Abstract
This study aimed to comprehensively delineate the clinical characteristics, surgical interventions, and evolving trends over the past decade among patients undergoing surgery for renal cell carcinoma (RCC). A retrospective analysis was conducted on the clinical records of 9,110 patients diagnosed with RCC who underwent surgical treatment at Peking University First Hospital between January 2013 and December 2022. Statistical analyses were performed using SPSS 21.0 software. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test, as appropriate. Numerical variables were assessed using the t-test or analysis of variance (ANOVA) for normally distributed data, while nonparametric tests were employed for non-normally distributed numerical variables or ordinal data. A p-value of less than 0.05 was considered statistically significant. The study cohort consisted of 6,416 males (70.4%) and 2,694 females (29.6%), with a median age of 55 years. Clear cell renal cell carcinoma (ccRCC) was the most prevalent histological subtype (87.6%), followed by chromophobe renal cell carcinoma (chRCC) (5.1%), papillary renal cell carcinoma (pRCC) (3.7%), and other subtypes (3.6%). Non-ccRCC patients exhibited a significantly higher proportion of advanced T3 + disease staging (19.4% vs. 15%, P < 0.001). Female patients demonstrated higher incidences of both non-ccRCC and special pathology types (P < 0.001), while non-ccRCC and advanced T-stage disease were more common in pediatric patients (P < 0.001) and were more likely to undergo radical nephrectomy (P < 0.001). Over the span of a decade, the demographic characteristics of RCC patients remained relatively stable; however, there was a notable decrease in tumor size over time (P < 0.001). Notably, partial nephrectomy rates surged between 2013 and 2016—reflecting growing acceptance of nephron-sparing techniques—but later balanced with radical nephrectomies as stricter selection criteria emerged, highlighting the dynamic evolution of RCC surgical management. Our study reveals dynamic shifts in RCC management over the past decade, marked by evolving surgical practices and a trend toward smaller tumor sizes at diagnosis, while distinct clinical features in pediatric patients underscore the need for continued refinement of early detection.
Keyword: Renal cell carcinoma; Surgery; Pathology; Decade-long study
Introduction
Renal cell carcinoma (RCC) is a significant malignancy that arises from the epithelial lining of the renal tubules. Within the spectrum of urinary tract malignancies encountered in clinical practice, RCC accounts for approximately 3% to 5% of all adult cancer diagnoses [1]. According to data from the Centers for Disease Control and Prevention (CDC) in the USA, as well as epidemiological studies conducted in our country, RCC ranks as the third most common tumor in the urinary system, following bladder and prostate cancers in terms of prevalence [2]. However, in the Beijing region, RCC surpasses these malignancies, making it the most frequent urinary tract cancer. Renal cell carcinoma (RCC) is a major malignancy that originates from the epithelial lining of the renal tubules, accounting for approximately 3% to 5% of all adult cancers. In both the USA and China, RCC is ranked as the third most common urinary tract malignancy—following bladder and prostate cancers. However, in the Beijing, RCC has emerged as the most frequent urinary tract cancer [3]. Research from American and European cohorts has consistently demonstrated a higher susceptibility in males, with an approximate male-to-female incidence ratio of 2:1 [1]. Although the disease can affect individuals across all age groups, it predominantly manifests in those aged between 50 and 70 years. Currently, surgical resection remains the cornerstone of treatment for localized and locally advanced RCC, with prior studies affirming its efficacy and safety profile [4]. The primary surgical options include partial nephrectomy and radical nephrectomy, selected based on the extent of the disease and individual patient characteristics. To date, however, a comprehensive epidemiological overview detailing the clinical presentation of RCC at diagnosis remains lacking in our country. This gap highlights the urgent need for a thorough investigation into the clinical profiles, pathological features, surgical approaches, and evolving trends associated with RCC.
At Peking University First Hospital, which serves as a high-volume center for RCC treatment, an exceptionally large number of cases have been managed over the past decade. This single-center retrospective study reviews the clinical data of 9,110 patients who underwent RCC surgery between January 2013 and December 2022. By comprehensively analyzing clinical characteristics, pathological features, surgical techniques, and their evolution over time, we aim to provide a detailed overview of the current status and emerging trends in RCC management.
Materials and methods
Study design and patient cohort
This study employed a retrospective cohort design, analyzing data from patients diagnosed with RCC who underwent surgical treatment at Peking University First Hospital, between January 2013 and December 2022. Inclusion criteria mandated primary RCC diagnosis confirmed by postoperative pathology following either partial nephrectomy or radical nephrectomy performed within the specified timeframe. Patients were excluded if they presented with recurrent RCC requiring reintervention, had incomplete essential clinical or pathological records, or did not undergo surgical resection as primary treatment. After applying these criteria, a total of 9,110 unique patient cases were included in the final analysis. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of Peking University First Hospital. Informed consent requirements were waived due to the retrospective nature of the analysis and the anonymization of patient data.
Data collection and variable definition
The dataset was subjected to rigorous statistical analysis, incorporating variables such as patient demographics (age, sex), tumor location, surgical approach, pathological classification, tumor stage and grade, tumor diameter, and disease progression patterns. This comprehensive evaluation aimed to analyze the characteristics and management of RCC within our clinical context. This investigation adheres to the 2016 edition of the World Health Organization (WHO) classification criteria for renal tumors, the eighth edition of the American Joint Committee on Cancer’s (AJCC) TNM staging and clinical staging guidelines [5], and the International Society of Urological Pathology (ISUP) grading system [6].
Statistical methods
Data analysis will be performed using SPSS 21.0 statistical software. Categorical variables will be evaluated employing the chi-square test (or Fisher’s Exact Test for small sample sizes), while numerical variables will be analyzed through the t-test or analysis of variance (ANOVA). For variables that do not adhere to a normal distribution or are ordinal in nature, nonparametric testing methods will be applied. A P value of less than 0.05 will be deemed to indicate statistical significance.
Results
Patients’ characteristics
Within the cohort of 9,110 patients included in this study, the median age was determined to be 55 years, with a marked predominance of male participants. The distribution of tumor locations across both kidneys was found to be relatively equitable, and the majority of cases were classified as solitary tumors. Clear cell renal cell carcinoma (ccRCC) constituted the predominant histological subtype, accounting for 87.6% of all cases, while 79.6% of the tumors were categorized as pathological stage T1 according to the TNM staging system. Regarding treatment modalities, the utilization rates of radical nephrectomy and partial nephrectomy were observed to be nearly equivalent. A detailed demographic and clinical characterization of the patient population is presented in Table 1. The patients’ geographic area distribution is revealed in Fig. 1.
Table 1.
Characteristic of patient population (N = 9110)
| Characteristic | N (%) |
|---|---|
| Median age(range) | 55 (10–95) |
| Gender | |
| Male | 6416 (70.4) |
| Female | 2694 (29.6) |
| Location | |
| Left | 4406 (48.4) |
| Right | 4686 (51.4) |
| Bilateral | 18 (0.2) |
| Site | |
| One site | 8923 (97.9) |
| Two sites | 157 (1.7) |
| Multiple sites | 30 (0.4) |
| Surgical approach | |
| Radical nephrectomy | 4359 (47.8) |
| Partial nephrectomy | 4751 (52.2) |
| Pathological classification | |
| ccRCC | 7979 (87.6) |
| chRCC | 468 (5.1) |
| pRCC | 334 (3.7) |
| TFE-3 related RCC | 79 (0.87) |
| Unclassified RCC | 65 (0.7) |
| ccpRCC | 21 (0.02) |
| Others | 164 (1.8) |
| pT stage | |
| pT1 | 7250 (79.6) |
| pT2 | 355 (3.9) |
| pT3 | 1366 (15.0) |
| pT4 | 30 (0.3) |
| pTx | 109 (1.2) |
| cT stage | |
| cT1a | 4851 (53.3) |
| cT1b | 2407 (26.4) |
| cT2 | 597 (6.6) |
| cT3a | 977 (10.6) |
| cT3b | 217 (2.4) |
| cT3c | 25 (0.3) |
| cT4 | 36 (0.4) |
| G grade | |
| G1 | 4330 (47.5) |
| G2 | 3468 (38.1) |
| G3 | 706 (7.7) |
| G4 | 60 (0.7) |
| Gx | 546 (6.0) |
| Sarcomatoid differentiation | |
| Yes | 202 (2.2) |
| No | 8908 (97.8) |
| Necrosis | |
| Yes | 1175 (12.9) |
| No | 7935 (87.1) |
| Venous tumor thrombus | |
| Yes | 182 (2.0) |
| No | 8928 (98.0) |
* ccRCC, clear cell renal cell carcinoma; chRCC, chromophobe renal cell carcinoma; pRCC, papillary renal cell carcinoma; ccpRCC, clear cell papillary renal cell carcinoma; pT stage, pathological T stage; cT stage, clinical T stage
Fig. 1.
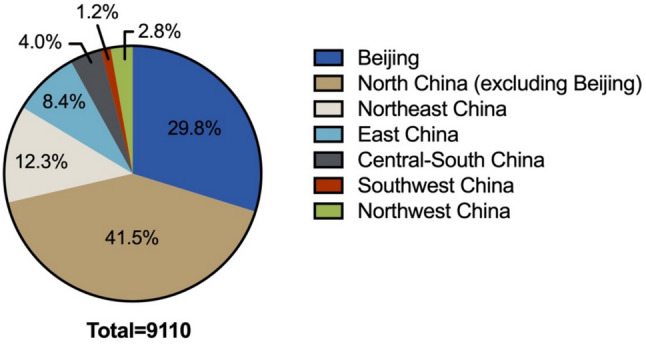
Patients’ geographic area distribution
Our urologic oncology was staffed by 15–20 surgeons subspecializing in kidney cancer (2013–2022). Procedures were performed or supervised by these surgeons, organized into laparoscopic, open and robotic surgery teams to leverage expertise. 20–35 surgical residents assisted as first assistants without primary operative roles. Cases were allocated by tumor complexity and surgeon proficiency, with a rotating schedule capping individual workloads at 10–20 cases/week to ensure quality and prevent overload.
Decadal trend analysis
Variability in the number of surgical procedures
Over the past decade, there had been a consistent increase in the number of patients eligible for nephrectomy, indicating a rising demand for surgical interventions. Notably, the year 2020 showed deviations primarily attributable to the effects of the global health crisis. However, beginning in 2019, the annual number of individuals undergoing surgery had consistently exceeded one thousand cases (Fig. 2).
Fig. 2.

Trends in the number of surgical operations for RCC patients
Alterations in age and gender
The median age of onset for operable RCC had remained stable, falling within the range of 54 to 57 years. A longitudinal analysis conducted over the past decade, with a focus on the median age, reveals no discernible trend toward either younger or older ages among patients undergoing surgical treatment for RCC. This consistency indicated that the demographic profile of individuals requiring surgical intervention for RCC had largely remained unchanged during the observed period (Fig. 3).
Fig. 3.

Trends in the age of RCC patients. * RCC, renal cell carcinoma; PSM, positive surgical margin
A trend analysis was performed to evaluate the age distribution of 9,110 RCC patients across five distinct biennial periods: 2013–2014, 2015–2016, 2017–2018, 2019–2020, and 2021–2022. The analysis demonstrated that the median age of patients varied slightly, ranging between 55 and 57 years across these intervals. However, this fluctuation was not statistically significant (P = 0.225), suggesting that the age distribution of patients undergoing surgical treatment for RCC has remained relatively stable over the examined decade (Table 2).
Table 2.
Comparison of the age composition of RCC patients in five periods
| Under 18 | 18–64 | Over 65 | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| N | Ratio/% | N | Ratio/% | N | Ratio/% | |||
| 2013–2014 (N = 1407) | 2 | 0.2 | 1108 | 78.7 | 297 | 21.1 | 10.615 | 0.225 |
| 2015–2016 (N = 1734) | 5 | 0.3 | 1370 | 79.0 | 359 | 20.7 | ||
| 2017–2018 (N = 1924) | 5 | 0.3 | 1472 | 76.5 | 447 | 23.2 | ||
| 2019–2020 (N = 1908) | 4 | 0.2 | 1440 | 75.5 | 464 | 24.3 | ||
| 2021–2022 (N = 2137) | 3 | 0.2 | 1659 | 77.6 | 475 | 22.2 | ||
* RCC, renal cell carcinoma
Surgical approaches
Between 2013 and 2015, a marked preference for partial nephrectomy was observed, with approximately 70% of patients opting for this surgical approach. Following this period, the proportion of partial nephrectomies underwent a gradual decline, characterized by intermittent fluctuations. Currently, the distribution between partial nephrectomy and radical nephrectomy has reached a state of equilibrium (Fig. 4).
Fig. 4.
Trends in the surgical approaches of RCC patients
Surgical modalities
Throughout the decade from 2013 to 2022, laparoscopic surgery remained the predominant surgical modality, though its proportion showed some fluctuation. Open surgery experienced a consistent decline, decreasing from over 20% in 2013 to below 10% by 2022. Concurrently, robotic-assisted surgery, starting from a negligible proportion, demonstrated a significant and rapid increase in utilization, particularly in the latter half of the decade, reaching over 20% by 2022 (Fig. 5).
Fig. 5.
Trends in the surgical modalities of RCC patients
Pathological T (pT) stages
Decade-long postoperative pathological analyses consistently demonstrated a predominant prevalence of pT1 stage tumors, accounting for 78.0% to 84.2% of all cases. In contrast, pT2, pT3, and pT4 stage tumors were significantly less frequent, representing 3.2% to 5.6%, 10.9% to 19.1%, and 0% to 0.8% of cases, respectively (Fig. 6). Over this ten-year timeframe, the proportional distribution of pT stages exhibited some variability. However, this fluctuation did not follow any clear or consistent trend (Table 3).
Fig. 6.
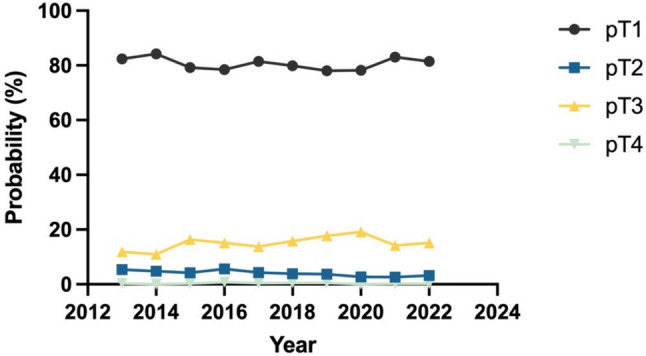
Trends in the T stages of RCC patients
Table 3.
Comparison of the pT stages of RCC patients in five periods
| pT1 + pT2 | pT3 + pT4 | χ2 | P | |||
|---|---|---|---|---|---|---|
| N | Ratio/% | N | Ratio/% | |||
| 2013–2014 (N = 1392) | 1232 | 87.5 | 160 | 11.5 | 32.628 | 0.000 |
| 2015–2016 (N = 1718) | 1439 | 83.8 | 279 | 16.2 | ||
| 2017–2018 (N = 1903) | 1612 | 84.7 | 291 | 15.3 | ||
| 2019–2020 (N = 1873) | 1524 | 81.4 | 349 | 18.6 | ||
| 2021–2022 (N = 2110) | 1797 | 85.1 | 313 | 14.9 | ||
* RCC, renal cell carcinoma; pT, pathological T
Tumor diameters
In the assessment of trends in average tumor diameters among patients with RCC undergoing surgical intervention, stratified into five-year intervals, the mean tumor diameters for the periods 2013–2014, 2015–2016, 2017–2018, 2019–2020, and 2021–2022 were recorded as (4.21 ± 2.47) cm, (4.38 ± 2.37) cm, (4.16 ± 2.28) cm, (4.05 ± 2.35) cm, and (3.83 ± 2.37) cm, respectively. These data indicated a noticeable trend of gradual reduction in tumor diameters over time among RCC patients undergoing surgery during the past decade, with statistically significant differences (P < 0.001) (Fig. 7).
Fig. 7.

Trends in the tumor diameters of RCC patients
Pathological classifications
Over the past decade, ccRCC had predominated in the pathological classification of RCC, accounting for 86.3% to 89.7% of all diagnosed cases (Fig. 8). Notably, the proportion of pathological subtypes among patients undergoing surgical treatment for RCC had remained remarkably consistent, with no significant variations observed in the distribution of these classifications (P = 0.162) (Table 4).
Fig. 8.
Trends in the pathological classifications of RCC patients
Table 4.
Comparison of the pathological classifications of RCC patients in five periods
| ccRCC | pRCC | chRCC | Others | χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Ratio/% | N | Ratio/% | N | Ratio/% | N | Ratio/% | |||
| 2013–2014 (N = 1407) | 1232 | 87.6 | 49 | 3.5 | 76 | 5.4 | 50 | 3.5 | 16.691 | 0.162 |
| 2015–2016 (N = 1734) | 1506 | 86.9 | 59 | 3.4 | 85 | 5.0 | 84 | 4.7 | ||
| 2017–2018 (N = 1924) | 1679 | 87.3 | 77 | 4.0 | 110 | 5.7 | 58 | 3.0 | ||
| 2019–2020 (N = 1908) | 1694 | 88.8 | 65 | 3.4 | 82 | 4.3 | 67 | 3.5 | ||
| 2021–2022 (N = 2137) | 1868 | 87.4 | 84 | 3.9 | 115 | 5.4 | 70 | 3.3 | ||
*RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; pRCC, papillary renal cell carcinoma; chRCC, chromophobe renal cell carcinoma
Positive surgical margin (PSM) rates
A significant downward trend in PSM rates was observed among surgically treated RCC patients over the past decade (P < 0.001) at our institution (Table 5), despite interim fluctuations (Fig. 9).
Table 5.
Comparison of the PSM rates of RCC patients in five periods
| PSM | χ2 | P | ||
|---|---|---|---|---|
| N | Ratio/% | |||
| 2013–2014 (N = 1407) | 30 | 2.1 | 20.032 | 0.000 |
| 2015–2016 (N = 1734) | 19 | 1.1 | ||
| 2017–2018 (N = 1924) | 19 | 1.0 | ||
| 2019–2020 (N = 1908) | 13 | 0.7 | ||
| 2021–2022 (N = 2137) | 16 | 0.7 | ||
Fig. 9.

Trends in the positive surgical margin rates of RCC patients
Correlation between demographic characteristics and postoperative pathological outcomes
Gender
The analysis of gender-based differences in surgical approaches and subsequent pathological findings uncovered significant distinctions. A markedly higher percentage of female patients underwent radical nephrectomy compared to male patients, with this difference being statistically significant (P < 0.001). Additionally, a notable association was observed between gender and the prevalence of specific pathological subtypes of RCC, with a significantly greater proportion affecting female patients (P < 0.001). However, no significant gender-related differences were detected regarding the number of primary lesions (P = 0.394) or pT-stage classification (P = 0.165) (Table 6).
Table 6.
Gender-based comparison of surgical method, number of primary lesions, pathological types, and pT staging in RCC Patients
| Male (N = 6416) | Female (N = 2694) | χ2 | P | ||
|---|---|---|---|---|---|
| Surgical approach | |||||
| Radical nephrectomy | N | 2927 | 1432 | 42.866 | 0.000 |
| Ratio/% | 45.6 | 53.2 | |||
| Partial nephrectomy | N | 3489 | 1262 | ||
| Ratio/% | 54.4 | 46.8 | |||
| Primary tumor site | |||||
| One site | N | 6276 | 2647 | 1.861 | 0.394 |
| Ratio/% | 97.8 | 98.3 | |||
| Two sites | N | 117 | 40 | ||
| Ratio/% | 1.8 | 1.5 | |||
| Multiple sites | N | 23 | 7 | ||
| Ratio/% | 0.4 | 0.2 | |||
| Pathological type | |||||
| ccRCC | N | 5742 | 2237 | 72.194 | 0.000 |
| Ratio/% | 89.5 | 83.0 | |||
| Non-ccRCC | N | 674 | 457 | ||
| Ratio/% | 10.5 | 17.0 | |||
| Male (N = 6343) | Female (N = 2658) | χ2 | P | ||
|---|---|---|---|---|---|
| pT stage | |||||
| pT1 + pT2 | N | 5537 | 2268 | 1.926 | 0.165 |
| Ratio/% | 84.1 | 85.3 | |||
| pT3 + pT4 | N | 1006 | 390 | ||
| Ratio/% | 15.9 | 14.7 | |||
‘* RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; pT, pathological T
Age
The patients treated in our center were stratified into three distinct age groups: "Under 18," "18–64," and "65 and older." We conducted an analysis of differences in surgical characteristics and pathological outcomes among these groups.
Our comparative findings revealed significant variations in surgical approaches (P < 0.001), pT-stage classification (P < 0.001), and pathological subtypes (P < 0.001) across the different age categories. Notably, a higher proportion of patients aged 18–64 underwent partial nephrectomy compared to those aged 65 and older (P < 0.001). In contrast, among patients under 18, a greater fraction underwent radical nephrectomy and were diagnosed with specific pathological subtypes, which may be associated with more advanced pT-stage disease (P < 0.001). However, the disparity in the number of primary foci among the three age groups did not reach statistical significance (P = 0.077) (Table 7).
Table 7.
Age-based comparison of surgical method, number of primary lesions, pathological types, and pT staging in RCC Patients
| Under 18 (N = 19) | 18–64 (N = 7049) | Over 65 (N = 2042) | χ2 | P | ||
|---|---|---|---|---|---|---|
| Surgical approach | ||||||
| Radical nephrectomy | N | 15 | 3209 | 1135 | 71.576 | 0.000 |
| Ratio/% | 78.9 | 45.5 | 55.6 | |||
| Partial nephrectomy | N | 4 | 3840 | 907 | ||
| Ratio/% | 21.1 | 54.5 | 44.4 | |||
| Primary tumor site | ||||||
| One site | N | 18 | 6895 | 2010 | 8.443 | 0.077 |
| Ratio/% | 94.7 | 97.8 | 98.4 | |||
| Two sites | N | 1 | 125 | 31 | ||
| Ratio/% | 5.3 | 1.8 | 1.5 | |||
| Multiple sites | N | 0 | 29 | 1 | ||
| Ratio/% | 0.0 | 0.4 | 0.1 | |||
| Pathological type | ||||||
| ccRCC | N | 5 | 6166 | 1808 | 132.594 | 0.000 |
| Ratio/% | 26.3 | 87.5 | 88.5 | |||
| Non-ccRCC | N | 14 | 883 | 234 | ||
| Ratio/% | 73.7 | 12.5 | 11.5 | |||
| Under 18 (N = 19) | 18–64 (N = 6970) | Over 65 (N = 2012) | χ2 | P | ||
|---|---|---|---|---|---|---|
| pT stage | ||||||
| pT1 + pT2 | N | 6 | 6041 | 1558 | 142.318 | 0.000 |
| Ratio/% | 31.6 | 86.7 | 77.4 | |||
| pT3 + pT4 | N | 13 | 929 | 454 | ||
| Ratio/% | 68.4 | 13.3 | 22.6 | |||
*RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; pT, pathological T
Correlation between pathological types, demographic characteristics, and staging
The analytical results indicated that the pT staging in non-ccRCC cases tends to be more advanced compared to ccRCC (P < 0.001). Nevertheless, no significant difference was observed in the number of primary lesions across various pathological types (P = 0.057) (Table 8).
Table 8.
Comparison of the composition ratio of the number of primary lesions and pT staging in patients undergoing surgery for different pathological types of RCC
| ccRCC (N = 7979) | non-ccRCC (N = 1131) | χ2 | P | ||
|---|---|---|---|---|---|
| Primary tumor site | |||||
| One site | N | 7818 | 1105 | 5.742 | 0.057 |
| Ratio/% | 98.0 | 97.7 | |||
| Two sites | N | 139 | 18 | ||
| Ratio/% | 1.7 | 1.6 | |||
| Multiple sites | N | 22 | 8 | ||
| Ratio/% | 0.3 | 0.7 | |||
| ccRCC (N = 7901) | non-ccRCC (N = 1100) | χ2 | P | ||
|---|---|---|---|---|---|
| pT stage | |||||
| pT1 + pT2 | N | 6718 | 887 | 13.873 | 0.000 |
| Ratio/% | 85.0 | 80.6 | |||
| pT3 + pT4 | N | 1183 | 213 | ||
| Ratio/% | 15.0 | 19.4 | |||
*RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; pT, pathological T
Furthermore, within this cohort, a subset of 79 patients (0.87%) was identified as having Xp11.2 translocation RCC, which belongs to the MiTF/TFE3 family of transcription factor fusion renal tumors. A marked gender disparity was observed, with females constituting the majority at 49 cases (62.0%), compared to males who accounted for 30 cases (38.0%). The median age at diagnosis within this subgroup was 32 years (range: 15–89 years), with a mean age of 35.86 years (standard deviation ± 3.48 years). Notably, the age of onset for patients with Xp11.2 translocation RCC was significantly younger than that of the broader RCC patient population treated at this institution (P < 0.001).
Discussion
Drawing on data from 9,110 RCC cases treated at our institution over the past decade—the largest single-center dataset reported to date—this manuscript provides a comprehensive analysis of patient demographics, surgical interventions, temporal trends, and associated prognostic factors. Our medical institution performed approximately 1,000 RCC surgeries annually, a volume that significantly exceeds the combined total of 3,331 surgeries conducted across 15 centers in 10 European countries over a five-year period, as reported by the European Association of Urology [7]. At our center, the median age at RCC diagnosis is 55 years—a figure that has remained stable over the past decade without a trend toward an older or younger cohort. In contrast, international reports indicate a median age of 64 years [8–10], suggesting that our patient population is relatively younger. This discrepancy may reflect broader variations in demographics and genetic diversity within our country. Furthermore, while data from the American Cancer Society (2023) show that individuals aged 60 and above account for 57.3% of RCC diagnoses globally [1], only 37.1% of cases at our institution fall into this age group. This difference is likely attributable to surgical contraindications or limited surgical accessibility among older patients. The gender distribution in our patient population demonstrated a male-to-female ratio of 2.38:1, which is consistent with the gender prevalence reported in existing literature [1].
In our center, ccRCC represented the predominant pathological subtype of RCC, comprising 87.6% of all cases, which is consistent with the proportions reported in previous studies [1, 11]. However, the incidence of pRCC, at 343 cases (3.77%), is lower than that reported in earlier literature [12, 13]. This discrepancy may be attributed to the significant tumor heterogeneity and the complex nature of the pathological classification of pRCC [14, 15]. Historically, pRCC had been clinically classified into Type I and Type II based on cytopathological characteristics [14]. However, the 2022 WHO classification of renal tumors did not incorporate this dichotomy due to its low interobserver reliability and the difficulty in categorizing certain papillary carcinomas into either type [16]. Despite this, since the 2022 classification criteria had yet to be universally adopted, our pathology department continued to utilize the original Type I and Type II classification system. This may partially explain the lower reported incidence of pRCC in our data. Consistent with the results of other studies, ChRCC was identified in 468 cases (5.14%) [12, 13]. Notably, the proportion of non-ccRCC at stage pT3 or higher was significantly greater than that of ccRCC, likely reflecting the generally more aggressive and invasive nature of non-ccRCC subtypes [17–20].
Following the widespread adoption of computerized tomography (CT) scanning for health assessments, our institution conducted a comprehensive analysis of data spanning a decade. This analysis demonstrated a progressive reduction in the maximum tumor diameter among surgical patients, a trend that aligns with findings reported by Junejo et al. in 2021 [21]. The decision to perform partial nephrectomy versus radical nephrectomy is primarily influenced by tumor size. Nevertheless, despite the observed annual decrease in tumor longitudinal dimensions, a direct correlation with the choice of surgical intervention has yet to be established. Notably, over the past decade, there had been a notable shift in the surgical management of RCC at our center. Initially, partial nephrectomies accounted for up to 70% of all procedures. However, this proportion had gradually shifted toward a more balanced distribution with radical nephrectomies over time. Radical nephrectomy, first introduced by Robson in 1963, has traditionally been regarded as the "gold standard" for the surgical treatment of localized RCC [22]. In contrast, the acceptance of partial nephrectomy as a viable alternative extended over a period of more than 110 years. Although the first partial nephrectomy was performed in 1887, it was not until the publication of extensive long-term follow-up studies by Herr in 1999 [23] and Hafez in 2000 [24] that this procedure received widespread endorsement in clinical guidelines and gained recognition within the academic community as a suitable treatment option. The implementation of nephron-sparing surgery (NSS) has gained favor due to its association with a lesser decline in glomerular filtration rate (GFR) and a reduced incidence of chronic kidney disease (CKD). As such, major clinical guidelines designate NSS as the preferred treatment modality for T1a RCC, with limited exceptions for cases involving a solitary kidney, bilateral tumors, or hereditary kidney cancer. However, the application of NSS for T1b and T2 RCC remains controversial, with no clear consensus established [25]. Recent advancements in robotic surgery, coupled with an increasing patient preference for procedures that prioritize tumor resection while preserving renal function, have encouraged clinicians to investigate the feasibility of NSS in patients with T2 RCC. This shift in approach may account for the observed rise in the proportion of NSS among all surgical interventions between 2013 and 2015. The subsequent development and widespread adoption of the R.E.N.A.L. nephrometry scoring system had facilitated a more prudent and discerning approach to the application of NSS. This underscored the necessity for careful consideration when expanding the surgical indications for NSS [26, 27]. As a result, the selection criteria for NSS have become increasingly stringent, which may account for the observed decline in the proportion of NSS procedures performed at our institution. Several center-specific factors may underlie the paradoxical drop in NSS despite smaller tumors. First, although many of our senior surgeons are highly experienced in open and laparoscopic NSS, in earlier years they received limited formal training in modern minimally invasive nephron-sparing surgery techniques, and in the early diffusion phase of laparoscopy both radical and partial approaches were adopted unevenly [28, 29]. Second, as a tertiary referral center, we manage a higher proportion of anatomically challenging tumors—multifocal, centrally located, or with sinus invasion—that favor radical over nephron-sparing resection (e.g., Medscape Urology Guidelines [30]). Finally, under current performance and quality metrics in China, NSS may be selected more often to shorten operative time, reduce perioperative morbidity, and expedite discharge. These factors, together with evolving surgeon preferences and policy incentives, likely account for the observed NSS decline and merit further prospective evaluation.
Beyond the fluctuation in surgical approaches, our center has also witnessed a transformation in surgical modalities over the past decade. The introduction of the Da Vinci robotic system in our center in 2015 marked a significant turning point in the adoption of robotic-assisted surgery. Robotic-assisted surgery was nearly absent in the early years of the study but experienced a substantial increase in utilization. We believe this trend analysis provides valuable context to our long-term experience and highlights the evolving surgical landscape within our institution over the past decade, reflecting the advancements and increasing adoption of minimally invasive techniques, significantly influenced by the integration of robotic surgery.
Furthermore, there have been no significant alterations in patient demographics, including age distribution, gender proportions, or pathological subtypes, at the time of surgery over the past decade. Although fluctuations in the proportions of different pT stages had been noted, no consistent trends had emerged. These findings suggest that the demographic and pathological profiles of RCC patients treated at our center have remained relatively stable during this period.
Upon analyzing the distribution of RCC across different genders and age groups, it had been observed that female patients demonstrated a higher prevalence of rare pathological subtypes of RCC compared to male patients, accompanied by an increased frequency of radical nephrectomy procedures. In pediatric populations, there was a significant rise in the incidence of non-ccRCC, which was characterized by more advanced pT-stage classifications and larger tumor dimensions. These factors collectively resulted in a higher probability of requiring radical nephrectomy. These trends may be attributed to the intrinsically higher malignancy, increased invasiveness, and poorer prognosis associated with specific pathological entities, such as collecting duct carcinomas and hereditary RCCs. Genetic mutations implicated in hereditary RCCs, including but not limited to fumarate hydratase (FH) gene defects, MiTF/TFE3-related translocation RCC, succinate dehydrogenase B (SDHB) gene mutations, and SMARCB1 gene abnormalities, were disproportionately observed in younger female patients [16, 19, 20, 31–34]. Despite their relatively low incidence, these distinct pathological subtypes, particularly hereditary cancers, were frequently diagnosed at an advanced stage and exhibit aggressive disease behavior and unfavorable prognostic outcomes. Consequently, they warranted heightened clinical vigilance due to their potentially life-threatening implications.
While formal time-to-event analyses by stage, grade, or margin status are beyond the scope of this descriptive cohort, we quantified PSM rates and identified a significant ten-year declining trend. This observed reduction in PSM incidence may be associated with advancements in surgical techniques. Subsequent studies will focus on long-term survival outcomes and recurrence patterns.
Conclusion
This investigation employs a comprehensive dataset obtained from RCC surgeries conducted over a decade at our single-center institution. Notably, the fluctuation in the rate of partial nephrectomies highlights an advancing comprehension of surgical techniques within the medical community. Additionally, specific pathological subtypes of RCC are observed with significant frequency among pediatric patients, who frequently present at advanced disease stages. This emphasizes the pressing need for enhanced early diagnostic efforts.
Author contribution
Ke Hu and Ming-Wei Ma wrote the main manuscript text. Ke Hu and Ming-Wei Ma contributed equally to this work. Xue-Song Li participate in the revision of the manuscript. Kai-Wei Yang, Jia-Yan Chen and Xue-Ying Ren collected statistics. Hong-Zhen Li and Xiao-Ying Li revised this work critically for important intellectual content. Xian-Shu Gao, Wei Yu and Qi Shen agreed to be accountable for all aspects of the work and ensure that questions related to the accuracy or completeness of any part of the work are appropriately investigated and resolved. All authors reviewed the manuscript.
Funding
National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital) (2023HQ11), Peking University Clinical Scientist Training Program (BMU2025PYJH033), Capital’s Funds for Health Improvement and Research (2024-4-40710).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ke Hu and Ming-Wei Ma have contributed equally to this work.
Contributor Information
Qi Shen, Email: sophiashen15@163.com.
Wei Yu, Email: yuweif@126.com.
Xian-Shu Gao, Email: doctorgaoxs@126.com.
References
- 1.Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Male Urologic Cancers. USCS Data Brief, no 21. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020.
- 3.Rongshou Z, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 2022;2(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–24. [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, E.S., Greene FL, et al, AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017.
- 6.Delahunt B, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–504. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Ghanem Y, et al. The impact of histological subtype on the incidence, timing, and patterns of recurrence in patients with renal cell carcinoma after surgery-results from RECUR consortium. Eur Urol Oncol. 2021;4(3):473–82. [DOI] [PubMed] [Google Scholar]
- 8.Siemer S, et al. Outcome of renal tumors in young adults. J Urol. 2006;175(4):1240–3. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, et al. Renal cell carcinoma in young and old patients–is there a difference? J Urol. 2008;180(4):1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook A, et al. Pediatric renal cell carcinoma: single institution 25-year case series and initial experience with partial nephrectomy. J Urol. 2006;175(4):1456–60. [DOI] [PubMed] [Google Scholar]
- 11.Capitanio U, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zibao X, Fuli W, Zheng Y, et al. Analysis of the clinical pathological characteristics, surgical conditions, and decade-long trends of renal cell carcinoma patients at a single center. J Clin Urol. 2019;34(01):27–31. 10.13201/j.issn.1001-1420.2019.01.007. [Google Scholar]
- 13.Wang Chenqing Ju, Yuquan YF, et al. Summary and analysis of renal cancer surgery cases over five years at a single center. J Modern Urol. 2021;26(06):514–8. [Google Scholar]
- 14.Linehan WM, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flippot R, et al. Papillary renal cell carcinoma: a family portrait. Eur Urol. 2018;73(1):79–80. [DOI] [PubMed] [Google Scholar]
- 16.Trpkov K, et al. New developments in existing WHO entities and evolving molecular concepts: the genitourinary pathology society (GUPS) update on renal neoplasia. Mod Pathol. 2021;34(7):1392–424. [DOI] [PubMed] [Google Scholar]
- 17.Volpe A, et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 2012;110(1):76–83. [DOI] [PubMed] [Google Scholar]
- 18.Wright JL, et al. Effect of collecting duct histology on renal cell cancer outcome. J Urol. 2009;182(6):2595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakouny Z, et al. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep. 2022;38(1): 110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibovich BC, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183(4):1309–15. [DOI] [PubMed] [Google Scholar]
- 21.Junejo NN, et al. Trends in the surgical management of renal cell carcinoma in a contemporary tertiary care setting. Urol Ann. 2021;13(2):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37–42. [DOI] [PubMed] [Google Scholar]
- 23.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161(1):33–4. [DOI] [PubMed] [Google Scholar]
- 24.Hafez KS, Fergany AF, Novick AC. Nephron sparing surgery for localized renal cell carcinoma: impact of tumor size on patient survival, tumor recurrence and TNM staging. J Urol. 1999;162(6):1930–3. [DOI] [PubMed] [Google Scholar]
- 25.Campbell SC, et al. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J Urol. 2021;206(2):199–208. [DOI] [PubMed] [Google Scholar]
- 26.Scosyrev E, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65(2):372–7. [DOI] [PubMed] [Google Scholar]
- 27.Pierorazio PM, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196(4):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon SA, et al. Trends in partial and radical nephrectomy: an analysis of case logs from certifying urologists. J Urol. 2013;190(2):464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harke NN, et al. Impact of surgical experience before robot-assisted partial nephrectomy on surgical outcomes: a multicenter analysis of 2500 patients. Eur Urol Open Sci. 2022;46:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.emedicine.medscape.com/article/446317-guidelines.
- 31.Malouf GG, et al. Transcription factor E3 and transcription factor EB renal cell carcinomas: clinical features, biological behavior and prognostic factors. J Urol. 2011;185(1):24–9. [DOI] [PubMed] [Google Scholar]
- 32.Tokuda N, et al. Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. 2006;176(1):40–3. [DOI] [PubMed] [Google Scholar]
- 33.Gleeson JP, et al. Comprehensive molecular characterization and response to therapy in fumarate hydratase-deficient renal cell carcinoma. Clin Cancer Res. 2021;27(10):2910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Msaouel P, et al. Comprehensive molecular characterization identifies distinct genomic and immune hallmarks of renal medullary carcinoma. Cancer Cell. 2020;37(5):720-734.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.





