Abstract
Background
Dexamethasone has been used extensively to prevent hypersensitivity reactions to paclitaxel. However, the optimal dose of dexamethasone is controversial, varying between 20 mg and 10 mg. We conducted this randomized controlled trial to illustrate that these 2 dosages of dexamethasone are non-inferior to the prevention of paclitaxel HSRs.
Methods
Gynecologic cancer patients who naively receive paclitaxel and carboplatin were invited to participate in this study. All participants received the same premedication with intravenous dexamethasone 20 mg, oral lorazepam 0.5 mg, and intravenous chlorpheniramine 10 mg at the first cycle. If they did not develop hypersensitivity reactions, they were randomized to receive either intravenous 20 mg or 10 mg dexamethasone with the same other premedication. The attending nurse recorded the patient’s symptoms regarding hypersensitivity reactions. The main outcome was hypersensitivity reaction events in each arm, with a non-inferiority margin of 0.11.
Results
A total of 122 patients were included and randomly assigned to receive dexamethasone 10 mg (n = 61) or dexamethasone 20 mg (n = 61). The overall incidence of hypersensitivity reactions in patients who received dexamethasone 10 mg and dexamethasone 20 mg was 9.8% and 13.1%, respectively, the risk difference between dexamethasone 10 mg and dexamethasone 20 mg not exceeding the non-inferiority margin of 0.11 (Risk Difference = -0.03, 95% confidence interval = -0.15 to 0.08).
Conclusion
Dexamethasone 10 mg was non-inferior to dexamethasone 20 mg in terms of prevention of paclitaxel hypersensitivity reactions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14769-7.
Keywords: Dexamethasone, Premedication, Paclitaxel, Hypersensitivity
Background
Paclitaxel is the most commonly used chemotherapy for gynecologic cancers [1]. Due to its poor water solubility, necessitates the use of polyoxyethylated castor oil (Cremophor EL) as a solvent. Unfortunately, Cremophor EL is associated with a high rate of infusion-related hypersensitivity reactions (HSRs) by triggering basophil/mast cell direct activation via C3a and C5a receptors, causing the release of histamines, and other inflammatory substances including prostaglandins, leukotrienes, and cytokines [2]. The paclitaxel HSRs event occurred reaching up to 40% [2]. To mitigate these reactions, corticosteroids, particularly dexamethasone acted by blocking the receptor of degranulated mast cell mediators in combination with an antihistamine, have been employed as premedication, reducing HSRs rates to below 20% [2–4]. The initial recommendation for paclitaxel premedication is as follows: 20 mg of dexamethasone orally or intravenously (IV) 12 and 6 h before treatment, 50 mg of diphenhydramine IV, 300 mg of cimetidine or 20 mg famotidine or 150 mg ranitidine IV 30 min before treatment [2]. However, due to the inconvenience of giving dexamethasone twice, a simplified schedule with a single 20 mg of dexamethasone IV administered with an anti-histamine 30 min before treatment was previously reported as having an incidence of HSRs of 4% [5]. Following that including our institute, premedication with a single 20 mg IV dosage of dexamethasone has been employed as the standard dosage for carboplatin plus paclitaxel premedication regimen [6–8]. We previously reported 18% paclitaxel HSRs in 44 patients or 4.2% in 213 cycles. These patients received the premedication with 20 mg dexamethasone (IV), 10 mg chlorpheniramine (IV), and 50 mg ranitidine (IV) 30 min before infusion [9]. However, the benefit of the H2 antagonist is not clear [8, 10] and due to it being available in our hospital since 2020 we subsequently omitted ranitidine in our paclitaxel premedication.
One major concern about the repeated and long-term administration of dexamethasone is the possibility of inducing steroid side effects such as osteoporosis, gastritis dermatitis, cataracts, Cushing syndrome, impaired glucose metabolism, and gastric ulcers [11–13]. Some institutions have attempted to reduce the dexamethasone dose from 20 mg to 10 mg, with a comparative study showing no increase in HSRs incidence [14]. Nevertheless, no randomized controlled study has confirmed the non-inferiority of the 10 mg dose. This trial aimed to address this gap by comparing 10 mg and 20 mg dexamethasone doses for HSRs prevention. Methods.
Trial design and setting
This prospective randomized controlled trial was conducted at the Gynecologic Oncology Unit of Chiang Mai University Hospital. The trial was approved by the Research Ethics Committee of the Faculty of Medicine of Chiang Mai University) (EC 116/2021), and prospectively registered as number TCTR20201215005 at the Thai Clinical Trials Registry (http://www.clinicaltrials.in.th). The full protocol in the Thai language is available from the corresponding author upon reasonable request. Written informed consent was obtained from all patients before participation in the study.
Trial participants
Eligible criteria for gynecologic cancer patients who could participate in this study were as follows: (1) aged 18 years of age or older, (2) planned to receive carboplatin and paclitaxel regimen (PT) for the first time, (3) normal bone marrow, renal, and liver function, (4) no history of dexamethasone or paclitaxel allergy, and (5) no current medication with any steroidal drugs. Patients were excluded if they had severe cardiac disease, a history of myocardial infarction within 6 months, or active pulmonary disease.
Sample size determination
For sample size determination, we focused on the non-inferior trial by comparing the proportion of HSRs between Dexamethasone 10 mg (test drug) and 20 mg (active control). The sample size was achieved using a web application developed by Sealed Envelope [15]. We discovered the success rate of the drugs to indicate the risk of HSRs in Dexamethasone 10 mg and 20 mg were 5% [4] and 4% [5], respectively. The non-inferiority margin is 11%, estimated from 85% of the minimum risk difference between Dexamethasone 20 mg [9] and placebo 13% [16]. The sample size required 122 cases (61 cases per group) with a dropout rate of 10%, a power of 80%, and a significance level of 5%.
Treatment allocation
For the first cycle of the PT regimen (carboplatin AUC 5 and generic paclitaxel 175 mg/m2: Intaxel® Fresenius Kabi Oncology Limited, India), all participants received the same premedication in addition to dexamethasone 20 mg IV, ondansetron 8 mg IV, lorazepam 0.5 mg oral, and chlorpheniramine 10 mg IV 30 min before the start of treatment with paclitaxel for 3 h followed by an hour of carboplatin. If they did not develop any HSRs, they were randomized into 2 groups. A control group received dexamethasone 20 mg IV and an experimental group received dexamethasone 10 mg IV 30 min before starting paclitaxel, the premedication for the next cycles being kept the same. During the study period, patients were withdrawn from the study if the following occurred: (1) refusing the next cycle of treatment or lost to follow-up, (2) changes in premedication or chemotherapy regimen due to any cause for example development of HSRs from the former cycle, progression of the disease, severe adverse effect.
The method for a randomized dose of dexamethasone was a block-of-four technique generated by the computer. Serial numbers were labeled on sealed opaque envelopes with the order forms of randomized dexamethasone doses inside. The enrollment, which occurred at the gynecologic oncology clinic, began with the investigator opening the envelope and putting the order forms from each envelope into the participant’s chart. All participants received a PT regimen at a one-day chemotherapy unit and they were blinded for the dose of dexamethasone. However, the attending nurses who administrated the premedication including chemotherapy, and observed the paclitaxel HSRs events were not blinded. When paclitaxel HSRs occurred, our center’s standard protocol was activated based on the severity by discontinuing the infusion and then administering oxygen therapy, rapid hydrocortisone 100 mg IV, and chlorpheniramine 10 mg IV for a mild form. If the clinical improvement occurred within 30 min, the paclitaxel infusion was resumed at a slower rate. For the severe form, in addition to hydrocortisone and antihistamine, rescue drugs such as epinephrine 1:1000 0.01 ml/kg IM and/or Salbutamol nebule (2.5 mg/2.5 ml) 1–2 nebules/dose were administered as well as endotracheal intubation and resuscitation if necessary. Following the development of severe paclitaxel HSRs, patients were treated with non-taxane chemotherapeutic agents, albumin-bound paclitaxel, or single carboplatin in the subsequent cycles.
All suspected HSRs symptoms were recorded by the attending nurses and verified by the attending physicians with the standard treatment as mentioned above. The patients were given antiemetic drugs with ondansetron and iron supplements to be taken at home. The chemotherapy schedule was repeated every 21 days with a total of 6 cycles.
Outcome assessment
Basic clinical characteristics, any reasons for withdrawing from the study and the number of paclitaxel HSRs were recorded and graded in terms of severity by using Common Terminology Criteria for Adverse Events (CTCAE) version 5 [17]. CTCAE grades 1 and 2 were categorized as a mild form, while grades 3 and 4 were categorized as a severe form. The HSRs that occurred during administered paclitaxel were classified as paclitaxel HSRs whereas the HSRS that occurred during or after administered carboplatin was classified as carboplatin HSRs.
Statistical analyses
Analyses of HSRs events were conducted using the per-protocol approach. Continuous variables were presented as mean ± SD and compared using the student’s t-test. Dichotomous variables are reported as frequencies. The chi-squared test or Fisher’s exact test was used as appropriate to compare the distribution of dichotomous variables between the two groups. Due to the dropout of the participants who withdrew in each cycle, the incidence of paclitaxel HSRs in each cycle between 2 allocated treatments was presented by using Kaplan Meier methods and compared with a log-rank test.
For assessment of non-inferiority, a comparison between the risk of development of paclitaxel HSRs in each group was conducted, with a pre-specified non-inferiority margin of 11% or 0.11. Non-inferiority was declared if the upper margin of the two-sided 95% confidence interval (CI) for the risk difference did not exceed a margin of 0.11. Statistical analysis was performed using SPSS version 22.0 (IBM, Armonk, NY, USA). A p-value of < 0.05 was considered as indicating statistical significance.
Results
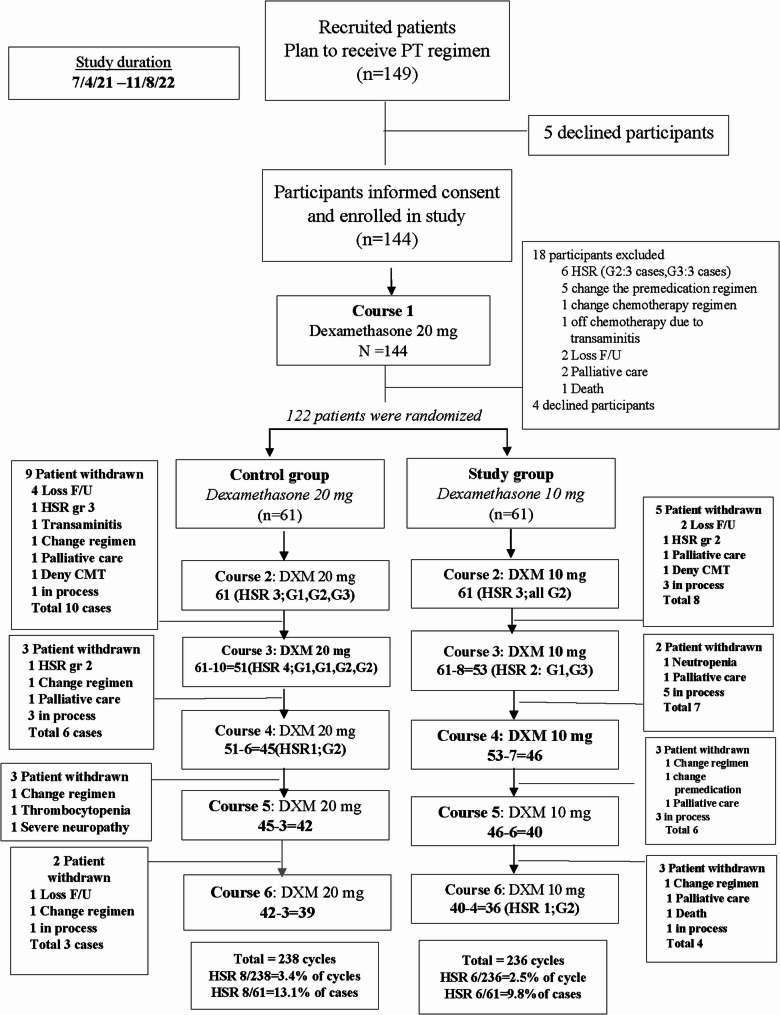
Between April 2021 and August 2022, a total of 149 gynecologic patients were assigned to receive the first cycle of the PT regimen. Five patients declined to participate in the study. Therefore, 144 patients completed informed consent forms and were enrolled in the study. However, after the first cycle of the PT regimen, 18 patients were excluded from the study due to the development of HSRs (6 cases), changes in the premedical regimen (5 cases), changes in the chemotherapy regimen (1 case), off chemotherapy due to transaminitis (1 case), lost to follow-up (2 cases), changes in the chemotherapy treatment to palliative care (2 case), death (1 case), and 4 patients declined to participate the study at this stage and subsequently withdrew from the protocol. Finally, 122 patients were randomized resulting in 61 in the control arm (dexamethasone 20 mg IV) and 61 in the experimental arm (dexamethasone 10 mg IV), basic clinical characteristics being well-balanced between the two groups as shown in Table 1.
Table 1.
Clinical characteristics of trials patients
| Variable | Comparison | ||
|---|---|---|---|
| DXM 20 mg n (%) |
DXM 10 mg n (%) |
p value† | |
| Patients enrolled and completed cycle 1 | 61 | 61 | |
| Mean age (±SD), years | 56.0 (10.5) | 57.3 (11.2) | 0.51 |
| Mean Body Surface Area (±SD), m2 | 1.5 (0.1) | 1.5 (0. 2) | 0.61 |
| Mean Body Mass Index (±SD) | 23.1 (4.0) | 23.2 (4.5) | 0.95 |
|
Concomitant diseases‡ Yes No |
34 (55.7) 27 (44.3) |
27 (44.3) 34 (55.7) |
0.21 |
| Smoker | 3 (4.9) | 1(1.6) | 0.62 |
|
Site of cancer diagnosis Ovary Uterine corpus Uterine cervix Fallopian tube Vagina Vulva Primary peritoneal carcinoma |
11(18.0) 21 (34.4) 22 (36.1) 5(8.2) 0 1(1.6) 1(1.6) |
13 (21.3) 23 (37.7) 16 (26.2) 5(8.1) 1(1.6) 0 3(4.8) |
0.72 |
|
Staging at enrollment I II III IV recurrence |
9 (14.8) 8 (13.1) 30 (49.2) 11(18.0) 3 (4.9) |
10 (16.4) 8 (13.1) 27 (44.3) 13 (21.3) 3 (4.9) |
0.99 |
| Paclitaxel was previously used | 4(50) | 4(50) | 1.00 |
|
Mean paclitaxel dosage prescribed (+ SD), mg Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 |
266.4 (28.1) 263.1 (28.2) 220.8 (98.2) 261.6 (31.4) 260.2 (30.1) 260.7 (31.7) |
264.5 (31.6) 261.2 (33.6) 238.1 (80.8) 257.9 (37.3) 261.2 (31.1) 261.9 (32.1) |
0.73 0.74 0.30 0.61 0.89 0.87 |
|
Mean carboplatin dosage prescribed (±SD), mg Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 |
496.3 (127.1) 506.9 (126.6) 509.4 (140.2) 488.1 (124.3) 495.0 (120.8) 494.6 (122.8) |
499.7 (112.7) 514.0 (116.1) 513.4 (102.6) 502.6 (111.4) 494.4 (108.7) 498.5 (100.6) |
0.88 0.75 0.87 0.56 0.98 0.88 |
DEX 20 mg: hypertension; HT(16),diabetic mellitus; DM(2),dyslipidemia;DLP (3),thyroid disease(3), human immunodeficiency virus (HIV) positive(2), VTE(1),respiratory disease(1),HT &venous thromboembolism(VTE) (1), HT& thalassemia(1), DM&VTE(1),DM &thyroid disease(1),HT &thyroid disease(1), viral hepatitis (1)
DEX 10 mg: HT(15),DLP(1), thyroid disease(4), HT&anti HIV positive(1), HT& breast cancer (2),HT &cardiac disease(3), thyroid disease & DLP(1)
SD Standard deviation, DXM Dexamethasone
† p values reflect comparisons between the two study groups within each analytical category, Statistical tests were as follows for differing variables: t-test compared means of age, BSA, BMI; paclitaxel and carboplatin dosage; cycle of nausea and cycle of vomiting; Chi-square and Fisher’s exact test used to compare categorical variables for concomitant disease, smoking, site of cancer diagnosis, and staging at enrollment
†† Concomitant diseases
During the study time, some patients were withdrawn from the study for various reasons resulting in a total of 238 cycles of the PT regimen in the control arm and 236 cycles in the experimental arm. The patients who received 6 completed cycles of the PT regimen in the control arm and experimental arm numbered 39 patients and 36 patients, respectively. In addition, some patients were “in process”, meaning they continued to receive the carboplatin plus paclitaxel regimen but did not have paclitaxel hypersensitivity reactions (HSRs) recorded within the study timeframe. It is noteworthy that none of these patients developed paclitaxel HSRs.
The rate of development of HSRs was 8 of 61 patients (13.1%) or 8 in 238 cycles (3.4%) in the control arm and 6 of 61 patients (9.8%) or 6 in 236 cycles (2.5%) in the experimental arm. All patients who developed paclitaxel HSRs had never received paclitaxel before. The details of these data are summarized in Fig. 1.
Fig. 1.
The consort of all participants. PT carboplatin and paclitaxel regimen, HSR hypersensitivity reaction, G grade,DXM dexamethasone
Regarding the primary objective, the risk difference in the rate of paclitaxel HSRs in the experimental arm (dexamethasone 10 mg) versus the control arm (dexamethasone 20 mg) was − 0.013 and the 95% CI was − 0.15 − 0.08. Therefore, the upper limit of the 95% CI for the comparison between both arms was within the prespecified margin of 0.11 as shown in Table 2; Fig. 2. We also compared the incidence of paclitaxel HSRs in each cycle and found no significant difference between the experimental and control arm (p = 0.643) as shown in Fig. 3.
Table 2.
Risk difference in patients who experienced hypersensitivity in the two arms (Dexamethasone 20 mg versus 10 mg)
| Dosage of Dexamethasone (mg) | HSRs | Risk difference* (95%CI) | |
|---|---|---|---|
| No (%) | Yes (%) | ||
| 10 | 55 (90.2) | 6 (9.8) | |
| 20 | 53 (86.9) | 8 (13.1) | −0.03(−0.15−0.08) |
| Total | 108 (88.5) | 14 (11.5) | |
HSRs Hypersensitivity reactions
*Risk difference = 9.8% -13.1% = -3.3% = -0.03
Fig. 2.
The 95% confidence interval of risk difference in patients with hypersensitivity reaction in each arm of the experiment (Dexamethasone 10 mg versus 20 mg) in relation to the non-inferior margin line. *Risk difference = −0.03 (−0.15−0.08)
Fig. 3.
A comparison of the incidence of paclitaxel hypersensitivity reactions (HSRs) in each cycle between intravenous dexamethasone 10 mg and 20 mg. Treatment Arm: Dexamethasone 20 mg, Incidence HSRs = 8/238 =3.4% of cycle; 8/61 = 13.1% of cases, Dexamethasone 10 mg, Incidence HSRs= 6/236 = 2.5% of cycle; 6/61 = 9.8% of cases, Log-rank test: p = 0.643, HSRs = hypersensitivity reaction
The details concerning the 14 patients who developed paclitaxel HSRs symptoms are summarized in Table 3. The mean onset of this event was 55.36 (± 38.36) minutes. Six patients who received dexamethasone 10 mg developed paclitaxel HSRs at grade 1 (1 case) and grade 2 (5 cases) while 8 patients who received dexamethasone 20 mg developed this event in grade 1 (2 cases), grade 2 (5 cases) and grade 3 (1 case). In this study, 1 patient developed grade 1 carboplatin HSRs with clinical symptoms of abdominal pain and diarrhea after starting carboplatin for 75 min at cycle 4. This case was in the experimental arm.
Table 3.
The details of patients who developed hypersensitivity reactions (N=14)
| SN | Age | Diagnosis | Dosage of Dexamethasone | cycle | Severity (grade) | The onset of HSR after paclitaxel start (min) | Symptoms | Continued next cycle in the protocol | Total cycle in the protocol |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | CA corpus | 20 | 3 | 2 | 20 | Flushing, chest tightness | No | 3 |
| 12 | 36 | CA cervix | 20 | 2 | 2 | 40 | Flushing, chest tightness | Yes | 6 |
| 17 | 36 | CA ovary | 20 | 3 | 1 | 100 | Flushing, chest tightness | Yes | 6 |
| 23 | 56 | CA ovary | 20 | 2 | 1 | 15 | Rash | Yes | 6 |
| 27 | 64 | CA ovary | 10 | 2 | 2 | 43 | Flushing, dyspnea | No | 6 |
| 36 | 62 | CA tube | 20 | 3 | 2 | 45 | Chest tightness, dyspnea, myalgia | Yes | 6 |
| 39 | 46 | CA ovary | 20 | 3 | 2 | 25 | Flushing, chest tightness | Yes | 6 |
| 47 | 62 | CA tube | 10 | 2 | 2 | 40 | Myalgia | Yes | 6 |
| 62 | 54 | CA corpus | 20 | 2 | 3 | 45 | Flushing, rash,stuffy nose | No | 2 |
| 68 | 71 | CA cervix | 10 | 6 | 2 | 85 | Flushing | Yes | 6 |
| 85 | 69 | CA corpus | 10 | 2 | 2 | 90 | Chest tightness, cough, pain | Yes | 3 |
| 86 | 70 | CA tube | 20 | 4 | 2 | 47 | Cough, dyspnea | Yes | 6 |
| 87 | 38 | CA tube | 10 | 3 | 1 | 25 | Flushing, headache | Yes | 6 |
| 110 | 55 | CA corpus | 10 | 3 | 2 | 155 | Flushing, chest tightness | Yes | 3 |
Mean (+SD) onset of HSR after paclitaxel start = 55.36(+38.86) minutes. 1 case developed grade 1 HSR (abdominal pain & diarrhea) after starting carboplatin for 75 minutes at cycle 4 (SN 97) Dexamethasone 10 mg
HSR Hypersensitivity reactions
Discussion
The paclitaxel used in this study was a generic version called Intaxel® from Fresenius Kabi Oncology Limited in India. Intaxel contains 6 mg of paclitaxel per milliliter along with 527 mg of polyoxyl 35 castor oil USNF and 49.7% v/v excipient alcohol. An earlier prospective study showed that this medication had a similar incidence of HSRs to the original paclitaxel, which is now rarely used in our country [18].
The incidence of HSRs to paclitaxel in this study was 13.1% in the control arm (dexamethasone 20 mg) and 9.8% in the experimental arm (dexamethasone 10 mg). However, only 2% of the control arm developed severe HSRs, classified as grade 3. This incidence was comparable to that reported by a previous study. Cathail et al. [3] conducted a systematic review of 9 papers that investigated the use of 20 mg dexamethasone IV with a total of 818 patients and found 46 patients who developed paclitaxel HSRs, accounting for 5.6% of individuals with a range of 2.2–24.2%. To reduce the side effects of a high dose of dexamethasone, many publications reported the favorable outcome of a decrease in dexamethasone dose in the premedication setting [4, 14, 19, 20]. Bookman et al. [20] carried out a retrospective study of paclitaxel HSRs in 238 patients who received intravenous 10 or 20 dexamethasone plus diphenhydramine 50 mg and cimetidine 300 mg or ranitidine 50 mg. In that study, they found that 4.6% of cases experienced HSRs related to paclitaxel in the first or second cycle of the therapy. However, the authors did not report the level of HSRs in each dosage of dexamethasone. Subsequently, Yenilmez et al. [4] conducted a retrospective study comparing two paclitaxel premedication protocols with 60 patients in each protocol. The first protocol involved the giving of the premedication with 20 mg of dexamethasone at 12 and 6 h for paclitaxel 175 mg/m2 (given every 2–3 weeks) and 8 mg of dexamethasone 30 min prior to weekly paclitaxel infusion in the first 2 cycles (158 cases). The second paclitaxel premedication protocol was administered in all paclitaxel infusions with 10 mg dexamethasone given for the first 2 cycles and then 8 mg for the subsequent cycles (200 cases). Both protocols were given diphenhydramine 25 mg and famotidine 20 mg 30 min before starting paclitaxel. The HSRs rate was 7% in the first protocol and 5% in the second protocol (p value = 0.7). All HSRs events occurred in association with the first infusion. The authors concluded that discontinuing dexamethasone in paclitaxel premedication after two uneventful infusions or decreasing the dose of dexamethasone was safe. The next study investigating the dose reduction of dexamethasone was conducted by Koppler et al. [14]. The authors reported the rate of HSRs in patients who received premedication with 40 mg (40 cases), 20 mg (48 cases), and 10 mg (52 cases) of dexamethasone in addition to ranitidine IV and clemastine IV were given prior to paclitaxel. The schedule of paclitaxel was 135–175 mg/m2 every 3 weeks in 76 patients and 100 mg/m2 weekly in 70 patients; in addition, 64% were given carboplatin. In this study, they reported only one case who received 10 mg of dexamethasone developed HSRs with bronchospasm, hypotension, and supraventricular tachycardia immediately after the start of the first cycle of paclitaxel (100 mg/m2). The authors concluded that a dosage of dexamethasone of either 20 mg or 10 mg did not increase the severity of HSRs. Recently, Lansinger et al. [19] performed a retrospective study to review the pattern of dexamethasone premedication in 3,181 patients. They reported the rate of paclitaxel and docetaxel HSRs as being 8.3% and following multivariate analysis they found no correlation between HSRs rate or severity between the various dosages or routes of dexamethasone. They recommended the routine use of a single dose of 10 mg of dexamethasone IV for the prevention of taxane HSRs.
Finally, our study also confirmed the non-inferiority between 20 mg and 10 mg of dexamethasone IV premedication for the prevention of paclitaxel HSRs as the patients did not develop any HSRs in the first cycle following the use of 20 mg of dexamethasone IV. The patients who did not develop the paclitaxel HSRs in the first cycle should be classified as a low-risk group and were safe to administer 10 mg of IV dexamethasone for premedication in the subsequent cycles. Incorporating this practice could potentially reduce healthcare costs associated with drug procurement and administration while maintaining patient safety and treatment efficacy. Further exploration of the health services impact and cost/benefit analysis of this approach would provide valuable insights into its implementation in clinical practice.
About the number of paclitaxel cycles that frequently developed HSRs, Rowinsky et al. [2] stated that the earlier cycles usually resulted in HSRs. The findings from this publication showed a correlation with our study which were that paclitaxel HSRs were commonly found in the first 3 cycles as follows: Cycle 1 (6 cases), cycle 2 (6 cases), cycle 3 (6 cases), cycle 4 (1 case), and cycle 6 (1 case) with almost all cases presenting with mild symptoms.
Regarding the onset time of paclitaxel HSRs, our study presented the mean onset time of this event was 55.36 (+ 38.36) minutes, contrasting with previous retrospective studies [21–24] that frequently reported onset within 5 min of paclitaxel infusion initiation. Discrepancies in onset times may be attributed to several factors as follows: 1) our study’s continuous observation throughout the infusion process,2) exclusive use of paclitaxel plus carboplatin with a fixed dose (paclitaxel 175 mg/m2 plus carboplatin AUC 5 mg/ml), 3) distinct premedication regimens involving 10 or 20 mg of intravenous dexamethasone 30 min before infusion, and 4) the exclusion of HSRs occurring in the first cycle of paclitaxel. In contrast, previous studies had retrospective designs that possibly led to missed recordings of later-onset HSRs., varying paclitaxel dosages and regimens (paclitaxel 180,175,80 mg/m2, paclitaxel plus carboplatin, weekly paclitaxel, paclitaxel plus cisplatin, paclitaxel plus ifosfamide), diverse premedication approaches (20 mg dexamethasone orally 12 and 6 h or once at 30 min intravenously before initiating paclitaxel), and reported all HSR events in every cycle [21–24].
In this present study, we identified 1 case of carboplatin HSRs at cycle 4 and successfully underwent the next 2 cycles of the PT regimen by adding hydrocortisone to the premedication. This was a rare event in patients who received carboplatin as a first-line treatment. A previous study reported that carboplatin HSRs usually developed at an incidence as high as 20% at 8 cycles but only 0.92% developed in the first 5 cycles [25]. The process involved in the HSRs of carboplatin is believed to result from prolonged exposure to platinum salt [25, 26].
One of the limitations of this study is the lack of double-blinding, which could introduce bias, particularly in the assessment of hypersensitivity reactions (HSRs). The strength of our study, however, lies in its randomized, single-blind, non-inferiority design, which reduces the risk of bias typically associated with non-randomized studies. While the single-blind design mitigates potential bias in outcome assessment, we acknowledge that blinding the attending nurses responsible for administering premedication could have further reduced the possibility of differential attention influencing the occurrence or reporting of HSRs.
To address this concern, we implemented several strategies to minimize the risk of bias in the assessment of HSRs. Both nurses and physicians were instructed to follow a standardized protocol using well-defined clinical criteria for diagnosing HSRs, which aimed to reduce subjective judgment and ensure consistency across assessments. In addition, we employed rigorous randomization procedures and blinded data analysis, further strengthening the reliability of our results. Adverse events were systematically recorded and independently adjudicated to verify the diagnosis of HSRs, providing additional confidence in the accuracy of our findings.
Despite the limitations inherent in the study design, these safeguards helped to mitigate potential biases, enhancing the scientific rigor and validity of the study outcomes. Further research using a double-blind design may offer additional insights into the impact of premedication administration on HSRs, but the current study provides valuable evidence within its design constraints.
Furthermore, it is essential to acknowledge certain 3 key constraints in our study. Firstly, we did not systematically document potential side effects associated with the administered steroids. Secondly, we lacked data on potential risk factors for paclitaxel HSRs such as a history of previous drug allergy or atopic disease [27]. Thirdly, a comprehensive presentation of participants’ histological details was omitted.
In conclusion, Premedication with 10 mg of dexamethasone was non-inferior concerning the prevention of paclitaxel HSRs in gynecologic cancer patients who naively received a paclitaxel& carboplatin regimen and no developed HSRs at the first cycle with 20 mg IV dexamethasone.
Supplementary Information
Acknowledgements
Our thanks go to the Faculty of Medicine, Chiang Mai University for supporting this project and to Assoc. Prof. Nut Koonrungsesomboon, M.D., Ph.D. and Mr. Tanarat Muangmool, MSc, for assistance with the statistical analysis.
Abbreviations
- IV
Intravenously
- HSRs
Hypersensitivity reactions
- PT
Carboplatin and paclitaxel regimen
Authors’ contributions
NS: Conceptualization, Data collection, Data interpretation, Format analysis, manuscript review, and editingPS: Conceptualization, Format analysis, Data interpretation, Manuscript writing, and editingCC: Conceptualization, manuscript review, and editing.
Funding
This study was supported by the Faculty of Medicine, Chiang Mai University Fund which mainly involved the cost of data recording.
Data availability
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Chiang Mai University Ethics Committee: Study Code: OBG-2563-07689/ EC 116/2021 and all the participants gave informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahaman J, Steiner N, Hayes MP, Chuang L, Fishman D, Gretz Iii H. Chemotherapy for gynecologic cancers. Mt Sinai J Med. 2009;76:577–88. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332:1004–14. [DOI] [PubMed] [Google Scholar]
- 3.O’Cathail SM, Shaboodien R, Mahmoud S, Carty K, O’Sullivan P, Blagden S, Gabra H, et al. Intravenous versus oral dexamethasone premedication in preventing Paclitaxel infusion hypersensitivity reactions in gynecological malignancies. Int J Gynecol Cancer. 2013;23:1318–25. [DOI] [PubMed] [Google Scholar]
- 4.Yenilmez A, Hood AP, Nguyen LH, Merl MY. Paclitaxel pre-medication: a comparison of two steroid pre-medication protocols. J Oncol Pharm Pract. 2017;23:491–5. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Simplified regimen for the prevention of paclitaxel-associated hypersensitivity reactions. J Clin Oncol. 1997;15:3517. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. An effective and more convenient drug regimen for prophylaxis against paclitaxel-associated hypersensitivity reactions. J Cancer Res Clin Oncol. 1999;125:427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The National. Comprehensive Cancer Network (NCCN) guideline 2022. Available from: https://www.nccn.org/ file:///C:/Users/Administrator/Downloads/nccn-chemotherapy-templates-userguide.pdf. Accessed 6 April 2023.
- 8.Chantharakhit C, Ruchakorn T, Mungkornkaew S, Amorntrakoon P, Tassanamethee S, Theeratrakul P, et al. Efficacy of premedication protocol without ranitidine for taxane regimen: a multicenter non-randomized historical controlled study. Asian Pac J Cancer Prev. 2022;23:1331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeerakornpassawat D, Suprasert P, Randomized. Controlled trial of dexamethasone versus dexamethasone plus hydrocortisone as prophylaxis for hypersensitivity reactions due to Paclitaxel treatment for gynecologic cancer. Int J Gynecol Cancer. 2017;27:1794–801. [DOI] [PubMed] [Google Scholar]
- 10.Cox JM, van Doorn L, Malmberg R, Oomen-de Hoop E, Bosch TM, van den Bemt, et al. The added value of H2 antagonists in premedication regimens during Paclitaxel treatment. Br J Cancer. 2021;124:1647–52. [DOI] [PMC free article] [PubMed]
- 11.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. [DOI] [PubMed] [Google Scholar]
- 12.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–65. [DOI] [PubMed] [Google Scholar]
- 13.Yanaranop M, Chaithongwongwatthana S. Intravenous versus oral dexamethasone for prophylaxis of paclitaxel-associated hypersensitivity reaction in patients with primary ovarian, fallopian tube and peritoneal cancer: a double-blind randomized controlled trial. Asia Pac J Clin Oncol. 2016;12:289–99. [DOI] [PubMed] [Google Scholar]
- 14.Köppler H, Heymanns J, Weide R. Dose reduction of steroid premedication for paclitaxel: no increase of hypersensitivity reactions. Onkologie. 2001;24:283–5. [DOI] [PubMed] [Google Scholar]
- 15.Sealed Envelope Ltd. Power calculator for binary outcome non-inferiority trial. 2012 Available from: https://www.sealedenvelope.com/power/binary-noninferior/ Accessed 12 Dec 2020.
- 16.Kris MG, O’Connell JP, Gralla RJ, Wertheim MS, Parente RM, Schiff PB, et al. Phase I trial of taxol given as a 3-hour infusion every 21 days. Cancer Treat Rep. 1986;70:605–7. [PubMed] [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events (CTCAE). Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017.
- 18.Ratanajarusiri T, Sriuranpong V, Sitthideatphaiboon P, Poovoravan N, Vinayanuwat C, Parinyanitikul N, et al. A difference in the incidences of hypersensitivity reactions to original and generic taxanes. Chemotherapy. 2017;62:134–9. [DOI] [PubMed] [Google Scholar]
- 19.Lansinger OM, Biedermann S, He Z, Colevas AD. Do steroids matter?? A retrospective review of premedication for taxane chemotherapy and hypersensitivity reactions. J Clin Oncol. 2021;39:3583–90. [DOI] [PubMed] [Google Scholar]
- 20.Bookman MA, Kloth DD, Kover PE, Smolinski S, Ozols RF. Short-course intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Ann Oncol. 1997;8:611–4. [DOI] [PubMed] [Google Scholar]
- 21.Sendo T, Sakai N, Itoh Y, Ikesue H, Kobayashi H, Hirakawa, et al. Incidence and risk factors for Paclitaxel hypersensitivity during ovarian cancer chemotherapy. Chemother Pharmacol. 2005;56:91–6. [DOI] [PubMed] [Google Scholar]
- 22.Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000;18:102–5. [DOI] [PubMed] [Google Scholar]
- 23.Aoyama T, Takano M, Miyamoto M, Yoshikawa T, Soyama H, Kato K, et al. Is there any predictor for hypersensitivity reactions in gynecologic cancer patients treated with paclitaxel-based therapy? Cancer Chemother Pharmacol. 2017;80:65–9. [DOI] [PubMed] [Google Scholar]
- 24.Thangwonglers T, Santimaleeworagun W, Therasakvichya S, Saengsukkasemsak N, Pimsi P. Characteristics of immediate hypersensitivity reaction to paclitaxel-based chemotherapy in gynecologic cancer patients. Asian Pac J Allergy Immunol. 2020. [DOI] [PubMed]
- 25.Sliesoraitis S, Chikhale PJ. Carboplatin hypersensitivity. Int J Gynecol Cancer. 2005;15:13–8. [DOI] [PubMed] [Google Scholar]
- 26.Pandey A, Bhosale B, Pandita V, Singh A, Ghosh J, Ghosh J, et al. Carboplatin hypersensitivity in relapsed ovarian carcinoma: a therapeutic challenge. Indian J Med Paediatr Oncol. 2014;35:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvão VR, Berlin ST, et al. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. 2016;137:1154–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.