Abstract
Background
Fractional flow reserve with computed tomography (FFR-CT) is influenced by calcified plaque artifacts, which can reduce its accuracy in predicting myocardial ischemia. Subtraction techniques can mitigate these artifacts, thereby enhancing diagnostic precision. However, the potential of subtraction FFR-CT and the pericoronary fat attenuation index (FAI) to improve the prediction of revascularization in patients with coronary artery disease (CAD) remains unclear. We aimed to evaluate the diagnostic performance of FFR-CT and pericoronary FAI in identifying the need for revascularization in patients with CAD.
Methods
We retrospectively analyzed coronary computed tomography angiography (CCTA) data from 76 patients with CAD (142 branches) using both conventional and subtraction CCTA images. The diagnostic performance of FFR-CT and FAI in identifying revascularization needs was assessed using receiver operating characteristic curves.
Results
Among the 76 patients, 54 underwent revascularization. Patients who underwent revascularization had higher pericoronary FAI and lower FFR-CT values. Subtraction FFR-CT values were higher than those obtained using conventional methods. Models 4 (subtraction CCTA), 5 (Model 4 + subtraction FFR-CT), and 6 (Model 5 + subtraction FAI) showed significantly better diagnostic efficacy for revascularization needs than compared to the Models 1 (conventional CCTA), 2 (Model 1 + conventional FFR-CT), and 3 (Model 2 + conventional FAI) (all p < 0.05). In the subtraction models, Model 6 and 5 were significantly more effective than Models 4 (all p < 0.05). Additionally, when clinical variables (male, age, body mass index, hypertension, dyslipidemia, diabetes mellitus, and smoking) were incorporated into Models 3 and 6, the resulting Models 7 and 8 performed significantly better than Model 3 (all p < 0.05).
Conclusion
Subtraction techniques have significantly improved the efficacy of CCTA with FFR-CT in assessing the need for revascularization in patients with CAD. By integrating clinical variables, CCTA, FFR-CT, and pericoronary FAI, individualized therapeutic decisions for CAD patients can be further optimized.
Keywords: Coronary computed tomography angiography, Coronary artery disease, Fat attenuation index, Fractional flow reserve, Subtraction
Background
Coronary computed tomography angiography (CCTA) provides a noninvasive method for identifying and excluding anatomical obstructive stenoses [1]. However, its reliability in correlating with coronary artery disease (CAD)-specific ischemia remains inconsistent [2]. Fractional flow reserve with computed tomography (FFR-CT) has emerged as a valuable tool for accurately diagnosing ischemic stenotic lesions, demonstrating high diagnostic performance and an improved ability to predict revascularization needs [2–4]. Moreover, the pericoronary fat attenuation index (FAI), which measures inflammation associated with myocardial ischemia, enhances the cardiac risk prediction capabilities of coronary CTA [5]. Nonetheless, the diagnostic performance of CCTA and FFR-CT is frequently compromised by coronary artery calcification, which leads to the “blooming” effect and beam-hardening artifacts, as well as challenges in recognizing vascular boundary conditions [6–9]. While subtraction techniques have demonstrated potential in enhancing the accuracy of CCTA [10] and improving the specificity and positive predictive value of FFR-CT for diagnosing significant stenosis [11], the impact of subtraction FAI remains insufficiently explored. Consequently, this study aimed to assess the diagnostic performance of subtraction FFR-CT and FAI in determining the necessity for revascularization necessity in patients with CAD.
Methods
Patients
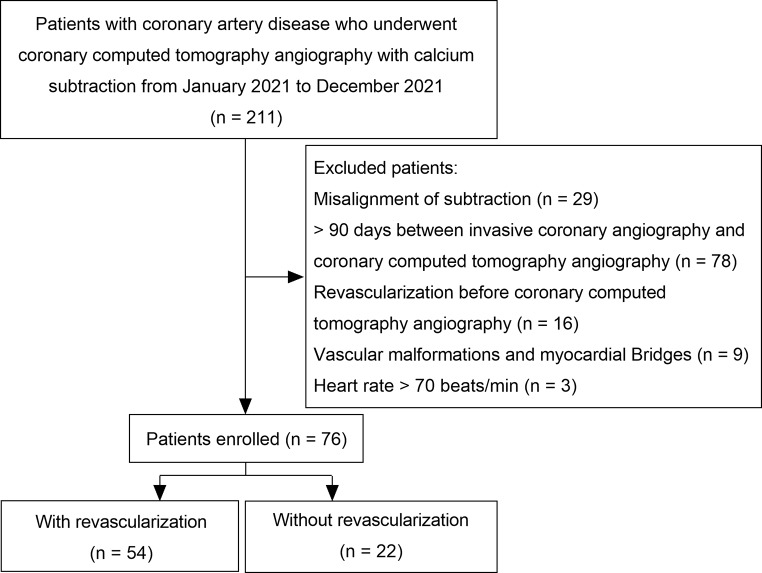
This retrospective study included patients with CAD who visited our hospital throughout 2021. All participants underwent CCTA and post-processing using the subtraction technique, followed by invasive coronary angiography (ICA) within 3 months after CCTA. Patient data, including clinical information and examination results, were extracted from electronic medical records at their initial visit. Inclusion was restricted to patients aged ≥ 18 years with a heart rate of 60–70 beats/min without arrhythmia, complete CCTA and ICA data, and high-quality conventional and subtracted CCTA images (Fig. 1). Patients were excluded if there was a mismatch in subtractive CCTA images, a history of myocardial infarction, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or coronary artery malformation or myocardial bridge. The study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science (February 1, 2020 /No. 2020-S336). Due to the retrospective nature of the study, the requirement for informed consent was waived.
Fig. 1.
Patient inclusion flowchart
CCTA examination
Scanning equipment
We employed a Canon 320-row CT scanner (Aquilion Vision; Canon Medical Systems, Otawara, Japan) for imaging. Patients underwent breathing training prior to scanning to minimize artifacts. Scans were performed using prospective cardiac gating, with sequential non-enhanced and enhanced phases post-breath-hold (to reduce motion artifacts, non-enhanced scanning and enhanced scanning are completed within a single breath hold). Scanning parameters included an area 1 cm below the tracheal bifurcation to the heart apex, intelligent mA control, 100 kV tube voltage, 0.5 mm layer thickness, and 0.25 mm layer spacing. The enhanced scan was triggered by artificial intelligence, with CT attenuation monitored by selecting an area of interest in the root of the patient’s aorta at a threshold of 100 Hounsfield units (HU). The scan was automatically triggered by a delay of 5 s when CT attenuation in the area of interest reached the threshold value. CCTA reconstruction was performed using adaptive iterative dose reduction technology (AIDR 3D Enhanced).
Subtraction CCTA
Subtraction CCTA utilized Volumetric CT Digital Subtraction Angiography software (Canon Medical Systems) to align unenhanced masks with the enhanced images. Both non-contrast and contrast-enhanced CT datasets were reconstructed across the entire cardiac cycle at 1% R-R intervals, with emphasis on the 70–80% phase to optimize motion matching. Diastolic phases exhibiting well-matched calcification morphology, sharpness, and density between the paired datasets were manually selected to minimize motion artifacts (requiring approximately 3–5 min per case). A two-step registration strategy was then applied: global non-rigid registration based on atlas segmentation aligned the overall cardiac structures, followed by local rigid registration of segmented coronary artery trees specifically targeting calcified plaques and stents. After registration, subtraction of the non-contrast from the contrast-enhanced images was performed to effectively suppress calcification-related artifacts and produce clean lumen subtraction images. The automated registration and subtraction process took approximately 2–3 min per case.
For clinical integration, dedicated subtraction software (e.g., Canon Medical Systems) and ≥ 256-detector row CT scanners are essential to ensure whole-heart coverage. Workstations require GPU acceleration (≥ 8 GB VRAM) for efficient processing. Specialist training should include a 2-day workshop covering dual-phase registration principles, motion artifact identification, and Focus ROI error correction, with competency assessed via 10 supervised cases. Routinely embed this protocol for severe-calcification pathways (Agatston score > 400 or stents < 3 mm).
Analysis of CCTA images
Two radiologists with 8 and 12 years of experience in cardiovascular imaging independently evaluated both conventional and subtraction CCTA images. Their assessments were blinded to the patients’ ICA results to ensure unbiased image interpretation. Discrepancies between the radiologists’ findings were resolved collaboratively to reach a consensus. To assess the degree of conventional and subtracted CCTA luminal stenosis in the three main branches of the right coronary artery (RCA), left anterior descending artery (LAD), and left circumflex artery (LCX), luminal stenosis was classified as mild (CCTA < 50%) or moderate-to-severe (CCTA ≥ 50%).
FFR-CT and FAI analysis
FFR-CT and FAI assessments were initially performed using an artificial intelligence-assisted diagnostic system (Shukun Technology Co., Ltd., Beijing, China), which has been granted a Class III Medical Device Registration Certificate by the State Drug Administration of China. Manual adjustments were applied when automated analysis yielded suboptimal results. This combined approach of automated and manual analysis ensured accuracy in the FFR-CT and FAI. The FFR-CT value is generally measured 2 cm distal to the final stenosis. FAI was measured as the CT value (-190 – -30 HU) of adipose tissue surrounding the proximal segment of the coronary artery equal to the diameter of the vessel, approximately 40 mm long and 10 mm from the opening. The FFR-CT and FAI values of all vessels were calculated by two investigators (Defu Li and Tingting Zhu) assessing each vessel independently. After 1 month, FFR-CT and FAI were re-assessed and calculated separately to assess intra- and interobserver agreement.
Therapeutic management strategy
All patients received hemodynamic treatment, including revascularization (PCI or CABG) or medical therapy, based on ICA findings, echocardiography, and clinical symptoms [12]. Patients were then grouped according to whether or not they were revascularized.
Statistical analysis
Data were analyzed using R software (version 4.3.3, Vienna, Austria) and SPSS (v26.0; IBM Corporation, Armonk, NY, USA). Based on previous literature [13, 14] and the study objectives, we performed a power analysis with PASS 15.0 software, which demonstrated high statistical power (0.999–1.000), confirming that the sample size was adequate for diagnostic analyses. Categorical variables, presented as frequencies and percentages, were compared using the chi-square test. Continuous variables, expressed as either median (interquartile range, IQR) or mean ± standard deviation, were determined by normality using the Shapiro–Wilk test. Comparisons between the groups were conducted using the Mann–Whitney U or independent samples t-tests.
The Wilcoxon test was used to analyze conventional FFR-CT and subtraction FFR-CT separately, as well as to compare conventional and subtraction FAI. The diagnostic efficacy of the conventional FFR-CT, subtractive FFR-CT, Model 1 (conventional CCTA), Model 2 (Model 1 + FFR-CT), Model 3 (Model 2 + FAI), Model 4 (subtractive CCTA), Model 5 (Model 4 + subtractive FFR-CT), Model 6 (Model 5 + subtractive FAI), Model 7 (Model 3 + clinical variables), and Model 8 (Model 6 + clinical variables, including male, age, body mass index, hypertension, dyslipidemia, diabetes mellitus, and smoking) for identifying patients with CAD with or without revascularization was analyzed using the receiver operating characteristic (ROC) curve, and sensitivity, specificity, and area under the curve (AUC) were calculated. CCTA ≥ 50% was considered a positive value. The FFR-CT value was taken as 0.8 for invasive FFR, and FFR-CT ≤ 0.8 was considered a positive value [15]. Prior to ROC analysis of the combined models, we conducted multivariate binary logistic regression with each variable in the combined model as an independent variable and revascularization status as the dependent variable. The logistic regression model was then fitted, and predicted probability values were calculated. These predicted probabilities were subsequently used for ROC curve analysis of the combined models. The Delong test was used to compare the differences in AUCs between the individual conventional and subtraction diagnostic models and between the combined diagnostic models of CCTA, FFR-CT, and FAI. To control for type I error inflation due to multiple comparisons, the Benjamini-Hochberg method was implemented to correct the rate of false discovery of the p-values obtained by DeLong’s test for the comparison of the respective ROC curves, and a corrected p-value of < 0.05 was considered statistically significant. Model improvements were assessed using net reclassification improvement (NRI) and integrated discrimination improvement (IDI). Bootstrap resampling (500 replicates) was used to generate 95% confidence intervals and p-values (two-sided Z-tests) for model comparisons. Intra- and inter-group variabilities were tested using the intragroup correlation coefficient (ICC; >0.80 was considered good agreement). Statistical significance was set at p < 0.05.
Results
Baseline patient characteristics
The study included 76 patients with CAD. Among these, 54 underwent revascularization within 3 months following CCTA, involving a total of 76 vessels (74 via PCI and 2 via CABG). The remaining 22 patients, encompassing 66 coronary arteries, did not undergo revascularization. Clinical baseline data showed no significant differences in hypertension, diabetes mellitus, or body mass index between patients with and without revascularization (p > 0.05; Table 1).
Table 1.
Clinical baseline data of patients with and without revascularization
| Without revascularization | With revascularization | p | |
|---|---|---|---|
| Male, n (%) | 15 (68.2) | 38 (70.4) | 0.851 |
| Age (years) | 62.6 ± 10.2 | 63.0 ± 9.5 | 0.877 |
| Body mass index (kg/m2) | 24.3 ± 2.8 | 24.4 ± 3.4 | 0.862 |
| Body height (cm) | 166.3 ± 9.1 | 164.7 ± 7.9 | 0.444 |
| Hypertension, n (%) | 16 (73) | 37 (68.5) | 0.717 |
| Dyslipidaemia, n (%) | 2 (9) | 14 (25.9) | 0.103 |
| Diabetes mellitus, n (%) | 9 (41) | 17 (31.2) | 0.432 |
| Family history of coronary heart disease, n (%) | 0 (0) | 2 (3.7) | 0.36 |
| Smoking, n (%) | 6 (27.3) | 18 (33.3) | 0.606 |
| Alcohol consumption, n (%) | 4 (18.2) | 10 (18.5) | 0.973 |
| Radiation dose (mSv) | 5.4 (4.0, 10.2) | 4.7 (3.7, 9.7) | 0.736 |
p, p-value
CCTA, FFR-CT, and pericoronary FAI characteristics
Patients who underwent revascularization displayed a significantly higher occurrence of both conventional and subtraction CCTA with stenosis ≥ 50% (p < 0.001 for both) than those without revascularization. Additionally, these patients exhibited higher conventional and subtraction FAI values (p = 0.005 and p = 0.020, respectively). In contrast, their conventional and subtraction FFR-CT values were significantly lower (p < 0.001 for both; Table 2). Figure 2 illustrates the CCTA, ICA, FFR-CT, and FAI profiles of these patients.
Table 2.
CCTA, FFR-CT, and pericoronary FAI in patients with and without revascularization
| Without revascularization | With revascularization | p | |
|---|---|---|---|
| Conventional FFR-CT | 0.88 (0.73, 0.94) | 0.68 (0.53, 0.84) | < 0.001 |
| Subtraction FFR-CT | 0.90 (0.82, 0.94) | 0.71 (0.59, 0.84) | < 0.001 |
| Conventional FAI (HU) | -87.2 ± 8.1 | -83.1 ± 8.7 | 0.005 |
| Subtraction FAI (HU) | -86.5 ± 8.4 | -83.0 ± 8.8 | 0.020 |
| Conventional CCTA ≥ 50% | 30 (45.5) | 72 (94.7) | < 0.001 |
| Subtraction CCTA ≥ 50% | 21 (31.8) | 72 (94.7) | < 0.001 |
CCTA, coronary computed tomography angiography; FAI, fat attenuation index; FFR-CT, fractional flow reserve with computed tomography; HU, Hounsfield units; p, p-value
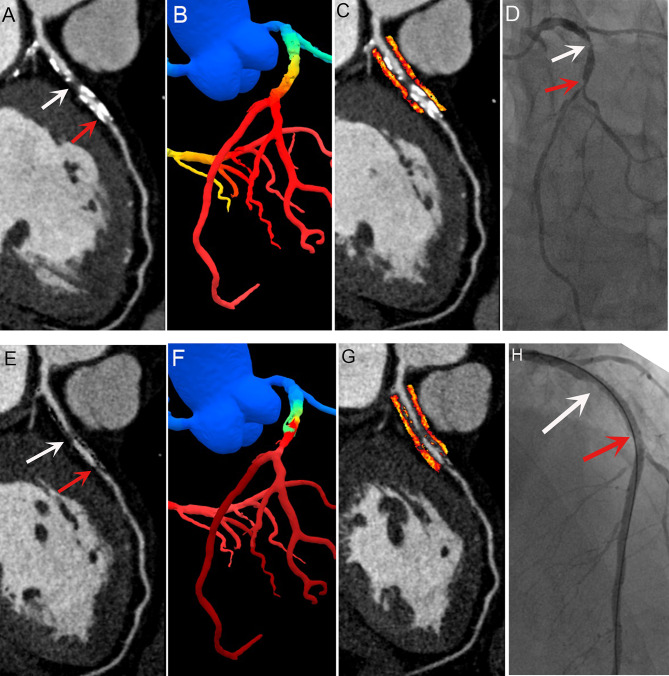
Fig. 2.
Representative images of a patient with coronary artery disease. Conventional coronary computed tomography angiography revealed both calcified and noncalcified plaques in the proximal LAD segment, and part of the lumen could not be evaluated because of occlusion of calcified plaques (A, red arrows). Post-subtraction, significant luminal stenosis became evident (E, white, and red arrows), aligning with invasive coronary angiography findings (D, white and red arrows). The lumen appeared patent and filled after PCI (H, white and red arrows). Conventional and subtraction FFR-CT were 0.63 (B) and 0.50 (F), and the conventional and subtraction fat attenuation indices were − 84 (C) and − 83 Hounsfield units (G), respectively. FFR-CT, fractional flow reserve with computed tomography; LAD, left anterior descending artery
Comparison of conventional and subtraction FFR-CT and conventional and subtraction pericoronary FAI
No significant difference was found in pericoronary FAI ([-85.0 ± 8.6] HU vs. [-84.6 ± 8.7] HU, p = 0.232) and CCTA ≥ 50% (102 [71.8%] vs. 93 [65.5%], p = 0.250) between conventional and subtraction methods. However, subtraction FFR-CT was significantly higher than conventional (0.83 [0.67, 0.90] vs. 0.77 [0.55, 0.92], p = 0.002).
Analysis of the ability of CCTA, FFR-CT, pericoronary FAI, and combined models to identify revascularization needs
We evaluated the diagnostic efficacy of eight diagnostic models incorporating CCTA, FFR-CT, pericoronary FAI, and clinical variables in identifying the need for revascularization. Conventional and subtraction FFR-CT and Models 1–6 performed well in terms of diagnostic efficacy in determining the need for revascularization in patients with CAD (AUC: 0.672–0.894, all p < 0.05; Table 3). The diagnostic efficacy of subtraction FFR-CT and Models 4–6 was better than that of conventional FFR-CT measurements and Models 1–3, respectively, and were significantly better in Model 8 and 7 than in Model 3 (all p < 0.05; Fig. 3A–D). In addition, Model 3 outperformed Models 1, Model 6 and 5 surpassed Model 4 (all p < 0.05; Figs. 3E and F). For model comparisons with statistically significant differences, we further analyzed the NRI and IDI. The results showed that, except for the comparisons between Model 5 vs. Model 4 (which showed no statistically significant differences in IDI), all other model comparisons demonstrated significant differences in both NRI and IDI. Notably, Model 4 showed the most significant improvement in net reclassification compared to Model 1 (NRI = 1.258, 95% CI: 0.973–1.492, p < 0.001), while Model 5 exhibited the most pronounced enhancement in overall discrimination compared to Model 2 (IDI = 0.108, 95% CI: 0.043–0.168, p < 0.001) (Fig. 3G).
Table 3.
Efficacy analysis of CCTA, FFR-CT, and pericoronary FAI in determining the need for revascularization
| Variable | Sensitivity (%) | Specificity (%) | AUC | 95% CI | p |
|---|---|---|---|---|---|
| Conventional FAI | 44.7 | 77.3 | 0.618 | 0.533–0.698 | 0.012 |
| Subtraction FAI | 57.9 | 60.6 | 0.598 | 0.513–0.680 | 0.040 |
| Conventional FFR-CT | 72.4 | 62.1 | 0.672 | 0.589–0.749 | < 0.001 |
| Subtraction FFR-CT | 64.5 | 81.8 | 0.731 | 0.651–0.802 | < 0.001 |
| Model 1 | 94.7 | 54.6 | 0.746 | 0.667–0.816 | < 0.001 |
| Model 2 | 94.7 | 54.6 | 0.752 | 0.673–0.821 | < 0.001 |
| Model 3 | 92.1 | 62.1 | 0.820 | 0.747–0.879 | < 0.001 |
| Model 4 | 94.7 | 68.2 | 0.815 | 0.741–0.875 | < 0.001 |
| Model 5 | 94.7 | 68.2 | 0.861 | 0.792–0.913 | < 0.001 |
| Model 6 | 82.9 | 84.9 | 0.894 | 0.832–0.940 | < 0.001 |
| Model 7 | 88.2 | 71.2 | 0.865 | 0.798–0.917 | < 0.001 |
| Model 8 | 84.2 | 86.4 | 0.913 | 0.854–0.953 | < 0.001 |
Model 1, conventional CCTA; Model 2, Model 1 + FFR-CT; Model 3, Model 2 + FAI; Model 4, subtraction CCTA; Model 5, Model 4 + subtraction FFR-CT; Model 6, Model 5 + subtraction FAI; and Models 7 and 8 were Models 3 and 6, respectively + male, age, body mass index, hypertension, dyslipidemia, diabetes mellitus, and smoking. FFR-CT ≤ 0.8 and CCTA ≥ 50% were considered positive. AUC, area under the curve; CCTA, coronary computed tomography angiography; CI, confidence interval; FAI, fat attenuation index; FFR-CT, fractional flow reserve with computed tomography; p, p-value
Fig. 3.
AUC comparisons for patients with and without revascularization identified by each diagnostic model. The definitions for Models 1–8 were as outlined in Table 3. Positive indicators for these models include FFR-CT measurements ≤ 0.8 and CCTA ≥ 50%. AUC, area under the curve; CCTA, coronary computed tomography angiography; FFR-CT, fractional flow reserve with computed tomography; NRI, net reclassification improvement; IDI, integrated discrimination improvement; CI, confidence interval; p, p-value
Results of consistency test analysis
The interobserver consistency for FFR-CT and FAI measurements was substantial, as indicated by ICC values ranging from 0.861 to 0.921 for FFR-CT and from 0.874 to 0.933 for FAI (Table 4).
Table 4.
Interobserver agreement between FFR-CT and FAI measurements
| FAI | FFR-CT | |||
|---|---|---|---|---|
| ICC (95% CI) | p | ICC (95% CI) | p | |
| TZ1 and TZ2 | 0.928 (0.914–0.940) | < 0.001 | 0.921(0.906–0.934) | < 0.001 |
| TZ1 and DL1 | 0.933 (0.920–0.944) | < 0.001 | 0.861(0.835–0.883) | < 0.001 |
| TZ1 and DL2 | 0.923 (0.909–0.936) | < 0.001 | 0.900(0.881–0.916) | < 0.001 |
| TZ2 and DL1 | 0.881 (0.859–0.900) | < 0.001 | 0.905(0.886–0.920) | < 0.001 |
| TZ2 and DL2 | 0.874 (0.850–0.894) | < 0.001 | 0.887(0.865–0.905) | < 0.001 |
| DL1 and DL2 | 0.890 (0.869–0.906) | < 0.001 | 0.865(0.840–0.886) | < 0.001 |
DL, Defu Li; TZ, Tingting Zhu; 1, 1st assessment; 2, 2nd assessment after 1 month interval; ICC, intra-class correlation coefficient; FFR-CT, fractional flow reserve with computed tomography; FAI, fat attenuation index; CI, Confidence interval; p, p-value
Discussion
In this retrospective study, we analyzed the role of subtraction FFR-CT in identifying revascularization needs in patients with CAD and, for the first time, explored the use of subtraction FAI in CAD. The main findings of this study are as follows: (1) Subtraction improved the ability of CCTA and FFR-CT to identify patients with CAD who required revascularization. (2) The diagnostic efficacy of CCTA in identifying patients with CAD who required revascularization was further improved by the combination of FFR-CT and pericoronary FAI, whether conventional or subtraction.3) The predictive power of image combinatorial models can be further optimized by integrating clinical variables such as age, male, body mass index, smoking, hypertension, dyslipidemia, and type 2 diabetes.
Current knowledge on using pericoronary FAI to identify the need for revascularization in patients with CAD is limited. This study indicates that pericoronary FAI was higher in patients undergoing revascularization than in those who did not, aligning with Duncker et al.’s findings that RCA-FAI was significantly higher in patients with myocardial ischemia than in those without myocardial ischemia and that RCA-FAI is a predictor of myocardial ischemia [16]. Therefore, high FAI values not only suggest plaque instability and perivascular adipose tissue inflammatory response, but also correlate with the degree of coronary artery stenosis [17]. In addition, pericoronary FAI serves as a biomarker of vascular inflammation, reflecting plaque inflammation and rupture in order to stratify patients at risk for future major adverse events [18, 19]. Our findings reveal a progressive enhancement in diagnostic performance upon the sequential integration of FFR-CT and pericoronary FAI into the CCTA model. Yan et al.’s similarly found found that combined pericoronary FAI improved the recognition of ischemic lesions using CCTA [20]. These findings indicate that pericoronary FAI not only provides a new perspective for assessing CAD but also compensates for the deficiencies in CCTA in identifying myocardial ischemia to some extent. Cardiovascular events can be caused by obstructive lesions, but may also be associated with “functional stenosis” due to abnormal lipid metabolism and endothelial dysfunction in inflammatory states of the vasculature [20]. Alternatively, rupture or erosion of vulnerable plaques can also lead to acute myocardial ischemia and cause major adverse cardiac events [21]. Therefore, pericoronary FAI is associated with myocardial ischemia [22], and its role in cardiovascular risk assessment should not be overlooked, especially in predicting the risk of “functional stenosis” and acute myocardial ischemia due to vascular inflammation.
Prior studies have demonstrated the improved accuracy of subtraction FFR-CT in diagnosing myocardial ischemia [11, 23, 24]. Our study confirms that subtraction FFR-CT is more effective than conventional methods. However, conventional FFR-CT did not significantly enhance the diagnostic efficacy of CCTA for revascularization needs in patients with CAD, and the diagnostic performance of FFR-CT was even lower than that of CCTA. Similar findings have been reported in two previous studies, where the diagnostic performance of FFR-CT was lower in a subgroup with severe calcification [9, 11]. We hypothesized that the higher diagnostic performance of subtraction FFR-CT and lower performance of conventional FFR-CT may be due to subtraction reducing the impact of the calcified plaque stenosis geometry on FFR-CT [25]. In contrast, calcified plaques complicate the differentiation between the lumen and vessel wall [11], hindering FFR-CT and reducing their diagnostic accuracy.
Although FFR-CT and invasive FFR correlate well, FFR-CT values are 0.03–0.06 lower than invasive FFR and present the potential for overestimation of stenosis [26, 27]. Our results showed that the subtraction of FFR-CT was significantly higher than that of conventional FFR-CT. The calcium subtraction technique addresses the blooming effect caused by calcified plaques, which can obscure the coronary lumen and overestimate stenosis severity in CCTA [28]. By aligning and subtracting the unenhanced and enhanced images, the technique effectively removes the artifacts caused by calcified plaques, providing a clearer and more accurate view of the coronary lumen [10]. The subtraction technique not only improves the anatomical accuracy of CCTA by clarifying luminal stenosis but also enhances the functional accuracy of FFR-CT by reducing the distortion caused by calcified plaques. This reduction in artifacts allows for more precise modeling of blood flow and pressure, ultimately leading to more accurate predictions regarding the need for revascularization. By improving both anatomical and functional assessments, the calcium subtraction technique enhances the overall ability to evaluate CAD and guides clinical decision-making. Therefore, the removal of calcified plaque artifacts via subtraction can help reduce the overestimation of ischemic status in FFR-CT, leading to more accurate clinical decisions and fewer unnecessary ICA procedures and revascularizations. Therefore, the removal of calcified plaque artifacts via subtraction may benefit patients by reducing the tendency of FFR-CT to overestimate the ischemic status, thereby altering clinical therapeutic decisions and reducing unnecessary ICA and revascularization.
To improve the accuracy of identifying whether revascularization had been performed, we used CCTA as the base model and constructed several combined models by adding FFR-CT and pericoronary FAI. We found that the diagnostic efficacy of CCTA improved with the addition of FFR-CT and pericoronary FAI to the two conventional models (Models 2 and 3). Takagi et al. also showed a trend of gradual improvement in diagnostic efficacy with the addition of FFR-CT and pericoronary FAI in predicting the need for early revascularization [12]. However, our findings differ from the aforementioned in that the diagnostic efficacy of Model 1 (CCTA) and Model 2 (CCTA + FFR-CT) did not show a significant difference, whereas the efficacies of models in the Takagi et al. study were significantly different. This subtle difference may be attributed to the use of different FFR-CT and FAI analysis software. In addition, Takagi et al. only included patients with > 30% CCTA stenosis, whereas our study population comprised all patients with ≤ 30% CCTA stenosis. Finally, differences in comorbidity distributions were observed: their cohort exhibited significantly higher prevalence rates of hypertension, diabetes, and hyperlipidemia in the revascularization group compared to the non-intervention group, whereas baseline comorbidities in our study showed no statistical differences between the two groups.
Another important finding regarding the diagnostic efficacy of the combined models was that both Models 5 and 6 showed significantly improved diagnostic efficacies compared to that of Model 4 (subtracted CCTA). These significant improvements in the diagnostic performance of the combined model after subtraction are, on the one hand, largely due to the use of subtraction techniques. Conversely, the combined model of CCTA, FFR-CT, and pericoronary FAI combines anatomy, physiology, and function to facilitate the effective identification of ischemic stenosis and to increase the likelihood of detecting acute myocardial ischemia caused by the rupture of vulnerable plaques using FAI. The enhanced diagnostic value of this combined model stems from its ability to simultaneously capture the structural, hemodynamic, and inflammatory components of coronary artery disease. While the subtraction technique improves FFR-CT by refining boundary conditions essential for flow modeling, it also optimizes pericoronary FAI assessment by reducing signal interference from calcified plaques. This enables more accurate quantification of perivascular inflammation, a key driver of plaque vulnerability and ischemia beyond stenotic severity alone. Thus, the synergy of anatomical stenosis (CCTA), functional flow limitation (FFR-CT), and inflammatory activity (FAI) reflects the complex, multifactorial pathophysiology of CAD, enhancing the precision of revascularization decision-making.
The coronary artificial intelligence-assisted diagnostic system used for this analysis is an open source platform based on PyRadiomics developed by van Griethuysen et al. [29] PyRadiomics uses a number of engineered hard-coded feature algorithms to process and extract radiological features from medical image data, allowing automated data processing, feature definition and batch processing. While the stability and reliability of the system has been validated in a variety of clinical settings [17, 30–33], its performance may be affected by different populations and clinical comorbidities. In addition, clinical variables such as age, male, body mass index, hypertension, diabetes mellitus, and smoking status are known to influence the progression of CAD and may also affect diagnostic accuracy [34]. In our study, we observed that integrating clinical variables into a diagnostic model (e.g., Model 7) improved the accuracy of identifying the need for revascularization. This phenomenon suggests that the performance of this companion diagnostic system is indeed affected by clinical variables. Therefore, subsequent personalized methods that incorporate imaging data as well as patient-specific clinical information should be used to further optimize diagnostic results and aid in clinical decision-making.
In addition to comparing AUCs, this study utilized NRI and IDI to quantify the incremental predictive value of each model. The results showed that, compared with conventional models, the subtraction models demonstrated significantly improved performance. Specifically, Model 4 showed the most notable NRI improvement over Model 1, while Model 5 had the greatest IDI enhancement compared to Model 2, indicating that the subtraction technique enhances the ability of corresponding conventional models to identify the need for revascularization. Additionally, model performance improved progressively with the addition of FFR-CT, FAI, and clinical variables—for instance, Model 7 outperformed Model 3, and Model 3 outperformed Model 1. These findings suggest that the application of subtraction technology, along with the integration of clinical variables, FAI, FFR-CT, and CCTA, improves the accuracy of assessing the need for revascularization. Importantly, this integrative approach may contribute to more precise clinical decision-making, reduce unnecessary invasive procedures, and ultimately lead to better patient outcomes in the management of coronary artery disease.
The integration of subtraction FFR-CT and pericoronary FAI into standard CCTA protocols offers a non-invasive and artifact-resistant diagnostic pathway that may reduce the need for invasive coronary angiography in patients with equivocal findings. Because revascularization decisions hinge not only on imaging evidence but also on a patient’s clinical risk profile, the incorporation of key clinical factors—such as age, sex, diabetes, hypertension, and smoking status—into diagnostic models is essential. This integrated approach enables more nuanced risk stratification and therapeutic planning. Furthermore, this dual-functional assessment can be applied to routine CCTA datasets using existing post-processing tools, suggesting ease of implementation in tertiary cardiac centers. The improved diagnostic accuracy and personalized assessment potential support its role in optimizing patient selection for invasive procedures and refining individualized revascularization strategies.
This study has some limitations. First, although the sample size was relatively small, the power analysis confirmed adequate statistical strength. Nonetheless, we acknowledge that this limitation may affect the generalizability of our findings. Small sample sizes may reduce statistical efficacy and lead to potential bias or underestimation of true effect sizes. Future studies should include a larger, multicenter cohort to validate these findings and improve the robustness of the statistical analysis. Increasing the sample size would also improve the reliability of the models and facilitate more detailed subgroup analyses, especially in different patient groups with varying degrees of co-morbidity. Second, our exclusion of patients with arrhythmias and prior revascularization may introduce selection bias. Patients with arrhythmias often change the prospective scanning mode, prolong the acquisition time, and affect the image quality of the subtraction. To address this, we began adopting the method combining ECG editing and virtual R waves to obtain stable end-diastolic (70–80%) reconstruction data and reduce motion artifacts. This approach achieved more than 90% phase-matching accuracy in our validation dataset, significantly reducing misalignment artifacts. Additionally, patients with prior PCI or CABG may exhibit altered vascular biology, which could affect image interpretation and limit the applicability of our findings in this subgroup. Nevertheless, future multicenter studies are needed to validate these technical refinements in patients with arrhythmia or prior revascularization. Third, a minimal number of subtraction mismatches occurred, rendering data from this segment of the study population incomplete. Lastly, there was limited support for invasive FFR due to its invasive nature and high costs, which reduced willingness among patients to undergo the procedure. However, given the strong agreement between FFR-CT and invasive FFR, the American Heart Association (AHA) joint Committee and American College of Cardiology (ACC) have recommended FFR-CT for coronary ischemia diagnosis and revascularization decisions [35, 36].
Conclusions
The use of subtraction significantly enhanced the diagnostic accuracy of FFR-CT in both revascularization and non-revascularization scenarios. Combining FFR-CT with pericoronary FAI notably improved the diagnostic capability of single CCTA scans. In addition, the inclusion of relevant clinical variables such as age, male, and comorbidities further improved diagnostic performance and allows for a more personalized assessment. Subtraction FFR-CT and peri-coronary FAI, along with clinical variables, provided a valuable foundation for clinical treatment planning and decision making in CAD management.
Acknowledgements
None.
Abbreviations
- CCTA
Coronary computed tomography angiography
- FAI
Fat attenuation index
- FFR-CT
Fractional flow reserve with computed tomography
- HU
Hounsfield units
- AUC
Area under the curve
- CI
Confidence interval
- ROC curve
Receiver operating characteristic curve
- ICA
Invasive coronary angiography
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- RCA
Right coronary artery
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- ICC
Intragroup correlation coefficient
Author contributions
Conceptualization: T Z; Data curation: Y L; Formal Analysis: H G; Funding acquisition: T Z; Investigation: H G; Methodology: Y L; Project administration: T Z; Resources: Y W; Software: H G; Supervision: Q L; Validation: Q L; Visualization: Y W; Writing – original draft: D L; Writing – review & editing: T Z and D L. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Hubei Province [grant number 2022CFB210]. The sponsor was not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tongji Medical College of Huazhong University of Science (February 1, 2020 /No. 2020-S336). The Ethics Committee of Tongji Medical College of Huazhong University of Science waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55:957–65. 10.1016/j.jacc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 2.Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCover-FLOW (Diagnosis of Ischemia-Causing stenoses obtained via noninvasive fractional flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–97. 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 3.Fairbairn TA, Nieman K, Akasaka T, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE registry. Eur Heart J. 2018;39:3701–11. 10.1093/eurheartj/ehy530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu T, Li D, Qiao J, et al. Accuracy of Subtraction fractional flow reserve with computed tomography in identifying early revascularization in patients with coronary artery disease. Scand Cardiovasc J. 2024;58:2373082. 10.1080/14017431.2024.2373082. [DOI] [PubMed] [Google Scholar]
- 5.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–39. 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coenen A, Lubbers MM, Kurata A, et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology. 2015;274:674–83. 10.1148/radiol.14140992. [DOI] [PubMed] [Google Scholar]
- 7.Vavere AL, Arbab-Zadeh A, Rochitte CE, et al. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification–a subanalysis of the CORE-64 trial. Radiology. 2011;261:100–8. 10.1148/radiol.11110537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto S, Kawasaki T, Kumamaru KK, et al. Diagnostic performance of on-site computed CT-fractional flow reserve based on fluid structure interactions: comparison with invasive fractional flow reserve and instantaneous wave–free ratio. Eur Heart J Cardiovasc Imaging. 2019;20:343–52. 10.1093/ehjci/jey104. [DOI] [PubMed] [Google Scholar]
- 9.Nørgaard BL, Gaur S, Leipsic J, et al. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging. 2015;8:1045–55. 10.1016/j.jcmg.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Qiao J, Li S, Yang H, et al. Subtraction improves the accuracy of coronary CT angiography in patients with severe calcifications in identifying moderate and severe stenosis: a multicenter study. Acad Radiol. 2023;30:2801–10. 10.1016/j.acra.2022.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Kamo Y, Fujimoto S, Nozaki YO, et al. Incremental diagnostic value of CT fractional flow reserve using Subtraction method in patients with severe calcification: a pilot study. J Clin Med. 2021;10:4398. 10.3390/jcm10194398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takagi H, Leipsic JA, McNamara N, et al. Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry. J Cardiovasc Comput Tomogr. 2022;16:19–26. 10.1016/j.jcct.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 14.Obuchowski NA, McClish DK. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med. 1997;16:1529–42. 10.1002/(sici)1097-0258(19970715)16:13%3C1529::aid-sim565%3E3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–21. 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 16.Duncker H, Achenbach S, Moshage M, et al. Computed tomography-derived characterization of pericoronary, epicardial, and paracardial adipose tissue and its association with myocardial ischemia as assessed by computed fractional flow reserve. J Thorac Imaging. 2023;38:46–53. 10.1097/RTI.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Li H, Wang Y, Zhu T. Quantitative plaque characteristics and pericoronary fat Attenuation index enhance risk prediction of unstable angina in nonobstructive lesions. Clin Radiol. 2025;80:106742. 10.1016/j.crad.2024.106742. [DOI] [PubMed] [Google Scholar]
- 18.Sagris M, Antonopoulos AS, Simantiris S, et al. Pericoronary fat Attenuation index-a new imaging biomarker and its diagnostic and prognostic utility: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2022;23:e526–36. 10.1093/ehjci/jeac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daghem M, Adamson PD, Wang KL, et al. Temporal changes in coronary (18)F-fluoride plaque uptake in patients with coronary atherosclerosis. J Nucl Med. 2023;64:1478–86. 10.2967/jnumed.122.264331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H, Zhao N, Geng W, Hou Z, Gao Y, Lu B. Pericoronary fat Attenuation index and coronary plaque quantified from coronary computed tomography angiography identify ischemia-causing lesions. Int J Cardiol. 2022;357:8–13. 10.1016/j.ijcard.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 21.van Veelen A, van der Sangen NMR, Delewi R, Beijk MAM, Henriques JPS, Claessen BEPM. Detection of vulnerable coronary plaques using invasive and non-invasive imaging modalities. J Clin Med. 2022;11:1361. 10.3390/jcm11051361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan B, Deng S, Xu R, Wang Y, He K. Correlation between hemodynamics assessed by FAI combined with CT-FFR and plaque characteristics in coronary artery stenosis. BMC Med Imaging. 2025;25(1):49. 10.1186/s12880-025-01590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, He Q, Xu L, et al. Diagnostic accuracy of Subtraction coronary CT angiography in severely calcified segments: comparison between readers with different levels of experience. Front Cardiovasc Med. 2022;9:828751. 10.3389/fcvm.2022.828751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Li F, Wu K, et al. Subtraction improves the accuracy of coronary CT angiography for detecting obstructive disease in severely calcified segments. Eur Radiol. 2021;31:6211–9. 10.1007/s00330-021-08092-5. [DOI] [PubMed] [Google Scholar]
- 25.Ilic I, Timcic S, Odanovic N, Otasevic P, Collet C. Serial stenosis assessment-can we rely on invasive coronary physiology. Front Cardiovasc Med. 2023;10:1172906. 10.3389/fcvm.2023.1172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of coronary blood flow using CT angiography: next Steps). J Am Coll Cardiol. 2014;63:1145–55. 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 27.Driessen RS, Danad I, Stuijfzand WJ, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73:161–73. 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Ichikawa K, Takahashi D, Sugaya T, Furuya J, Igarashi K. A new contrast enhancement protocol for Subtraction coronary computed tomography requiring a short breath-holding time. Acad Radiol. 2017;24:38–44. 10.1016/j.acra.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 29.van Griethuysen JJM, Fedorov A, Parmar C et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104e107.c 10.1158/0008-5472.Can-17-0339. [DOI] [PMC free article] [PubMed]
- 30.Guo B, Xing W, Hu C, et al. Clinical effectiveness of automated coronary CT-derived fractional flow reserve: A Chinese randomized controlled trial. Radiology. 2024;313(1):e233354. 10.1148/radiol.233354. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Guan H, Wang Y, Zhu T. Quantitative plaque characterization, pericoronary fat Attenuation index, and fractional flow reserve: a novel method for differentiating between stable and unstable angina pectoris in a case-control study. Quant Imaging Med Surg. 2025;15(2):1139–50. 10.21037/qims-24-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong X, Li N, Zhu C, et al. Diagnosis of coronary artery disease in patients with type 2 diabetes mellitus based on computed tomography and pericoronary adipose tissue radiomics: a retrospective cross-sectional study. Cardiovasc Diabetol. 2023;22(1):14. 10.1186/s12933-023-01748-0. Published 2023 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing M, Xi H, Wang Y, et al. Association between pericoronary fat Attenuation index values and plaque composition volume fraction measured by coronary computed tomography angiography. Acad Radiol. 2024;31(9):3579–89. 10.1016/j.acra.2024.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Virani SS, Newby LK, Arnold SV, etal, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: A report of the American heart association/american college of cardiology joint committee on clinical practice guidelines. Circulation. 2023;148:e9–119. 10.1161/CIR.0000000000001168. Erratum in: Circulation. 2023;148(13):e148. 10.1161/CIR.0000000000001183. Erratum in: Circulation. 2023;148(23):e186. 10.1161/CIR.0000000000001195. [DOI] [PubMed]
- 35.Cury RC, Leipsic J, Abbara S, et al. CAD-RADS™ 2.0–2022 Coronary artery disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). J Am Coll Radiol. 2022;19(11):1185–212. 10.1016/j.jacr.2022.09.012 [DOI] [PubMed]
- 36.Guo B, Jiang M, Guo X, et al. Diagnostic and prognostic performance of artificial intelligence-based fully-automated on-site CT-FFR in patients with CAD. Sci Bull (Beijing). 2024;69(10):1472–85. 10.1016/j.scib.2024.03.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cury RC, Leipsic J, Abbara S, et al. CAD-RADS™ 2.0–2022 Coronary artery disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). J Am Coll Radiol. 2022;19(11):1185–212. 10.1016/j.jacr.2022.09.012 [DOI] [PubMed]
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.