (18.9) | 0.61 (0.28–1.36) | 0.334 | - | - |
| Depression | 12 (18.8) | 77 (24.3) | 0.72 (0.37–1.42) | 0.588 | - | - |
| Anxiety | 10 (15.6) | 55 (17.4) | 0.89 (0.42–1.85) | 0.864 | - | - |
| Bipolar disorder | 1 (1.6) | 7 (2.2) | 0.70 (0.09–5.83) | 0.999 | - | - |
| Other psychiatric disorders | - | 14 (4.4) | - | - | - | - |
| Thyroiditis | 7 (10.9) | 38 (12.0) | 0.90 (0.38–2.13) | 0.712 | - | - |
| Urticaria | 3 (4.7) | 11 (3.5) | 1.37 (0.37–5.07) | 0.762 | - | - |
| Gastrointestinal disease | 1 (1.6) | 4 (1.3) | 1.25 (0.14–11.34) | 0.446 | - | - |
| Celiac disease | - | 4 (1.3) | - | - | - | - |
| Other autoimmune diseases | 1 (1.6) | 7 (2.2) | 0.71 (0.09–5.83) | 0.999 | - | - |
| Sleep disturbances | 17 (26.6) | 110 (34.7) | 0.68 (0.38–1.25) | 0.353 | - | - |
| Previous preventive treatment failures (median – IQR) | 1 (0–3) | 3 (0–4) | - | 0.001 | 0.97 (0.96–0.99) | 0.001 |
| BMI, kg/m2 (median – IQR) | 25 (22–30) | 24 (21–27) | - | 0.038 | 1.01 (1.00-1.02) | 0.060 |
| Age at migraine onset, years (median – IQR) | 18 (14–25) | 16 (13–22) | - | 0.247 | - | - |
| Migraine duration, years (median– IQR) | 24 (14–37) | 24 (15–35) | - | 0.240 | - | - |
| Monthly migraine days (median – IQR) | 15 (8–24) | 10 (5–17) | - | 0.007 | 1.00 (1.00-1.01) | 0.002 |
| Chronic migraine, n (%) | 37 (57.8) | 158 (49.5) | 1.40 (0.81–2.40) | 0.226 | - | - |
| Medication overuse, n (%) | 26 (40.6) | 96 (30.2) | 1.58 (0.91–2.75) | 0.102 | - | - |
| HIT-6 score, median (IQR) | 64 (60–68) | 64 (58–67) | - | 0.293 | - | - |
| HALT score, median (IQR) | 48 (18–76) | 28 (10–60) | - | 0.149 | - | - |
| HADS-A score, median (IQR) | 8 (4–11) | 7 (5–11) | - | 0.523 | - | - |
| HADS-D score, median (IQR) | 5 (3–9) | 5 (2–9) | - | 0.539 | - | - |
*Proportions are reported on the total of women in each group
**Either habitual or abuse
***During the previous three months BMI: Body Mass Index. CGRP-mAbs: monoclonal antibodies targeting the calcitonin gene-related peptide pathway. HIT-6: 6-item Headache Impact Test. HALT: Headache-Attributed Lost Time. HADS: Hospital Anxiety and Depression Scale
NRNRM: Non-refractory, Non-resistant Migraine. ResM: Resistant Migraine. RefM: Refractory Migraine
CGRP: calcitonin gene-related peptide
Supplementary Table 1 Comparisons between baseline sociodemographic and clinical characteristics of participants with and without study follow-up
Discussion
In our multicenter prospective cohort study, which longitudinally assessed individuals with NRNRM, ResM, and RefM as defined by the EHF categories, we found that (i) more participants with ResM improved their treatment responsiveness condition to NRNRM compared to those with RefM; (ii) participants with ResM or RefM had a higher disability burden compared with those with NRNRM, (iii) disability levels in RefM remained relatively stable over time, whereas ResM showed the most significant improvement, and (iv) presence of trigger points, number of treatment failures and baseline monthly migraine days were the only independent predictors of changes of treatment responsiveness status.
This is the first real-life longitudinal study reporting the change in categories of treatment responsiveness in tertiary headache centers. We found that approximately 40% of participants with ResM reverted to NRNRM, while two out of every three participants with RefM remained in their category. Our finding confirms the difference between ResM, which can improve if individuals are prescribed effective migraine prevention, and RefM, in which some specific mechanisms might determine the ineffectiveness of preventive treatments [6]. In our study, participants with RefM had a heavy and relatively stable migraine burden, while those with ResM experienced a decrease in the migraine burden. It is also relevant that no group of participants experienced increased migraine disability burden. Notably, most participants with ResM were prescribed monoclonal antibodies targeting the CGRP pathway or OnabotulinumtoxinA for the first time when entering the REFINE study follow-up, which might have been important in overcoming resistance to preventive treatments.
As we showed that ResM and RefM are associated with a higher migraine disability burden compared with NRNRM, we confirmed that individuals with these difficult-to-treat migraine states experience a disease-related disability that extends beyond the simple lack of response to preventive treatments. Our data contribute to identifying characteristics typical of individuals with ResM and RefM, which deserve further exploration as they might be associated with mechanisms underlying resistance or refractoriness to preventive treatments [6]. Our findings also highlight the importance of timely recognition and treatment of ResM, as this condition is more prone to respond to treatments. On the other hand, it is also important to correctly identify individuals with RefM as they might represent suitable candidates for research on the mechanisms of migraine and candidates for combination treatments and/or non-pharmacological treatments [6, 18]. These findings could inform headache specialists and policy decision-makers to streamline referral processes for individuals with ResM and RefM to specialist care. Our data validate the effectiveness of a classification system that differentiates ResM from RefM, enabling clinicians to provide specialist care to individuals most likely to benefit.
A lower number of prior treatment failures and fewer baseline monthly migraine days, indicating a lower overall migraine burden, were associated with worsening in treatment responsiveness status. While these findings may seem counterintuitive, they likely reflect features of individuals with non-refractory migraine who can still worsen toward a severe migraine responsiveness status. Conversely, individuals with RefM cannot worsen further. On the other hand, a higher number of prior treatment failures and the presence of trigger points were associated with improved treatment responsiveness status. While the former might still be interpreted with the same conceptual framework, it might also be partially explained by the widespread use of anti-CGRP monoclonal antibodies as a last resource—after all or most other classes of drugs have failed [6]. Notably, trigger points might represent a clinically meaningful treatment responsiveness marker consistent with previous literature [19].
The severity and burden of migraine have historically been assessed based on the frequency of headache attacks by distinguishing individuals with episodic migraine from those with chronic migraine [3, 17]. However, this classification provides only a snapshot of a highly dynamic condition, does not account for the intensity of the attacks, and offers limited insights into the predictive response to future therapies [20–22]. A classification framework based on treatment responsiveness might better reflect migraine history and provide complementary information to the standard frequency-based definition, thereby enhancing management strategies [23].
The main strength of our study is that it represents the first one to provide a longitudinal assessment of individuals with ResM and RefM over six months. Additional strengths include its multicenter prospective design and the adoption of a standardized definition of ResM and RefM established by a large international consensus. However, our study has also some limitations. First, the proportions among categories were unbalanced, with RefM being relatively underrepresented. Second, due to the observational nature of the study, participants received heterogeneous management after baseline, depending on local protocols and clinical practice. This might have had an impact on the change from ResM to RefM, as some participants classified as ResM might have remained in their category only due to an insufficient number of drug trials within the six-month-period. Third, almost half of the initial participants were lost to follow-up, and participants lost to follow-up had different characteristics from those who were followed-up (Supplementary Table 1). The proportion of participants lost to follow-up might have introduced selection bias and limited the generalizability of our findings; nevertheless, the REFINE study had an observational design with no compulsory procedure and did not adopt strategies to enhance participants’ adherence to follow-up. Fourth, our purely observational study design could not allow to assess the exact contribution of each medication and especially injectable treatments – i.e., onabotulinumtoxinA and CGRP-mAbs – to migraine prevention. Finally, migraine follows a naturally fluctuating course that is highly individualized and sometimes seasonally patterned, such as holidays, work/school routines, or weather changes. Therefore, treatment responsiveness categories might partially reflect these natural fluctuations rather than the results of management.
Conclusions
We found that many individuals with ResM who receive care in tertiary headache centers and are treated with migraine-specific preventive medication can revert their resistance to treatment, while individuals with RefM tend to have a stable condition. The categories of ResM and RefM have a high migraine burden and reflect the treatment responsiveness of individuals, providing complementary information to the standard frequency-based definition (i.e., chronic and episodic migraine) to inform management. Timely recognition and treatment of individuals with ResM and identification of those with RefM are crucial to managing those conditions. Longer observational studies are warranted to draw definite conclusions about the natural history of ResM and RefM.
Supplementary Information
Acknowledgements
This work was partially supported by “Ricerca Corrente” funding from Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Abbreviations
- EHF
European Headache Federation
- CGRP
Calcitonin Gene-related Peptide
- HADS
Hospital Anxiety and Depression Scale (HADS)
- HALT
Headache-Attributed Lost Time (HALT)
- HIT-6
6-item Headache Impact Test
- RefM
Refractory Migraine
- ResM
Resistant Migraine
- NRNRM
Non-Refractory Non-Resistant Migraine
Author contributions
SS, VC, and ZK conceived the study and supervised the project. UP and RO drafted the initial manuscript. All authors contributed to data collection, reviewed the manuscript for content, and approved the final version of the manuscript.
Funding
None.
Data availability
The corresponding author will grant access to the study data upon reasonable request and after approval by the REFINE executive committee.
Declarations
Consent for publication
Participants or their authorized representatives provided written informed consent.
Competing interests
PM serves as the Editor-in-Chief of The Journal of Headache and Pain and SN Comprehensive Clinical Medicine. M.W-P is a member of the Editorial Board of The Journal of Headache and Pain. RO reports personal fees or non-financial support from AbbVie, Bayer, Eli Lilly, Lundbeck, Novartis, Organon, Pfizer, and Teva; he is an Editorial Board Member of The Journal of Headache and Pain, Confinia Cephalalgica, and Associate Editor for Frontiers in Neurology and Arquivos de Neuropsiquiatria. PP-R reports in the last 36 months, fees as a consultant and speaker for: AbbVie, Dr. Reddy’s, Eli Lilly, Lundbeck, Medscape, Novartis, Organon, Pfizer and Teva. Her research group has received research grants from AbbVie, Novartis and Teva; as well as, InstitutoSalud Carlos III, EraNet Neuron, European Regional Development Fund (001-P-001682) under theframework of the FEDER Operative Programme for Catalunya 2014-2020 - RIS3CAT; has receivedfunding for clinical trials from AbbVie, Amgen, Biohaven, Eli Lilly, Lundbeck, Novartis, Pfizer Teva. SS reports personal fees as speaker or advisor from Abbott, Allergan-Abbvie, AstraZeneca, Bayer, Boehringer, Eli Lilly, Lundbeck, Pfizer, and Teva; she received research grants from Novartis and Uriach; she is President of the European Stroke Organisation, Editor-in-Chief of Cephalalgia and Cephalalgia Reports, and Assistant Editor for Stroke. DM has received fees and travel grants from AbbVie, Bristol Myers Squibb, Cross Pharma, Eli Lilly, Genesis Pharma, Haleon, Merck, Lundbeck, Novartis, Orion, Roche, Pfizer, Viatris, AstraZeneca, and Teva Pharmaceuticals; has participated in clinical trials for Amgen, Eli Lily, Lundberg, Novartis, Pfizer, and Teva Pharmaceuticals, as principal investigator; is president of the Hellenic Headache Society; is a member of the Management Group of the Headache Scientific Panel and of the Coordinating panel for the Functional Neurological Disorders at the European Academy of Neurology; and a past-President of the European Headache Federation. AO reports receiving personal fees as a speaker or advisor from AbbVie, Eli Lilly, TEVA, Pfizer, Organon, Ali Raif, İlko, and Neutec. She is a board member of IHS. She is the current president of the Global Migraine and Pain Society and MENAA Headache Society. She serves as a guest editor or reviewer for several Journals, including Cephalalgia, Cephalalgia Reports, TJHP, BMC Neurology, Headache, and Frontiers in Neurology. FV received travel grants, honoraria for advisory board, speaker panels, or clinical investigation studies from Allergan/AbbVie, Angelini, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva Pharmaceuticals; he serves as Specialty Chief Editor of Frontiers in Neurology Headache and Neurogenic Pain section. MB previously received sponsorship, financial support, fees from: Abbott Laboratories, AbbVie Inc, Allergan Inc, Berlin-Chemie AG (Menarini Group), Boehringer Ingelheim Pharma GmbH, Desitin Arzneimittel GmbH, EV3, Gedeon Richter Ltd, GlaxoSmithKline, KBM Pharma Ltd., H. Lundbeck A/S, Novartis Pharma Services Inc, Nycomed SEFA, Orion Pharma, Pfizer Inc, Sanofi-Aventis, Sandoz d.d., Scanmed Group, Solvay Pharmaceuticals, Teva Pharmaceutical Industries Ltd./Sicor Biotech UAB, Zentiva International.
Ethical approval and consent to participate
was obtained from the Institutional Review Board and/or relevant Ethics Committees at all participating centers (protocol number from coordinating center: 45/2020-21). Participants or their authorized representatives provided written informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Umberto Pensato and Raffaele Ornello contributed equally to this work.
References
- 1.Global incidence (2024) prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet 403:2133–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global (2024) regional, and National burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol 23:344–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari MD, Goadsby PJ, Burstein R et al (2022) Migraine Nat Rev Dis Primers 8:2 [DOI] [PubMed] [Google Scholar]
- 4.Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018) CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 14:338–350 [DOI] [PubMed] [Google Scholar]
- 5.Al-Hassany L, Boucherie DM, Creeney H et al (2023) Future targets for migraine treatment beyond CGRP. J Headache Pain 24:76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ornello R, Andreou AP, De Matteis E, Jürgens TP, Minen MT, Sacco S (2024) Resistant and refractory migraine: clinical presentation, pathophysiology, and management. EBioMedicine 99:104943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro JD, Moon PK, Han M et al (2022) Early onset diffusion abnormalities in refractory headache disorders. Front Neurol 13:898219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodick D, Silberstein S (2006) Central sensitization theory of migraine: clinical implications. Headache 46(Suppl 4):S182–S191 [DOI] [PubMed] [Google Scholar]
- 9.D’Antona L, Matharu M (2019) Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain 20:89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman EA, Brahin EJ (2008) Refractory headache: historical perspective, need, and purposes for an operational definition. Headache 48:770–777 [DOI] [PubMed] [Google Scholar]
- 11.Pensato U, Cevoli S, Pierangeli G, Cortelli P (2023) The evolutionary meaning of migraine. Cephalalgia 43:3331024231209303 [DOI] [PubMed] [Google Scholar]
- 12.Martelletti P, Katsarava Z, Lampl C et al (2014) Refractory chronic migraine: a consensus statement on clinical definition from the European headache federation. J Headache Pain 15:47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulman EA, Lake AE 3rd, Goadsby PJ et al (2008) Defining refractory migraine and refractory chronic migraine: proposed criteria from the Refractory Headache Special Interest Section of the American Headache Society. Headache.;48:778– 82 [DOI] [PubMed]
- 14.Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick D (2006) Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia 26:1168–1170 [DOI] [PubMed] [Google Scholar]
- 15.Sacco S, Braschinsky M, Ducros A et al (2020) European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European migraine & headache alliance (EMHA). J Headache Pain 21:76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosignoli C, Ornello R, Caponnetto V et al (2024) Resistant and refractory migraine - two different entities with different comorbidities? Results from the REFINE study. J Headache Pain 25:212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headache Classification Committee of the International Headache Society (IHS) (2018);38:1-211 [DOI] [PubMed]
- 18.Voloshin AG, Moiseeva IV (2021) Combined interventional treatment of refractory chronic migraine. SN Compr Clin Med 3:1320–1326 [Google Scholar]
- 19.Do TP, Heldarskard GF, Kolding LT, Hvedstrup J, Schytz HW (2018) Myofascial trigger points in migraine and tension-type headache. J Headache Pain 19:84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favoni V, Mascarella D, Giannini G et al (2023) Prevalence, natural history and dynamic nature of chronic headache and medication overuse headache in italy: the SPARTACUS study. Cephalalgia 43:3331024231157677 [DOI] [PubMed] [Google Scholar]
- 21.Serrano D, Lipton RB, Scher AI et al (2017) Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain 18:101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White CS (2023) Unique considerations in episodic migraine: underserved populations. Curr Pain Headache Rep 27:503–509 [DOI] [PubMed] [Google Scholar]
- 23.Ishii R, Schwedt TJ, Dumkrieger G et al (2021) Chronic versus episodic migraine: the 15-day threshold does not adequately reflect substantial differences in disability across the full spectrum of headache frequency. Headache 61:992–1003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author will grant access to the study data upon reasonable request and after approval by the REFINE executive committee.