Abstract
Introduction
Helicobacter pylori infection can cause peptic ulcer disease, chronic gastritis, primary gastric lymphoma, and gastric cancer. Treatment with bismuth-based quadruple therapy is typically the first line of treatment but can be challenging due to increased pill burden and adverse effects, leading to nonadherence to therapy. Recent studies have shown that vonoprazan can be used in combination with amoxicillin as a potential treatment option. We conducted a systematic review and meta-analysis to assess the efficacy and tolerability of vonoprazan-based dual therapy as compared to bismuth-based therapy (BBT).
Methodology
We conducted a comprehensive search of multiple electronic databases including PubMed, Embase, and Cochrane Library to identify randomized controlled studies assessing vonoprazan and amoxicillin (VA) in comparison to BBT for H. pylori treatment in adults >18 years of age. Studies with pediatric populations, written in languages other than English, or without control groups were excluded.
Results
Out of the 1968 citations, 9 studies including 2039 patients were included in the final analysis. There were 463 and 481 men in the VA and the BBT groups, respectively. The mean age ranged from 38.1 to 48.7 years in the VA group and from 38.6 to 46.1 in the BBT group. The VA group had an eradication rate similar to that of the BBT group (odds ratio [OR]: 0.32, 95% confidence interval [CI]: 0.26–0.40; P = 0.08). The VA group had a lower incidence of total adverse events than the BBT group (OR: 0.32, 95% CI: 0.26–0.40; P = 0.0001), including a reduced occurrence of nausea and vomiting (OR: 0.42, 95% CI: 0.28–0.65; P = 0.0001). There was no difference in compliance between the VA and BBT groups (OR: 1.16, 95% CI: 0.76–1.76; P = 0.50).
Conclusion
Our study showed a similar eradication rate of H. pylori but a significantly lower incidence of adverse events in the VA group compared with the BBT group. Our analysis suggests that a VA-based regimen is an acceptable treatment option for H. pylori patients who cannot tolerate BBT.
Keywords: Bismuth, Helicobacter pylori, vonoprazan
Helicobacter pylori is a leading cause of peptic ulcer disease and can be associated with chronic gastritis, gastric cancer, and lymphoma, with an over 50% global prevalence.1 In addition, H. pylori has been shown to have extragastric manifestations, e.g., in the central nervous system, as a trigger for autoimmune diseases, and cardiovascular disorders, and has been classified as a class I carcinogen by the World Health Organization.2,3 Therefore, its eradication is paramount to reduce overall morbidity and mortality.4
Treatment strategies differ by geographic location, prevalence, bacterial resistance, cost of drugs, and availability. The American College of Gastroenterology recommends bismuth-based quadruple therapy (BQT) (bismuth, metronidazole, high-dose proton pump inhibitors [PPI], tetracycline) and clarithromycin-based triple therapy (PPI, amoxicillin, and clarithromycin) as the first-line treatment for H. pylori infection.5 The efficacy of clarithromycin-based triple therapy is limited by the widespread resistance to clarithromycin, and BQT is considered first-line therapy in regions with high clarithromycin resistance.6
BQT is composed of multiple drugs with a high pill burden, which can lead to adverse effects and gut microbiome dysbiosis. These adverse effects and the higher pill burden lead to low adherence rates.4,5 This has led to research on dual therapy as a potential alternative.7 Previous studies reported similar eradication rates with fewer adverse effects with dual therapy composed of PPI and high-dose amoxicillin (2 g divided 3–4× per day) compared to BBT.8,9
Vonoprazan, an H/K ATPase pump inhibitor, has a rapid onset of action, has fewer nocturnal acid breakthroughs, and is not affected by the CYP2C19 gene polymorphism when compared to PPI.10,11 Vonoprazan has a higher 24-hour pH holding time ratio over high-dose PPIs, endorsing stronger potency.12 We conducted a systematic review and meta-analysis to assess the efficacy and safety of vonoprazan-amoxicillin–based dual therapy (VA) as compared to bismuth-based regimens.
METHODS
The study was performed in compliance with Cochrane guidelines for conducting systematic reviews and meta-analyses and the Preferred Reporting Items for Systematic Reviews and Meta-analysis.13,14 The study protocol was registered at PROSPERO with reference number CRD42023455492.
A literature search of multiple electronic databases (including Embase, Scopus, Web of Science, and Medline/PubMed) was conducted on October 15, 2024, using keywords [vonoprazan] AND [“H. pylori” OR “Helicobacter pylori”]. Two independent reviewers initially reviewed all articles for inclusion using titles and abstracts. In case of disagreements, a third reviewer reviewed the study for inclusion. Full-text manuscripts were reviewed for short-listed articles. The bibliographic section of the selected articles, as well as systematic and narrative articles on the topic, were manually searched for additional relevant articles.
We included randomized controlled clinical trials comparing VA dual therapy to BBT as a treatment for H. pylori infection in adults >18 years of age. We excluded studies performed in pediatric populations, as well as animal studies and studies in language other than English. We excluded abstracts, posters, letters to the editor, narrative reviews, comments, systematic reviews, meta-analyses, case reports, case series, and observational studies.
The primary outcome was the H. pylori eradication rate confirmed by a negative test 4 weeks or more after the end of the antimicrobial medication.15 Secondary outcomes were medication compliance, overall adverse events, diarrhea, dizziness, headache, skin rash, abdominal pain, and abdominal distension. Two independent coauthors extracted the data using a form, and a third coauthor verified the data prior to analysis. Review Manager 5.3 was used to run the analysis, and forest plots were generated. The Cochrane risk-of-bias tool for randomized trials, version 2, was utilized to assess the risk of bias and the quality of the studies (Table 1).16
Table 1.
Risk of bias assessment summary
| Author name, year | Design | Tool used | Overall ROB |
|---|---|---|---|
| Chen, 202428 | RCT | Cochrane RoB 2 | Low |
| Hu, 202313 | RCT | Cochrane RoB 2 | Low |
| Huang, 202125 | RCT | Cochrane RoB 2 | High |
| Li, 202329 | RCT | Cochrane RoB 2 | High |
| Peng, 202314 | RCT | Cochrane RoB 2 | Low |
| Qian, 202317 | RCT | Cochrane RoB 2 | Low |
| Wang, 202230 | RCT | Cochrane RoB 2 | Low |
| Yan, 202028 | RCT | Cochrane RoB 2 | Low |
| Yang, 20198 | RCT | Cochrane RoB 2 | High |
RCT indicates randomized controlled trial; ROB, risk of bias.
RESULTS
Out of 1968 citations, nine randomized clinical trials comparing the efficacy and safety of VA to BBT were included in this meta-analysis, as shown on the PRISMA flow chart (Figure 1).15–17 A total of 2038 patients were included in the study. BBT included PPI, bismuth, and amoxicillin along with clarithromycin or metronidazole or furazolidone.16,17 When quality assessment of included studies was assessed using the Cochrane ROB 2 tool, six studies had an overall low risk of bias, and three studies had an overall high risk of bias.
Figure 1.
PRISMA flow chart showing different stages of screening and number of studies included.
Eradication rate
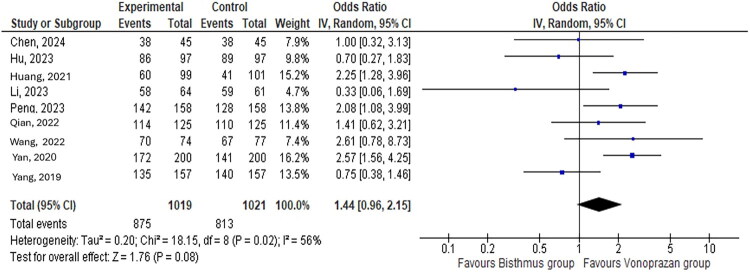
The meta-analysis showed a similar H. pylori eradication rate in the VA group compared with the BBT group, with moderate levels of heterogeneity indicating nonsignificant variation in the outcomes (odds ratio [OR]: 1.44, 95% confidence interval [CI]: 0.96–2.14; I2 = 56%) (Figure 2). With the exception of one sensitivity analysis with the study conducted by Yang et al, the VA group had a significantly higher eradication rate than the BBT group without a heterogeneity change in the results (OR: 1.64, 95% CI: 1.12–2.41; I2 = 43%, P = 0.01) (Figure 3).
Figure 2.
H. pylori eradication rate in the vonoprazan and amoxicillin group compared to the bismuth-based therapy group.
Figure 3.
Leave-one-out sensitivity analysis comparing eradication of H. pylori in the vonoprazan and amoxicillin group compared to the bismuth-based therapy group.
Adverse events
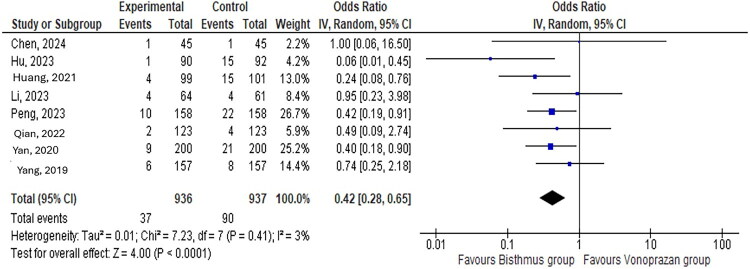
The VA group had a lower incidence of overall adverse events than the BBT group (OR: 0.32, 95% CI: 0.26–0.40; I2 = 0%) (Figure 4). The BBT group had a significantly higher rate of nausea and vomiting than the VA group with low heterogeneity between study results (OR: 0.42, 95% CI: 0.28–0.65; I2 = 0%, P = 0.0001) (Figure 5). The BBT and VA groups had similar rates of dizziness and headache (OR: 0.56, 95% CI: 0.21–1.49; I2 = 29%, P = 0.24), skin rash (OR: 0.77, 95% CI: 0.33–1.76; I2 = 29%, P = 0.35), abdominal pain (OR: 0.83, 95% CI: 0.48–1.43; I2 = 26%, P = 0.50), abdominal distension and early satiety (OR: 1.47, 95% CI: 0.32–6.67; I2 = 38%, P = 0.62), and diarrhea (OR: 1.10, 95% CI: 0.65–1.84; I2 = 29%, P = 0.73) (Supplemental Figures 1–5).
Figure 4.
Incidence of overall adverse events in the vonoprazan and amoxicillin group compared to the bismuth-based therapy group.
Figure 5.
Incidence of nausea and vomiting in the vonoprazan and amoxicillin group compared to the bismuth-based therapy group.
Compliance
There was no difference in compliance between the VA and BBT groups (OR: 1.16, 95% CI: 0.76–1.76; I2 = 0%) (Figure 6).
Figure 6.
Compliance in the vonoprazan and amoxicillin group compared to the bismuth-based therapy group.
DISCUSSION
The effectiveness of H. pylori eradication therapies is declining, primarily due to antimicrobial resistance, with acid-suppressive drugs also playing a partial role.18 Studies have reported a general rise in primary resistance to H. pylori therapies, attributed to gene mutations, and incomplete adherence to therapy, leading to the need for newer treatment strategies.19,20 Uses of multiple antibiotics are also limited by their side effects, including the effect on gut microbiota.21,22
PPI and amoxicillin dual therapy is a reasonable strategy since amoxicillin has a low resistance rate and PPIs provide a nonacidic environment for higher amoxicillin efficacy.23 However, the lack of American College of Gastroenterology adoption and lack of sufficient studies limit its widespread adoption.24 In addition, a higher PPI and antibiotics dose in the therapy is required to improve efficacy and achieve desired eradication rates >90%.8,25 Amoxicillin is pH dependent, and H. pylori is highly susceptible to amoxicillin at pH > 6; thus, it requires potent and sustained gastric acid suppression for eradication.26 However, prolonged or higher-dose use of PPIs and antibiotics ultimately affects gut microbiota and leads to gastrointestinal disturbances.21,23,25,27 Our meta-analysis showed that VA had similar rates of H. pylori eradication and offers an advantage of providing sustained and reliable acid suppression, since it is not subject to polymorphisms in CYP2C19
Adherence to H. pylori treatment is often impacted by adverse events, particularly nausea and vomiting, which are the most common side effects of eradication therapy. Our study found that the VA group experienced a lower overall rate of adverse events, including nausea and vomiting. This suggests that the VA regimen may be a preferable option for patients who have difficulty tolerating H. pylori treatment.
This study has several strengths, including a systematic literature search with well-defined inclusion and exclusion criteria and detailed data extraction. We included only randomized controlled trials, which leads to homogenous data and a decreased risk of bias compared with observational studies. Our meta-analysis has some limitations. First, all the included studies were based in China. Even though data were homogenous, the results may not be widely applicable. There was a paucity of literature from other parts of the world owing to costs and nonapproval. Second, the overall sample size was smaller; hence, the study may not have detected small differences. We could not evaluate the efficacy and safety of two therapies stratified by the dosages and regimen. Third, we could not consider the resistance pattern of the included populations, and antimicrobial susceptibility testing was not performed. Lastly, 24-hour intragastric pH was not monitored or considered and, hence, rates of “true acid suppression” are not known.
In conclusion, VA-based dual therapy is an acceptable treatment strategy, as evidenced by a similar H. pylori eradication rate and a lower adverse event rate in our study. VA therapy may have improved tolerability and, hence, overall patient satisfaction. Future multinational randomized controlled trials with larger sample sizes are required to find the optimal dosage, efficacy, and cost-effectiveness of VA-based therapy in different patient populations.
Supplementary Material
Disclosure statement/Funding
The authors report no funding or conflicts of interest.
References
- 1.den Hollander WJ, Sostres C, Kuipers EJ, Lanas A.. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2013;18 Suppl 1:24–27. doi: 10.1111/hel.12074. [DOI] [PubMed] [Google Scholar]
- 2.Baj J, Forma A, Flieger W, et al. Helicobacter pylori infection and extragastric diseases-a focus on the central nervous system. Cells. 2021;10(9):2191. doi: 10.3390/cells10092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe SE. Helicobacter pylori infection. N Engl J Med. 2019;380(12):1158–1165. doi: 10.1056/NEJMcp1710945. [DOI] [PubMed] [Google Scholar]
- 4.Liou JM, Chen CC, Chang CM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019;19(10):1109–1120. doi: 10.1016/S1473-3099(19)30272-5. [DOI] [PubMed] [Google Scholar]
- 5.Chey WD, Leontiadis GI, Howden CW, Moss SF.. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 7.Gao CP, Zhou Z, Wang JZ, Han SX, Li LP, Lu H.. Efficacy and safety of high-dose dual therapy for Helicobacter pylori rescue therapy: a systematic review and meta-analysis. J Dig Dis. 2016;17(12):811–819. doi: 10.1111/1751-2980.12432. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Zhang Y, Fan L, et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114(3):437–445. doi: 10.14309/ajg.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z, Li J, Huang W, et al. High-dose dual therapy versus bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection: a systematic review with meta-analysis. Turk J Gastroenterol. 2022;33(6):454–462. doi: 10.5152/tjg.2022.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strand DS, Kim D, Peura DA.. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiyotoki S, Nishikawa J, Sakaida I.. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern Med. 2020;59(2):153–161. doi: 10.2169/internalmedicine.2521-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi T, Furuta T, Fujiwara Y, et al. Randomised trial of acid inhibition by vonoprazan 10/20 mg once daily vs rabeprazole 10/20 mg twice daily in healthy Japanese volunteers (SAMURAI pH study). Aliment Pharmacol Ther. 2020;51(5):534–543. doi: 10.1111/apt.15641. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Mei H, Su NY, et al. Eradication rates of Helicobacter pylori in treatment-naive patients following 14-day vonoprazan-amoxicillin dual therapy: a multicenter randomized controlled trial in China. Helicobacter. 2023;28(4):e12970. doi: 10.1111/hel.12970. [DOI] [PubMed] [Google Scholar]
- 14.Peng X, Chen HW, Wan Y, et al. Combination of vonoprazan and amoxicillin as the first-line Helicobacter pylori eradication therapy: a multicenter, prospective, randomized, parallel-controlled study. Clin Exp Med. 2023;23(7):4011–4019. doi: 10.1007/s10238-023-01074-5. [DOI] [PubMed] [Google Scholar]
- 15.Romano M, Cuomo A.. Eradication of Helicobacter pylori: a clinical update. MedGenMed. 2004;176(1):19. [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Qian HS, Li WJ, Dang YN, et al. Ten-day vonoprazan-amoxicillin dual therapy as a first-line treatment of Helicobacter pylori infection compared with bismuth-containing quadruple therapy. Am J Gastroenterol. 2023;118(4):627–634. doi: 10.14309/ajg.0000000000002086. [DOI] [PubMed] [Google Scholar]
- 18.Yan TL, Gao JG, Wang JH, Chen D, Lu C, Xu CF.. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J Gastroenterol. 2020;26(32):4846–4856. doi: 10.3748/wjg.v26.i32.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, O’Morain C, McNamara D.. Helicobacter pylori resistance to current therapies. Curr Opin Gastroenterol. 2019;35(1):6–13. doi: 10.1097/MOG.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 20.Mladenova I. Epidemiology of Helicobacter pylori resistance to antibiotics (a narrative review). Antibiotics (Basel). 2023;12(7):1184. doi: 10.3390/antibiotics12071184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Xu W, Lee A, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 23.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong BC, Xiao SD, Hu FL, et al. Comparison of lansoprazole-based triple and dual therapy for treatment of Helicobacter pylori-related duodenal ulcer: an Asian multicentre double-blind randomized placebo controlled study. Aliment Pharmacol Ther. 2000;14(2):217–224. doi: 10.1046/j.1365-2036.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang Q, Shi Z, Cheng H, Ye H, Zhang X.. Efficacy and safety of modified dual therapy as the first-line regimen for the treatment of Helicobacter pylori infection: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2021;55(10):856–864. doi: 10.1097/MCG.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labenz J. Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract Res Clin Gastroenterol. 2001;15(3):413–431. doi: 10.1053/bega.2001.0188. [DOI] [PubMed] [Google Scholar]
- 27.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Zhang D, Huang S, et al. Comparison of vonoprazan dual therapy, quadruple therapy and standard quadruple therapy for Helicobacter pylori infection in Hainan: a single-center, open-label, non-inferiority, randomized controlled trial. BMC Gastroenterol. 2024;24(1):131. doi: 10.1186/s12876-024-03225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Lv L, Zhu Y, Zhou Z, He S. A modified 14-day dual therapy with vonoprazan and amoxicillin amplified the advantages over conventional therapies for eradication of Helicobacter pylori: a non-inferiority clinical trial. Infect Drug Resist. 2023;16:5637–5645. doi: 10.2147/IDR.S417711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Yu J. IDDF2022-ABS-0149: Effectiveness and safety of novel vonoprazan-based regimen for H. Pylori eradication in China: a single-centre, prospective, randomized trial. Gut 2022;71:A145. doi: 10.1136/gutjnl-2022-IDDF.199. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.