Abstract
Acute aerobic exercise (AEX) enhances motor learning and promotes neuroplasticity. Although previous studies examined the impact of AEX on primary motor cortex (M1) excitability, the dose–response relationship across a broad intensity spectrum with consistent exercise parameters remains to be fully elucidated. This study investigated the impact of AEX intensity on distinct M1 circuits using transcranial magnetic stimulation (TMS). Thirty right-handed adults underwent four experimental sessions: 20-min rest (control), light (LIIT: 35% heart rate reserve [HRR]), moderate (MIIT; 55% HRR), and high-intensity interval training (HIIT; 80% HRR) AEX. The interval cycling sessions alternated between 3 min at the target intensity and 2 min of active recovery (25% HRR). We performed TMS measures before (Pre), immediately post (Post0), and 20-min post (Post20) AEX/rest to assess corticospinal excitability and short-interval intracortical inhibition (SICI). We found that: (i) HIIT and MIIT increased corticospinal excitability, with HIIT eliciting a sustained increase, and (ii) all AEX intensities (LIIT, MIIT, HIIT) decreased SICI, with the greatest sustained reduction following MIIT. Altogether, our results demonstrate the impact of HIIT and MIIT to enhance corticospinal excitability and reduce SICI in M1. This study provides evidence for a dose–response effect of AEX intensity on the modulation of distinct M1 circuits.
Keywords: acute exercise, exercise intensity, neuroplasticity, primary motor cortex
Introduction
An acute bout of aerobic exercise can enhance motor learning and promote neuroplasticity (Roig et al. 2012; Mang et al. 2014; Stavrinos and Coxon 2017; Opie and Semmler 2019; Neva et al. 2021). Studies using transcranial magnetic stimulation (TMS) have shed light on the impact of acute aerobic exercise (AEX) on the human brain (Singh et al. 2014; Smith et al. 2014; Mooney et al. 2016; Lulic et al. 2017; Neva et al. 2017; Yamazaki et al. 2019; Andrews et al. 2020). A single session of exercise increases the responsiveness to neuroplasticity-inducing repetitive TMS protocols, indicating a potential preparatory or “priming” effect of AEX on neuroplasticity (Mang et al. 2014; Singh et al. 2014; Andrews et al. 2020).
Further investigations have delved into the neurophysiological mechanisms underlying these effects, employing both single- and paired-pulse TMS techniques to explore AEX-induced changes in primary motor cortex (M1) excitability (Singh et al. 2014; Smith et al. 2014; Mooney et al. 2016; Lulic et al. 2017; Stavrinos and Coxon 2017; Yamazaki et al. 2019; El-Sayes et al. 2019b; Turco and Nelson 2021). Following an acute bout of lower limb cycling exercise, various inhibitory and facilitatory circuits within the upper-limb representation of M1 exhibit excitability changes. Although there is variability in the effects of AEX on these various inhibitory and facilitatory M1 circuits, there are two TMS-based measures that are emerging as more consistent and robust (Youssef et al. 2024). Gathering data shows that AEX decreases GABAA-related inhibition, as reflected by a reduction in short-interval intracortical inhibition (SICI; Singh et al. 2014; Smith et al. 2014; Lulic et al. 2017; Stavrinos and Coxon 2017; El-Sayes et al. 2019b, Yamazaki et al. 2019). In fact, a recent meta-analysis demonstrated that decreased SICI was the most consistent and robust effect following AEX, particularly for moderate- and high-intensity exercise (Youssef et al. 2024). Other studies have shown that M1 output excitability, or corticospinal excitability, can be impacted by AEX (Ostadan et al. 2016; Lulic et al. 2017; El-Sayes et al. 2019b; Opie and Semmler 2019). Importantly, even though most studies observed no modulation of corticospinal excitability following AEX across multiple exercise intensities and types (Mang et al. 2014; Singh et al. 2014; Neva et al. 2017; Stavrinos and Coxon 2017; Smith et al. 2018; Yamazaki et al. 2019; Andrews et al. 2020; El-Sayes et al. 2020; Morris et al. 2020), a meta-analysis demonstrated that only high-intensity cycling exercise increased corticospinal excitability (Youssef et al. 2024). Thus, it appears that AEX parameters, such as intensity, play an important role in the impact of exercise on M1 excitability modulation (Youssef et al. 2024). Although prior studies have compared various intensities of AEX, they often varied in exercise type or structure (eg comparing high-intensity interval training to continuous moderate-intensity exercise) and duration. To date, no single study has systematically examined the effects of exercise intensity on M1 cortical circuit modulation while controlling for both exercise type or structure (ie interval) and duration [eg 15 to 30 min; (Youssef et al. 2024)].
The AEX parameters across studies have been considerably different, which may contribute to the variability of the impact of exercise on M1 excitability reported in the literature. Specifically, previous work used different intensities (eg light, moderate, or high intensity), types (eg continuous or interval exercise) and durations (eg 15 min, 30 min) of exercise, or different combinations of these parameters in a single study (Youssef et al. 2024). For instance, the majority of studies investigating exercise-induced effects on M1 excitability showed decreased SICI using moderate-intensity continuous exercise (Singh et al. 2014; Smith et al. 2014; Lulic et al. 2017; El-Sayes et al. 2019b) or high-intensity interval training exercise (HIIT; Stavrinos and Coxon 2017, Opie and Semmler 2019, Hendy et al. 2022, Kuo et al. 2023). Only a few studies have reported no significant change in SICI following moderate-intensity continuous cycling (Mooney et al. 2016; Morris et al. 2020) or HIIT (Andrews et al. 2020; Nicolini et al. 2020). For instance, Andrews et al. (2020) compared both moderate- and high-intensity protocols but employed different types/structures (continuous vs. interval cycling) across exercise conditions. While they found a pattern of SICI modulation suggestive of a dose–response effect of exercise, it was not significant based on the statistical model used. This underscores the necessity to isolate the impact of exercise intensity while keeping other exercise parameters, such as type/structure and duration, constant. Only two studies investigated the impact of light-intensity continuous cycling exercise on SICI, which showed inconsistent results (Opie and Semmler 2019; Yamazaki et al. 2019).
On the other hand, most studies that used moderate intensity continuous exercise did not find an effect on corticospinal excitability (Singh et al. 2014; Smith et al. 2014; Singh et al. 2016; Neva et al. 2017; Smith et al. 2018; Brown et al. 2020; Morris et al. 2020), with few studies showing an increase (Lulic et al. 2017; El-Sayes et al. 2020). Some studies have shown that HIIT can increase corticospinal excitability (Ostadan et al. 2016; Opie and Semmler 2019; Nicolini et al. 2020; Hendy et al. 2022), whereas others have not shown this effect (Mang et al. 2014; Stavrinos and Coxon 2017; Andrews et al. 2020; El-Sayes et al. 2020). Finally, only a few studies investigated the effect of light-intensity continuous exercise on corticospinal excitability and reported no effect (Yamazaki et al. 2019). Thus, it is probable that exercise intensity plays an important role in the impact of AEX on SICI and corticospinal excitability modulation, along with other exercise parameters (ie type and duration).
Taken together, the current state of the literature supports the potential of a dose–response relationship between exercise intensity and M1 cortical excitability changes. Crucially, no previous study has investigated the effect of AEX intensity on M1 excitability comparing a comprehensive spectrum of three distinct exercise intensities (light, moderate, and high) while controlling for both exercise type (continuous vs interval) and duration. Moreover, as demonstrated in previous work (Youssef et al. 2024), some exercise-induced effects on M1 excitability emerge immediately, while others may manifest with a slight delay (eg 20 min postexercise). However, few studies have assessed the time course of M1 excitability changes postexercise or examined interneuron-specific effects using directionally sensitive TMS approaches. Thus, the time-sensitive and interneuron circuit effects post-AEX remain unclear.
It is possible that variability in exercise-induced effects on M1 excitability is due to the TMS parameters used. All previous studies have explored the impact of AEX on M1 excitability changes using a posterior-to-anterior (PA) TMS current, except for one previous study (Neva et al. 2021). In that study, both PA and anterior-to-posterior (AP) TMS currents were used to investigate the unique M1 interneurons (Hanajima et al. 1998; Ziemann and Rothwell 2000; Di Lazzaro et al. 2001) that may be impacted by AEX (Neva et al. 2021). It was found that moderate-intensity cycling exercise increased corticospinal excitability and decreased SICI as measured with AP, but not with PA, TMS current. These results suggested that interneuron excitability sensitive to an AP TMS current may play an important role underlying the exercise-induced effects on neuroplasticity (Mirdamadi et al. 2017; Hannah et al. 2018; Spampinato 2020). Specifically, a PA current predominantly elicits early I-waves (eg I1), which are thought to arise from direct, monosynaptic inputs to corticospinal neurons. In contrast, an AP current preferentially recruits later I-waves (eg I3), which are believed to involve polysynaptic circuits and a distinct population of interneurons (Hanajima et al. 1998; Ziemann and Rothwell 2000; Di Lazzaro et al. 2012).
Importantly, the interneuron circuits preferentially activated by PA and AP TMS currents demonstrate unique neurophysiological characteristics. For instance, AP TMS preferentially recruits interneuron circuits that receive modulatory inputs from frontal regions such as the premotor and prefrontal cortex (Hanajima et al. 1998; Ziemann and Rothwell 2000; Paulus et al. 2008; Di Lazzaro et al. 2012; Hamada et al. 2013; Hamada et al. 2014). This connectivity suggests that AP interneuron circuits may play a more prominent role in higher-order aspects of motor control, including motor planning, attention, and cognitive regulation of movement. Indeed, prior research has demonstrated that the excitability of AP interneuron circuits is preferentially modulated during motor tasks involving greater attentional demand, motor preparation, or visuomotor adaptation (Mirdamadi et al. 2017; Hannah et al. 2018; Spampinato et al. 2020). Thus, it is perhaps not surprising that corticospinal and intracortical excitability sensitive to AP TMS is more susceptible to the impact of acute exercise (Neva et al. 2021), as other work has shown that acute exercise can enhance motor learning, adaptation, and cognitive regulation of movement (Roig et al. 2012; Thomas et al. 2016; Herrera et al. 2024; Neva 2024).
Taken together, the neurophysiological distinction between PA- and AP-sensitive interneuron circuits indicates the potential of a differential impact on their excitability following varying AEX intensities. By probing these distinct circuits, we can determine if exercise intensity differentially engage specific M1 interneuron excitability, thereby providing a nuanced understanding of how exercise impacts motor cortical excitability.
The overarching aim of the current study was to examine the dose–response relationship between AEX and M1 cortical circuit modulation using a single session of interval cycling exercise across a spectrum of different intensities (ie light, moderate, high), while holding exercise type/structure (ie interval) and duration (ie 20 min) constant. The primary objective of this study was to examine the impact of AEX intensity on M1 excitability modulation on SICI and corticospinal excitability. Additionally, this study aimed to assess M1 excitability at two postexercise time points to capture both immediate and delayed neurophysiological effects, addressing a key limitation of earlier work that focused on immediate AEX effects when comparing different exercise intensities (Opie and Semmler 2019; Andrews et al. 2020; El-Sayes et al. 2020). We hypothesized that HIIT would increase corticospinal excitability and that MIIT and HIIT would decrease SICI.
The secondary objective was to examine the impact of AEX intensity on M1 cortical circuit excitability as measured by PA and AP TMS currents. While studies using conventional PA TMS have yielded mixed results regarding the effect of acute exercise on corticospinal excitability, emerging evidence suggests that AP-directed TMS may be more sensitive to exercise-induced modulation. Our prior study showed that moderate-intensity exercise selectively enhanced corticospinal excitability with AP but not PA TMS current (Neva et al. 2021), suggesting that later I-wave generating pathways may be preferentially engaged by this form of exercise. Moreover, a recent meta-analysis from our group demonstrated that high-intensity exercise is associated with increased corticospinal excitability, with all studies using PA TMS current (Youssef et al. 2024). Despite these findings, it is vital to note that many studies showing no change could not be included due to insufficient or missing data (eg pre- and post-MEP amplitude averages). The pervasive finding that high-intensity exercise typically does not alter corticospinal excitability (Mang et al. 2014, Smith et al. 2014, Stavrinos and Coxon 2017, Smith et al. 2018, El-Sayes et al. 2020) is a pivotal insight informing our specific hypotheses regarding corticospinal excitability measured with the conventional PA TMS current. Considering all these findings, we hypothesized that corticospinal excitability measured with AP TMS current would increase following both MIIT and HIIT, while remaining unchanged with PA TMS current. Further, we expected to observe decreased SICI measured with AP current to a greater extent than the PA current following MIIT and HIIT.
Materials and methods
Participants
Characteristics of participants are presented in Table 1. All data are presented as mean (SD) unless otherwise noted. Thirty healthy right-handed (93 [11]; Edinburgh Handedness Inventory [EHI]; Oldfield 1971) young adults (27 [5] years, 50% females) took part in the study. A sensitivity analysis performed in G*Power with an alpha risk of 0.05 and a sample size of 30 indicated that we had a 90% chance of observing a medium effect size of f(U) = 0.262 (~ η2p = 0.064) or higher (Lakens 2022).
Table 1.
Participant characteristics.
| Characteristic for N = 30 | |
|---|---|
| Sex (female/male) | 15/15 |
| Age (years) | 27 (5) |
| Handedness (EHI score) | Right-handed, 93 (11) |
| Weight (kg) | 68 (12) |
| Height (cm) | 171 (9) |
| Body mass index (kg/m2) | 23 (3) |
| Peak power output (W) Power:weight ratio (W/kg) |
173 (44) 3 (1) |
| IPAQ score (total METs) | 4871 (5222) |
| IPAQ categories for levels of physical activity (n/N) | |
| High | 8/30 |
| Moderate | 8/30 |
| Low | 14/30 |
All data are reported as mean (SD) unless otherwise noted. Abbreviations: BMI, body mass index; cm, centimeters; EHI, Edinburgh Handedness Inventory; IPAQ, International Physical Activity Questionnaire; kg, kilograms; m2, meters square; METs, metabolic equivalents; N, total number of participants; n, number of participants in the indicated subgroup; W, watts.
Informed consent was obtained before the administration of any experimental protocol. Participants were screened for any potential contraindications to TMS using standard screening forms. Participants reported no neurological disorders and were otherwise healthy. The Ethics Committee of the Centre de recherche de l'Institut Universitaire de Gériatrie de Montréal (CRIUGM) approved all experimental procedures under the approval number “CER VN 22-23-19.”
Experimental design
This study used a within-subject design. Each participant completed a preliminary visit and 4 experimental sessions with a minimum of 2 d between each session (Fig. 1). The experimental sessions aimed to assess (i) the impact of AEX intensity on M1 excitability and (ii) the distinct effects of AEX intensity on M1 excitability measured by PA and AP TMS currents. Following 5 min at rest to measure resting heart rate (HR), an incremental exercise test to exhaustion was performed during the preliminary visit to obtain peak power output and peak HR. The resting HR and peak HR were used to prescribe the exercise intensity during the subsequent sessions based on HRR (see Heart rate measurement section for more details), and peak power output was used for further categorization of exercise intensity for the AEX sessions. During the 4 experimental sessions, the following four conditions were performed: 20 min of (i) seated rest, (ii) light-intensity interval training (LIIT), (iii) moderate-intensity interval training (MIIT), or (iv) high-intensity interval training (HIIT) AEX. These conditions were performed in a pseudorandomized order, with each session lasting 2 to 2.5 h. Neurophysiological measurements were conducted before (Pre), immediately after (Post0), and 20 min after (Post20) AEX or rest. Postexercise assessments were timed to capture both immediate and delayed effects on motor cortical excitability, as prior studies have shown that TMS-based measures can be modulated either immediately (Lulic et al. 2017; Neva et al. 2017; El-Sayes et al. 2019a; Yamazaki et al. 2020; Neva et al. 2021) or a delay (Singh et al. 2014; Smith et al. 2014; Mooney et al. 2016; Brown et al. 2020; Yamazaki et al. 2020; Neva et al. 2021) following acute exercise. The order of neurophysiological testing across sessions was pseudorandomized to ensure that participants began with different conditions on their first visit and completed the remaining sessions in a randomized order. The session order for each participant is provided in Supplementary Table 1. To accommodate diurnal fluctuations in corticospinal excitability (Merrell et al. 2024), sessions were scheduled at the same time of day (±3 h) for each participant (Tamm et al. 2009).
Fig. 1.
Study design. An incremental exercise test to exhaustion was conducted in visit 1 (preliminary visit) to establish the exercise intensities for the four subsequent visits (experimental sessions) following ACSM recommendations based on the heart rate reserve. The four experimental sessions involved 20 min of (1) seated rest, (2) light-intensity interval cycling (LIIT), (3) moderate-intensity interval cycling (MIIT), or (4) high-intensity interval cycling (HIIT). The order of each session was randomized. Neurophysiological measurements using transcranial magnetic stimulation (TMS) were taken pre (Pre), immediately post (Post0), and 20 min post (Post20) AEX or rest. Abbreviations: AEX, acute aerobic exercise (4 blocs of 3 min cycling followed by 2 min active recovery at 25% heart rate reserve); HIIT, high interval training cycling acute exercise (80% heart rate reserve); LIIT, light interval training cycling acute exercise (35% heart rate reserve); MIIT, moderate interval training cycling acute exercise (55% heart rate reserve); Post0, immediately after exercise/rest; Post20, 20 min after exercise/rest; pre, B\before exercise/rest.
International Physical Activity Questionnaire
Daily levels of physical activity over the 7 d preceding the visit were assessed using the International Physical Activity Questionnaire (IPAQ; Booth 2000). The IPAQ assesses physical activity across various domains including leisure time, household chores, and gardening, as well as work- and transportation-related activities. Frequency and duration of engagement in each of these activities are collected. These activities were classified according to the following categories: (i) walking, (ii) moderate-intensity activities, and (iii) high-intensity activities. Physical activity levels among participants were examined to characterize the sample of participants.
Incremental test
During the first visit, participants performed an incremental exercise test on an upright cycle ergometer (Cyclus2, CY00100) to establish the AEX intensity for subsequent sessions. The participant started pedaling at a power output equivalent to their body mass (eg 70 W for 70 kg) (Myers et al. 2009, ACSM 2018). Every 2 min, the power output increased by 15, 20, 25, or 30 W, depending on the participant’s body mass. Participants were instructed to maintain a pedaling cadence between 60 and 80 rotations per minute (rpm). They were asked to maintain the same pedaling cadence until exhaustion, defined as being unable to maintain a cadence higher than 60 rpm for 10 s despite verbal encouragement. During the last 20 s of each stage, the participant reported their perception of effort intensity using the CR100 scale (Borg and Kaijser 2006), muscle (thigh) pain using a numerical rating scale ranging from 0 (no pain) to 10 (maximum pain) (Safikhani et al. 2018), and affective response using the Feeling scale (Hardy and Rejeski 1989).
Heart rate measurement
Prior to and during the incremental test, the HR was recorded using a chest belt (Cyclus2) during the exercise and at rest (HRrest). We used the percentage of HRR (%HRR) method to prescribe the cycling exercise intensities. HRR refers to the difference between the peak HR (HRpeak) recorded during an incremental test and the HRrest measured during 5 min of seated rest before the incremental test. To prescribe exercise intensity, we used the Karvonen formula (Karvonen and Vuorimaa 1988; Garnier et al. 2018):
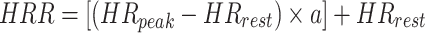 |
The intensities were prescribed via the constant a with the following values corresponding to the exercise intensities as described in the next section: 0.25, 0.35, 0.55, and 0.80.
Acute exercise session
Following a 5-min warm-up at 25% HRR on the same cycle ergometer used in the incremental exercise test, participants engaged in 20 min of interval cycling, involving four 5-min blocks. Each block involved 3 min of pedaling at the target intensity of (i) light (35% HRR, low-intensity interval training [LIIT] condition), (ii) moderate (55% HRR, moderate-intensity interval training [MIIT] condition), or (iii) high (80% HRR, high-intensity training [HIIT] condition), followed by 2 min of active rest (25% HRR). These %HRRs were selected according to the American College of Sports Medicine (ACSM) guidelines (ACSM 2018). Participants were instructed to maintain a cycling cadence of 60 to 80 rpm throughout the acute cycling exercise sessions. Perception of effort using the CR100 scale (Borg and Kaijser 2006), muscle (thigh) pain perception using a numerical rating scale ranging from 0 to 10 (Safikhani et al. 2018), and affective response using the Feeling scale (Hardy and Rejeski 1989) was recorded throughout the exercise and rest periods. Specifically, perception of effort, muscle pain, and affective responses were reported by the participants at two time points during each block: (i) at 0 to 20 s and (ii) at 2 min 30 s, from the beginning of each block. HR was continuously monitored using an HR belt (Polar T31C HR sensor). At the beginning and end of each cycling exercise interval and the warm-up, the experimenters recorded HR. Throughout the cycling exercise, participants were instructed to maintain their hands in a relaxed position, resting them on top of the handlebars and to avoid gripping them. This instruction aimed to minimize contraction of the nonexercised intrinsic hand muscles. Continuous electromyography (EMG) was recorded from both the right and left first dorsal intraosseous (FDI) muscles to ensure participants were resting the non-exercised hand muscles.
Rest
The rest condition lasted for 25 min to align with the duration of the AEX. Participants sat comfortably in a chair while watching an emotionally neutral documentary. They were instructed not to engage in any tasks involving their upper limbs, such as using a mobile device. HR, quadriceps pain perception using a numerical rating scale ranging from 0 to 10, and affective response using the Feeling scale (Hardy and Rejeski 1989) were recorded at the same time points as the AEX conditions.
Electromyographic recording
For all TMS measurements, EMG was recorded from the right FDI as the muscle of interest and from the right abductor pollicis brevis muscle to further monitor muscle activity of the hand. Electrodes measuring 1 cm in diameter were placed over the FDI in a belly–tendon configuration. The ground electrode was placed on the ulnar styloid (Covidien, Mansfield, MA, USA). LabChart software (LabChart 8.0) was used to record EMG data. EMG signals were sampled using a PowerLab (PL3516 PowerLab, 16/35 16 Channel Recorder, AD Instruments, Colorado Springs, CO, USA) data acquisition system and a bioamplifier (Dual Bio Amp, AD Instruments, Colorado Springs, CO, USA) with an acquisition rate of 2 KHz and bandpass (20 to 400 Hz) and notch filtered (center frequency of 50 Hz). Data were captured in a 500-ms sweep from 100 ms before to 400 ms after TMS delivery.
Transcranial magnetic stimulation
While undergoing transcranial magnetic stimulation (TMS) measurements, participants were comfortably seated on an adjustable chair and remained at rest. A Magstim BiStim 2002 stimulator (Magstim Co., UK) connected to a figure-of-eight coil (Magstim 70 mm P/N 9790, Magstim Co., UK) was used to deliver monophasic TMS pulses. TMS current was manipulated to generate current flow either in a PA or AP direction. A standard coil for PA TMS measures delivered a current that was directed in a PA direction. A custom coil was made to produce an AP current, delivering a TMS current in the 180° reverse direction from PA (ie AP). The TMS coils were oriented 45° to the mid-sagittal plane with the handle facing posteriorly. Finally, the standard coil was used to deliver pulses in the lateral–medial (LM) current direction, which was performed by orienting the coil handle 90° to the mid-sagittal plane (Fig. 2). This orientation was used to estimate D-wave activation, which reflects direct activation of corticospinal output neurons typically found in layer 5 of the cortex. Following established procedures, MEP onset latencies were recorded following LM stimulation at 150% of resting motor threshold (RMT), allowing us to indirectly distinguish between D- and I-wave contributions to MEPs (Day et al. 1989; Di Lazzaro et al. 2001; Cirillo et al. 2018). Together with PA and AP currents, this approach ensured that we probed distinct pools of corticospinal neurons. Coil positioning and monitoring were performed using Brainsight neuronavigation software (Rogue Research Inc., Montreal, QC, Canada). By positioning the coil over M1 in the PA orientation, the “hotspot” for the M1 FDI representation was identified. This hotspot was used for the PA, AP, and LM TMS current directions. The RMT was determined at the hotspot for all TMS current directions (PA, AP, and LM). RMT was defined as the lowest stimulus intensity needed to elicit 5 out of 10 consecutive MEPs with a peak-to-peak amplitude of 50 μV or more. TMS pulses were administered at a random rate between 0.15 and 0.2 Hz (with ~20% variation) throughout the study.
Fig. 2.

Transcranial magnetic stimulation (TMS) current directions and motor evoked potential (MEP) onset latency results. (A, left panel) TMS current directions are displayed. LM TMS is represented at the top, PA TMS is represented in the middle, and AP TMS is represented at the bottom. The figure displays TMS coil current directions, represented with arrows, and orientations over the left M1 (dominant) first dorsal intraosseous muscle (FDI) representation. We used a standard 8-figure TMS coil for both LM and PA stimulations, and a reversed current coil for AP stimulations. The coil was oriented 90° for LM TMS current and 45° for both PA and AP TMS currents, relative to the longitudinal fissure. (A, right panel) Electromyographic (EMG) traces are displayed from a representative participant, recorded from the right (dominant) FDI. Vertical dashed lines represent MEP onset latency elicited by each TMS current direction (LM, PA, AP). B) MEP onset latency for each TMS current (LM, PA, AP) using boxplots, with dots representing individual data. C) MEP onset latency differences (LM-PA, LM-AP) using boxplots, with individual data connected with lines. Abbreviations: AP, anterior-to-posterior; LM, lateral-to-medial; ms: milliseconds; PA, posterior-to-anterior; TMS, transcranial magnetic stimulation; ***P < 0.001.
MEP amplitudes were evaluated in both the PA and AP current directions to examine potential changes in corticospinal excitability following AEX. MEPs were assessed at 110% and 130% RMT in both PA and AP current directions. MEPs were assessed at 110% RMT since previous work has shown that lower intensity suprathreshold TMS pulses preferentially activate distinct interneuron circuits targeted with PA and AP currents (Day et al. 1989; Di Lazzaro et al. 2001; Cirillo et al. 2018). Our previous work showed that moderate-intensity AEX increased corticospinal excitability measured with this lower intensity (ie 110% RMT) in the AP but not the PA TMS current direction and not at the higher TMS intensities (ie 130% RMT and above) in either current direction (Neva et al. 2021). MEPs were also assessed at 130% RMT to reproduce our previous findings that AEX enhances corticospinal excitability assessed in the AP current direction only at the lower stimulus intensities (ie 110% RMT) to preferentially activate a distinct group of interneurons (Neva et al. 2021). Here, we aimed to identify distinct intensity-related AEX-induced modulation of these interneuron populations. Ten stimuli were administered at each TMS intensity and current direction, with the order of intensities randomized. The breaks between TMS measurements were about 10 to 15 s and under 1 min when switching between PA and AP TMS coils.
SICI was assessed to investigate the effect of AEX intensity on intracortical inhibition, measured in both PA and AP directions, like our previous work (Neva et al. 2021). A subthreshold conditioning stimulus (CS) was delivered prior to a suprathreshold test stimulus (TS) over the M1 FDI hotspot. The CS was delivered at 80% RMT. The TS delivered at a percentage of maximum stimulator output (%MSO) was individually adjusted at each timepoint and across all conditions to produce an average peak-to-peak MEP amplitude of ~1 mV across 10 stimulations. The interstimulus interval (ISI) between the CS and TS was set at 2 milliseconds (ms) for the PA TMS current direction. The decision to use a 2-ms ISI for PA while measuring SICI was based on previous research indicating a significant reduction in inhibition after acute exercise (Smith et al. 2014; Lulic et al. 2017; Stavrinos and Coxon 2017; Opie and Semmler 2019; Yamazaki et al. 2019; Neva et al. 2021; Hendy et al. 2022). Additionally, other studies showed that employing a 3-ms ISI during PA SICI assessment could potentially be affected by facilitation mechanisms (Peurala et al. 2008). Since previous work demonstrated enhanced intracortical facilitatory mechanisms following AEX (Smith et al. 2014; Neva et al. 2017; Morris et al. 2020), this was a particularly important confound to avoid. Conversely, we used a 3-ms ISI to measure SICI in the AP current direction based on previous work that showed consistent and pronounced inhibition compared to a 2-ms ISI (Sale et al. 2016; Cirillo et al. 2018; Neva et al. 2021). Moreover, considering the likely longer cortical transmission pathways with AP TMS (Spampinato 2020), the 3-ms ISI was deemed appropriate for assessing SICI in this current direction (Neva et al. 2021). To evaluate SICI at each timepoint (Pre, Post0, and Post20), 10 paired pulses (CS followed by TS) and 10 TS were administered in a randomized order for both PA and AP TMS current directions.
MEP onset latencies were identified as the earliest latency among the block of 10 MEPs to indirectly evaluate I-wave recruitment and infer preferential activation of distinct interneuron populations. This procedure was conducted for both PA and AP TMS current directions (assessed at 110% RMT). Finally, to estimate D-wave activation, MEP onset latencies were identified in the same way as described above, except that it was assessed using an LM TMS current at 150% RMT (Day et al. 1989, Di Lazzaro et al. 2001, Cirillo et al. 2018).
Critically, previous work has demonstrated the reliability of recording 5 to 10 MEPs when assessing corticospinal excitability and SICI (McGinley et al. 2010; Cavaleri et al. 2017; Ammann et al. 2020). Indeed, these studies have shown excellent within-session reliability (eg ICC = 0.92; Cavaleri et al. (2017)). Given the comprehensive scope of the current study, including multiple TMS measures, currents, and time points across four experimental sessions, we prioritized a design that minimized participant burden and postexercise fatigue while maintaining reliable data quality.
Data processing and statistical analysis
Repeated-measures analysis of variance (RM-ANOVA) was used to analyze the AEX/rest-related data (HR, perception of effort, muscle pain, and affect) and the neurophysiological data as measured using TMS (MEP amplitude, SICI, and MEP onset latency). The specific analyses will be described below in more detail. Post hoc analyses were conducted using Holm–Bonferroni correction where appropriate. Residual statistics, skewness, and kurtosis values, along with plots, were generated to assess the normality and homoscedasticity of the data. Statistical procedures were performed using Jamovi software (version 2.2.5), with significance set at P < 0.05. Effect sizes were calculated and reported as partial eta squared (η2p) following established guidelines for interpretation (Cohen 2013), where 0.01 indicates a small effect, 0.06 a moderate effect, and 0.14 a large effect. All data are reported as mean (SD).
Aerobic exercise/rest data
We assessed HR, perception of effort, muscle pain, and affective response data during the AEX and rest sessions to confirm that the AEX intensities elicited different physiological/psychological responses corresponding to each AEX condition (HIIT, MIIT, LIIT) or rest. Thus, one-way RM-ANOVAs using the within-subjects factor of exercise CONDITION (HIIT, MIIT, LIIT, REST) were conducted separately on average values for HR, perception of effort, muscle pain, and affective response.
Neurophysiological data
RMT, TS %MSO, and TS MEP amplitudes
Consistent RMT (%MSO), determined at the beginning of each session (pre-AEX/rest), was ensured by conducting a two-way RM-ANOVA using within-subjects factors CONDITION (HIIT, MIIT, LIIT, REST) and TMS CURRENT (PA, AP). Stable TS %MSO values during SICI assessment pre- and post-AEX/rest were ensured using a three-way RM-ANOVA, considering within-subject factors TIME (Pre, Post0, Post20), CONDITION (HIIT, MIIT, LIIT, REST), and TMS CURRENT (PA, AP). This ensured stable TMS intensity used during assessment of SICI pre- and post-AEX/rest, across the different AEX conditions and TMS currents. Stable TS MEP amplitudes pre- and post-AEX/rest were ensured using a three-way RM-ANOVA, considering within-subject factors TIME (Pre, Post0, Post20), CONDITION (HIIT, MIIT, LIIT, REST), and TMS CURRENT (PA, AP). This confirmed consistent corticospinal output excitability throughout the assessment of SICI pre- and post-AEX/rest, across the different AEX conditions and TMS currents.
MEP onset latency
We used a semi-automated system to determine MEP onset latency, defined as the point in time where the rectified EMG signal surpassed 5 times the mean prestimulus EMG. We calculated MEP latencies induced by single-pulse TMS in each current direction (PA, AP, LM). Additionally, we calculated MEP latency differences (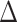 PA-LM,
PA-LM, 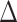 AP-LM) as further indirect indicators of I-wave recruitment, thereby confirming specific interneuron activation as elicited by PA and AP TMS (Di Lazzaro et al. 2001; Cirillo et al. 2018; Spampinato 2020). We used the earliest MEP latency response in a two-way RM-ANOVA, incorporating within-subjects factors TMS CURRENT (PA, AP, LM) and CONDITION (HIIT, MIIT, LIIT, REST). Similarly, MEP onset latency differences between PA-LM and AP-LM were compared using a two-way RM-ANOVA, incorporating the within-subjects factor of MEP ONSET DIFFERENCE (∆PA-LM, ∆AP-LM) and CONDITION (HIIT, MIIT, LIIT, REST).
AP-LM) as further indirect indicators of I-wave recruitment, thereby confirming specific interneuron activation as elicited by PA and AP TMS (Di Lazzaro et al. 2001; Cirillo et al. 2018; Spampinato 2020). We used the earliest MEP latency response in a two-way RM-ANOVA, incorporating within-subjects factors TMS CURRENT (PA, AP, LM) and CONDITION (HIIT, MIIT, LIIT, REST). Similarly, MEP onset latency differences between PA-LM and AP-LM were compared using a two-way RM-ANOVA, incorporating the within-subjects factor of MEP ONSET DIFFERENCE (∆PA-LM, ∆AP-LM) and CONDITION (HIIT, MIIT, LIIT, REST).
EMG and MEP data processing for corticospinal excitability and SICI
For both MEPs and SICI, we visually inspected the EMG data for voluntary muscle activity. Peak-to-peak MEP amplitudes (measured in millivolts, mV) were analyzed using custom MATLAB scripts. Any trials showing visible voluntary prestimulus EMG activity were excluded from the analysis (constituting 0.73% of trials). Pre-trigger EMG root-mean-square (RMS) values were calculated over an 80-ms window, from 90 to 10 ms before the TMS pulse. This time window was chosen to capture background muscle activity immediately preceding stimulation, without including the TMS artifact. SICI was quantified as a ratio of CS + TS MEP amplitude over TS MEP amplitude:
SICI ratio =  , where smaller SICI ratio values indicate greater inhibition, while larger values indicate less inhibition (disinhibition).
, where smaller SICI ratio values indicate greater inhibition, while larger values indicate less inhibition (disinhibition).
Corticospinal excitability
To assess the impact of AEX intensity on corticospinal excitability, the average of 10 MEP amplitudes was computed for each of the two TMS intensities (110%, 130% RMT). Each stimulus intensity was analyzed separately, as the utilization of lower intensities (eg 110% RMT) enhances the likelihood of preferentially activating distinct interneuron circuits with both PA and AP TMS current directions (Mirdamadi et al. 2017; Cirillo et al. 2018). Thus, we performed three-way RM-ANOVAs using within-subjects factors TIME (Pre, Post0, Post20), CONDITION (HIIT, MIIT, LIIT, REST), and TMS CURRENT (PA, AP) for each TMS intensity (110, 130% RMT).
Short-interval intracortical inhibition
To assess the impact of AEX intensity on SICI, the average MEP amplitude was computed for each of the ten TS and CS + TS pulses, as assessed with each TMS current (PA, AP), to determine the SICI ratio. Thus, a three-way RM-ANOVA was conducted, incorporating within-subjects factors TIME (Pre, Post0, Post20), CONDITION (HIIT, MIIT, LIIT, REST), and TMS CURRENT (PA, AP) using the SICI ratio as the dependent measure.
Results
Physical activity data (IPAQ)
Participants demonstrated a large range of physical activity levels as assessed by the long-form IPAQ (Craig et al. 2003). There was a mean (SD) metabolic equivalents-min/week of 4871 (5222) for all participants, with 26.7% (8 participants) categorized as demonstrating high, 26.7% (8 participants) as moderate, and 46.6% (14 participants) as low (see Table 1) levels of physical activity.
Aerobic exercise/rest data
Incremental exercise test
The average incremental test duration was 11.72 (4) min. The average peak power output was 173 (44) W. Average peak HR was 180 (13) bpm. The average resting HR was 67 (10) bpm. The power output used in each exercise condition are illustrated in Fig. 3A as a percentage of peak power output.
Fig. 3.

Exercise-related data. A) Displays the power output normalized to the percent of the peak value reached during the incremental exercise test. B) Displays the average of raw heart rate data in bpm over 20 min of exercise or rest. C) Displays the heart rate data normalized to the percent of the peak value reached during the incremental exercise test. D) Displays the perception of effort data on CR-100 scale (0 to 100). E) Displays the reported pain data on a 0 to 10 scale. F) Displays the affective response to exercise assessed using the feeling scale. Large dark gray circles represent the average for each condition (REST, LIIT, MIIT, and HIIT), and the colored dots represent the individual data. Abbreviations: % peak value, percent of the peak value; bpm, beat per minute; HIIT, high-intensity interval training cycling acute exercise; LIIT, light-intensity interval training cycling acute exercise; MIIT, moderate-intensity interval training cycling acute exercise. **P < 0.01; ***P < 0.001.
Acute aerobic exercise/rest sessions
All participants successfully completed the LIIT, MIIT, and HIIT sessions (see Supplementary Fig. 1). Based on the incremental test, the light-intensity exercise during LIIT was set at 64 (16) W, the moderate-intensity exercise during MIIT was set at 99 (25) W, and the high-intensity exercise during HIIT was set at 143 (40) W. The active rest intensity set for all exercise conditions (LIIT, MIIT, and HIIT) was set at 46 (14) W. Supplementary Table 2 displays the HR, perception of effort, and power output outcomes during each exercise/rest condition at different key timepoints. Supplementary Tables 3 and 4 provide the details of the statistical results for the exercise-related data.
Raw heart rate data
HR values (see Fig. 3B) showed a significant effect of CONDITION (F3,87 = 268.07, P < 0.001, η2p = 0.902), with post hoc analyses demonstrating significant differences between all conditions (all Ps < 0.001). Specifically, when averaged over the 20-min AEX/Rest period, HIIT showed the highest HR (151 [15]), followed by MIIT (131 [7]), LIIT (122 [4]), and REST (73 [1]).
Heart rate data as a percentage of peak value
For HR (% peak value; see Fig. 3C), a main effect of CONDITION (F3,87 = 361.5, P < 0.001, η2p = 0.93) was found. Post hoc analysis indicated that all conditions were different from each other (all Ps < 0.001). Specifically, HIIT showed the highest peak % HR (85 [8]), followed by MIIT (74 [9]), LIIT (68 [10]), and REST (41 [6]). Further, the peak HR during these sessions was 172 (14) bpm for HIIT, 143 (16) bpm for MIIT, 129 (16) bpm for LIIT, and 77 (9) bpm for REST.
Perception of effort data
For the perception of effort (Fig. 3D), a main effect of CONDITION (F3,58 = 51.56, P < 0.001, η2p = 0.643) was found. Post hoc analysis indicated that all conditions were different from each other (all Ps < 0.001). Specifically, HIIT showed the highest perception of effort (51 [14]), followed by MIIT (34 [6]) and LIIT (20 [3]).
Muscle pain data
For reported pain (Fig. 3E), a main effect of CONDITION (F3,87 = 59.45, P < 0.001, η2p = 0.67) was found. Post hoc analysis indicated that all conditions were different from each other (all Ps < 0.002). Specifically, HIIT showed the highest reported pain (4 [1]), followed by MIIT (3 [1]), LIIT (2 [0.4]), and REST (0 [0.02]).
Affective response data
For affective responses (Fig. 3F), a main effect of CONDITION (F3,87 = 31.54, P < 0.001, η2p = 0.52) was found. Post hoc analysis indicated that all conditions were different from each other (all Ps < 0.001). Specifically, HIIT showed the lowest score on the Feeling Scale (1 [1]), followed by MIIT (2 [0.4]), LIIT (3 [0.2]), and REST (4 [0.1]).
Neurophysiological data
Baseline neurophysiological data
For RMT values, a main effect of CURRENT (F2,58 = 81.602, P < 0.001, η2p = 0.74) was found (see Supplementary Table 5). Post hoc analysis revealed that RMT for PA TMS current (45 [6] %MSO) was significantly lower than both AP (57 [9] %MSO; t29 = −5.743, P < 0.001) and LM (51 [60] %MSO; t29 = −7.173, P < 0.001) TMS currents. Further, RMT values for LM current were significantly lower than AP TMS current (t29 = −5.743, P < 0.001). There was no CONDITION main effect (F3,87 = 0.665, P = 0.576, η2p = 0.02) or CURRENT × CONDITION interaction (F6,174 = 0.408, P = 0.873, η2p = 0.01).
TS %MSO values used during SICI were stable across time and exercise conditions but were different between TMS currents (see Supplementary Table 6). Specifically, there was a main effect of CURRENT (F1,294 = 402.61, P < 0.001, η2p = 0.93), no significant effect of TIME (F2,58 = 0.510, P = 0.650, η2p = 0.02), CONDITION (F3,87 = 0.3, P = 0.822, η2p = 0.01), TIME × CONDITION interaction (F6,174 = 1.35, P = 0.239, η2p = 0.04), or TIME × CONDITION × CURRENT interaction (F6, 174 = 1.07, P = 0.379, η2p = 0.04).
TS MEP amplitudes during SICI were stable across time and exercise conditions but were different between TMS currents (see Supplementary Table 7). Specifically, there was a main effect of CURRENT (F1,294 = 402.61, P = 0.001, η2p = 0.93), no significant effect of TIME (F2,58 = 0.23, P = 0.797, η2p = 0.01), CONDITION (F3,87 = 2.33, P = 0.079, η2p = 0.07), nor was there CONDITION × CURRENT interaction (F3,87 = 0.68, P = 0.567, η2p = 0.02) or TIME × CONDITION × CURRENT interaction (F6,174 = 1.35, P = 0.239, η2p = 0.04).
There was no significant difference in EMG RMS prestimulation values across conditions, time points, or their interaction (all Ps > 0.087 and all η2p < 0.07; see Supplementary Table 8).
For MEP onset latency (see Fig. 2), a significant main effect of CURRENT (F2,58 = 243.81 P < 0.001, η2p = 0.91) was found. Post hoc analysis showed that MEP onset latency was shorter using LM current (20 [1] ms) compared to PA (21 [1] ms; t29 = −11.06, P < 0.001) and AP TMS current (23 [1] ms; t29 = −24.29, P < 0.001). Further, MEP onset latency for using PA TMS current was shorter than AP TMS current (t29 = −14.47, P < 0.001). There was no significant effect of CONDITION or CURRENT × CONDITION interaction (all Ps > 0.147).
For MEP onset latency difference (∆PA-LM, ∆AP-LM; see Fig. 2), statistical analysis revealed a significant effect of MEP ONSET DIFFERENCE (F1,29 = 257.51, P < 0.001, η2p = 0.90). Post hoc analysis showed that ∆PA-LM (2 [1] ms) was greater than ∆AP-LM (3 [1] ms; t29 = −19.15, P < 0.001). There was no significant effect of CONDITION or MEP ONSET DIFFERENCE × CONDITION interaction (all Ps > 0.142).
Corticospinal excitability
MEP data are displayed for each TMS intensity (Fig. 4A, C, and D: 110% RMT; Fig. 4B, E, and F: 130% RMT), and TMS currents collapsed (Fig. 4A and B) and separated into PA (Fig. 4C and E: “left panel” average data, “right panel” individual data plots with means overlayed) and AP (Fig. 4D and F: “left panel” average data, “right panel” individual data plots with means overlayed). For MEPs at 110% RMT (Fig. 4A), a TIME × CONDITION interaction was found (F1,29 = 22.16, P < 0.001, η2p = 0.2). Post hoc analysis indicated greater MEP amplitude at Post0 compared to Pre for HIIT (t29 = −6.42, P < 0.001) and MIIT (t29 = −4.41, P = 0.008). At Post0, MEP amplitude was greater for HIIT (t29 = 4.88, P < 0.001) and MIIT (t29 = 3.42, P = 0.047) compared to rest. Also, there was greater MEP amplitude at Post20 compared to Pre for HIIT (t29 = −5.5, P < 0.001). Finally, at T2, MEP amplitude was greater for HIIT compared to rest (t29 = 4.06, P = 0.009). Additionally, we found main effects of TIME (F2,58 = 27.64, P < 0.001 η2p = 0.49), CONDITION (F3,87 = 7.53, P < 0.001, η2p = 0.21), CURRENT (F3,87 = 7.53, P < 0.001, η2p = 0.43), with no other effects or interactions (all Ps < 0.106).
Fig. 4.
Corticospinal excitability results. A, B) Display average peak-to-peak MEP amplitudes elicited with TMS currents collapsed for 110% RMT A) and 130% RMT B) at each timepoint (Pre, before exercise/rest; Post0, immediately after exercise/rest; Post20, 20 min after exercise/rest). Vertical gray lines show significant differences between rest and the condition represented by its color, with green for LIIT, orange for MIIT, and red for HIIT average peak-to-peak MEP amplitudes elicited with PA current for 110% RMT (C, left panel), AP current for 110% RMT (D, left panel), PA current for 130% RMT (E, left panel), and AP current for 130% RMT (F, left panel) are displayed at each timepoint. (Right panels of C–F) Box plots display individual data at each timepoint for MEP amplitudes at 110% RMT elicited with PA and AP currents, and for 130% RMT with PA and AP currents, in which the box depicts the median, 25th, and 75th percentiles, and the individual data are overlayed. HIIT is displayed in red triangles, MIIT is displayed in orange triangles, LIIT is displayed in green triangles, and the rest is displayed in gray rings. Bars represent the standard deviation of the mean. Abbreviations: AP, anterior-to-posterior; HIIT, high-intensity interval training cycling acute exercise; LIIT, light-intensity interval training cycling acute exercise; MEP, motor evoked potential; MIIT, moderate-intensity interval training cycling acute exercise; mV, millivolt; RMT, resting motor threshold; PA, posterior-to-anterior; Post0, immediately after exercise/rest; Post20, 20 min after exercise/rest; Pre, before exercise/rest; TMS, transcranial magnetic stimulation. Within-subjects: *P < 0.05; **P < 0.01; ***P < 0.001. Between-subjects: $ P < 0.05; $$ P < 0.01; $$$ P < 0.001.
For MEPs at 130% RMT (Fig. 4B), a TIME × CONDITION interaction was found (F1,29 = 8.43, P < 0.001, η2p = 0.23). Post hoc analysis indicated greater MEP amplitude at Post0 compared to Pre for HIIT (t29 = −5.97, P = 0.001) and MIIT (t29 = −5.32, P = 0.007). At Post0, MEP amplitude was greater for HIIT (t29 = 6.05, P = 0.001) and MIIT (t29 = 4.84, P = 0.001) compared to rest. Also, there was greater MEP amplitude at Post20 compared to Pre for HIIT (t29 = −5.82, P = 0.002). Finally, at Post20, MEP amplitude was greater for HIIT compared to rest (t29 = 5.98, P = 0.001). Additionally, we found main effects of TIME (F2,58 = 25.27, P < 0.001 η2p = 0.47), CONDITION (F3,87 = 17.98, P < 0.001, η2p = 0.38), CURRENT (F3,87 = 8.4, P = 0.007, η2p = 0.22), with no other effects or interactions (all Ps < 0.577).
Short-interval intracortical inhibition
SICI data are displayed for TMS currents collapsed (Fig. 5A) and separated into PA (Fig. 4B: “left panel” average data, “right panel” individual data plots with means overlayed) and AP (Fig. 4D: “left panel” average data, “right panel” individual data plots with means overlayed). For SICI (Fig. 5A), a TIME × CONDITION interaction was found (F6,174 = 7.28, P < 0.001, η2p = 0.2). Post hoc analysis indicated less SICI at Post0 compared to Pre for HIIT (t29 = −7.38, P < 0.001), MIIT (t29 = −6.06, P < 0.001) and LIIT (t29 = −4.88, P < 0.001). Also, there was less SICI at Post20 compared to Pre for HIIT (t29 = −5.38, P < 0.001), MIIT (t29 = −5.77, P < 0.001) and LIIT (t29 = −3.68, P = 0.022). At Post0, there was less SICI for HIIT (t29 = 4.58, P = 0.002) and MIIT (t29 = 3.67, P = 0.023) compared to rest. Finally, at Post20, there was less SICI for MIIT compared to rest (t29 = 3.6, P = 0.026).
Fig. 5.
Short-interval intracortical inhibition results. A) Displays average SICI ratios, where greater values represent less GABAergic inhibition, at each timepoint (pre, before exercise/rest; Post0, immediately after exercise/rest; Post20, 20 min after exercise/rest). Vertical gray lines show significant differences between rest and the condition represented by its color, with green for LIIT, orange for MIIT, and red for HIIT. (Left panels of B, D) average SICI ratios at each timepoint elicited with PA (B, left panel) and AP (D, left panel) currents. (Right panels of B, D) Box plots for SICI ratios display individual data at each timepoint elicited with PA (B, right panel) and AP (D, right panel) currents, in which the box depicts the median, 25th, and 75th percentiles, and the individual data are overlayed. HIIT is displayed in red triangles, MIIT is displayed in orange triangles, LIIT is displayed in green triangles, and the rest is displayed in gray rings. Bars represent the standard deviation of the mean. Abbreviations: AP, anterior-to-posterior; HIIT, high-intensity interval training cycling acute exercise; LIIT, light-intensity interval training cycling acute exercise; MIIT, moderate-intensity interval training cycling acute exercise; PA, posterior-to-anterior; Post0, immediately after exercise/rest; Post20, 20 min after exercise/rest; Pre, before exercise/rest; SICI, short intracortical inhibition; TMS, transcranial magnetic stimulation. Within-subjects: *P < 0.05; **P < 0.01; ***P < 0.001. Between-subjects: $ P < 0.05; $$ P < 0.01; $$$ P < 0.001.
We also found a CONDITION × CURRENT interaction (F3,87 = 2.83, P = 0.043, η2p = 0.09; Fig. 5C). Post hoc analyses demonstrated that MIIT showed less SICI compared to rest with AP current (t29 = 4.07, P = 0.005), but not PA TMS current (t29 = 0.8, P = 0.429). We conducted follow-up pairwise comparisons to understand what was driving this particular effect (Fig. 5B; see Supplementary Table 9). We found that MIIT showed less SICI than rest at Post0 and Post20 using AP TMS current (all Ps < 0.0168), but not with PA current (all Ps > 0.490), and there were no differences at Pre for either TMS current (all Ps > 0.057; Holm-Bonferroni correction applied for 16 comparisons). Finally, we found a main effect of TIME (F2,58 = 48.71, P = 0.001, η2p = 0.63), CONDITION (F3,87 = 5.73, P < 0.001, η2p = 0.16), and CURRENT (F1,29 = 106.42, P < 0.001, η2p = 0.79).
Discussion
We investigated the impact of AEX intensity on distinct motor cortical circuits. There are two main findings of the current study: (i) HIIT and MIIT increased corticospinal excitability, with HIIT eliciting a sustained increase in the excitable output of M1, and (ii) LIIT, MIIT, and HIIT demonstrated a reduction in GABAergic inhibition as measured by SICI, with a sustained reduction after MIIT. A secondary finding was that GABAergic inhibition, as measured by SICI, showed a greater reduction when assessed with AP than PA TMS current following MIIT compared to rest. Collectively, the current results demonstrate the capacity of an acute session of high- and moderate-intensity interval exercise to enhance corticospinal excitability and reduce GABAergic inhibition of the motor cortex. These results provide evidence for a dose–response effect of exercise intensity on the modulation of distinct motor cortical circuits.
We collected physiological and perceptual responses during the AEX. HR, perception of effort, and muscle pain were higher during the three exercise conditions compared to rest, and affective responses were lower during the three exercise conditions compared to rest. Second, there were clear, distinct physiological and perceptual responses between the three exercise conditions. We observed an increased HR, perception of effort, and muscle pain with increased exercise intensity. Additionally, affective responses decreased with increasing exercise intensity. These differences between conditions across our variables attest that we were successful in our experimental manipulation of three distinct exercise intensities.
Increased corticospinal excitability following acute exercise
We found that HIIT increased corticospinal output excitability to the greatest extent and duration relative to the other exercise intensities (MIIT and LIIT) and rest. Both MIIT and HIIT increased corticospinal excitability immediately after exercise, but only HIIT maintained this increase for 20 min postexercise. Further, HIIT alone showed increased corticospinal excitability when compared to rest 20 min postexercise. Importantly, these results were found regardless of the measured TMS intensity (110%, 130% RMT) and current direction (PA, AP). These results suggest that intensity plays an important role in the ability of acute exercise to modulate the excitability of motor cortex output. In line with the current results, our recent systematic review and meta-analysis demonstrated that only high-intensity exercise increases corticospinal excitability, whereas moderate- and light-intensity exercise does not (Youssef et al. 2024). However, a limitation of this meta-analysis was that each study performed different acute exercise types, durations, and intensities (eg % maximum power output, max HR). Relatedly, no study systematically examined the impact of acute exercise on M1 excitability across a spectrum of exercise intensities (eg light, moderate, high) while also simultaneously controlling for exercise type/structure (eg continuous vs. interval) and duration. Importantly, we addressed these limitations by examining the effect of acute exercise across three different intensities (ie light, moderate, high; based on ACSM guidelines) along with a rest condition, while controlling for exercise type (ie interval training exercise) and duration (ie 20 min). Thus, the present study represents an experimental confirmation of several results in our meta-analysis (Youssef et al. 2024).
In fact, the current results further extend our previous findings (Youssef et al. 2024) by demonstrating that HIIT increased corticospinal excitability for up to 20 min following exercise compared to the other exercise conditions (ie MIIT and LIIT). Evidence has been accumulating to demonstrate that HIIT can increase corticospinal excitability of the non-exercised upper limb (Ostadan et al. 2016; Nicolini et al. 2020; Hendy et al. 2022). The current findings suggest that acute exercise intensity, along with other exercise parameters such as type and duration, are important factors influencing the impact of acute exercise on M1 excitable output. Previous work showed increased corticospinal excitability following HIIT when the intensity was high compared to similar studies (eg 105% to 120% VO2peak) and exercise duration was relatively short (eg 15 min) (Ostadan et al. 2016, Nicolini et al. 2020, Hendy et al. 2022). In contrast, other studies with slightly lower intensities than the previous examples (Ostadan et al. 2016, Nicolini et al. 2020, Hendy et al. 2022) did not observe an effect of exercise on corticospinal excitability (Mang et al. 2014; Stavrinos and Coxon 2017; Andrews et al. 2020; El-Sayes et al. 2020). Overall, this suggests that exercise parameters like exercise intensity, type, and duration play a key role in exercise-enhanced corticospinal excitability.
Contrary to our hypothesis, MIIT increased corticospinal excitability immediately postexercise. While our meta-analysis showed that moderate-intensity exercise did not change corticospinal excitability (Youssef et al. 2024), there are individual studies that suggested moderate-intensity exercise can increase corticospinal excitability (MacDonald et al. 2019; El-Sayes et al. 2019b). However, it is important to highlight that the majority of studies using moderate-intensity exercise did not find an effect on corticospinal excitability (Singh et al. 2014; Smith et al. 2014; Neva et al. 2017; Smith et al. 2018; Andrews et al. 2020; Brown et al. 2020; El-Sayes et al. 2020; Morris et al. 2020; Neva et al. 2021; Kuo et al. 2023). It may be that our current findings relate to employing MIIT (ie moderate-intensity “interval” training exercise) as opposed to all but one study using moderate-intensity “continuous” exercise (El-Sayes et al. 2020). Notably, the vast majority of studies using moderate continuous cycling exercise did not show an impact of AEX on corticospinal excitability (McDonnell et al. 2013; Singh et al. 2014; Smith et al. 2014; Singh et al. 2016; Neva et al. 2017; Brown et al. 2020; Morris et al. 2020; Kuo et al. 2023). The moderate-intensity exercise parameters used in the current study, such as incorporating active recovery intervals instead of cycling at a constant load, might partially explain the differing findings with previous work using moderate-intensity continuous exercise (McDonnell et al. 2013; Singh et al. 2014; Smith et al. 2014; Singh et al. 2016; Neva et al. 2017; Brown et al. 2020; Morris et al. 2020; Kuo et al. 2023). In fact, the only study that used MIIT showed increased corticospinal excitability following exercise (El-Sayes et al. 2020). Studies comparing continuous versus intermittent cycling at a moderate intensity have shown that MIIT results in higher blood lactate levels compared to continuous exercise (Grossl et al. 2012; Kanthack et al. 2020). It was shown that performing moderate aerobic exercise in intervals (ie alternating lower and higher exercise intensities) leads to a lack of stabilization of physiological changes for the same duration as continuous exercise, resulting in greater blood lactate accumulation (Kanthack et al. 2020). This has been suggested to trigger signaling cascades that increase M1 excitability (Coco et al. 2010; Coco et al. 2014; Coco et al. 2020). Since the current study did not measure these specific physiological changes (eg blood lactate), the mechanisms underlying these exercise-induced changes in corticospinal excitability remain speculative.
Short-interval intracortical inhibition decreased following acute exercise
Our findings indicate a dose–response relationship between exercise intensity and modulation of SICI. All three exercise conditions (HIIT, MIIT, and LIIT) decrease SICI immediately and 20 min following exercise compared to pre-exercise. However, only HIIT and MIIT decreased SICI to a greater extent than rest immediately following exercise, and only MIIT showed a decrease in SICI compared to rest 20 min postexercise. Thus, while each of our exercise intensities were sufficient to modulate SICI, there was a nuanced effect that includes a greater decrease immediately following exercise for HIIT and MIIT than LIIT, with MIIT demonstrating the greatest decrease in SICI.
Exercise-induced decreases in SICI have been observed in many studies (Singh et al. 2014; Smith et al. 2014; Lulic et al. 2017; Stavrinos and Coxon 2017; El-Sayes et al. 2019b; Opie and Semmler 2019; Neva et al. 2021; Hendy et al. 2022; Kuo et al. 2023) and were revealed to be the most consistent and robust effect in our meta-analysis (Youssef et al. 2024). Our moderator analysis showed that moderate- and high-intensity acute exercise contributed to this decrease in SICI, which lasted up to 30 min post-AEX (Youssef et al. 2024). The current results confirm and extend these findings, by showing that MIIT and HIIT reduce SICI immediately following exercise, with MIIT demonstrating a prolonged effect up to 20 min postexercise compared to rest.
Our findings also extend our previous findings by demonstrating that LIIT was also sufficient to elicit a decrease in SICI up to 20 min postexercise. While our meta-analysis demonstrated that light-intensity exercise did not show decreased SICI, it is likely that the low number of studies employing this intensity contributed to the lack of effect (Youssef et al. 2024). Studies demonstrating exercise-induced disinhibition of SICI hypothesized that this effect is associated with modulation of GABAA-receptor-mediated activity. Previous pharmacological studies have demonstrated the role of these receptors in the paradigm of SICI measured using TMS (Hanajima et al. 1998; Di Lazzaro et al. 2012). Therefore, exercise-induced changes in SICI might result from a series of physiological events from AEX that lead to modulation of GABAergic functioning within M1. Intriguingly, recent work has shown increased GABA concentration within areas like the visual cortex (Maddock et al. 2016), sensorimotor cortex (Coxon et al. 2018), and other regions (Ryberg et al. 2023) following aerobic exercise using magnetic resonance spectroscopy. The apparent discrepancy between decreased SICI (reflecting GABAA-receptor related inhibition; Hanajima et al. 1998, Ziemann et al. 1998, Di Lazzaro et al. 2012) and increased GABA concentration (Maddock et al. 2016; Coxon et al. 2018; Ryberg et al. 2023) may be attributed to the fundamental differences in what each technique measures. SICI is understood to reflect GABAA-receptor-related inhibition (Hanajima et al. 1998; Ziemann et al. 1998; Di Lazzaro et al. 2012; Ziemann et al. 2015) where Ziemann et al. (2015) outline the impact of GABA-blocking drugs specifically reducing SICI, without affecting other measures of inhibition, facilitation, or corticospinal excitability (Ziemann et al. 2015). In contrast, magnetic resonance spectroscopy captures overall GABA concentrations, encompassing both synaptic and extrasynaptic GABA within he measured brain tissue (Coxon et al. 2018; Li et al. 2022; Ryberg et al. 2023). Thus, an increase in GABA concentration does not necessarily indicate enhanced inhibitory function and may coexist with reduced synaptic inhibition as captured by TMS measures.
Both blood lactate and dopamine levels may play a role in the exercise-induced reduction of GABAergic activity underlying SICI. Specifically, blood lactate accumulation is suggested to be associated with a reduction in GABAergic neuronal activity (Kann 2024), which might partly explain the reduction in SICI following HIIT and MIIT. Similarly, higher exercise intensities (moderate, high) are shown to induce greater levels of dopamine in the brain (Sacheli et al. 2019), and increased dopamine release can contribute to reduced GABAA receptor activity (Flores-Hernandez et al. 2000). Interestingly, recent research has more directly demonstrated the contribution of dopamine receptor activity in exercise-induced modulation of M1 inhibitory circuitry, such as SICI (Curtin et al. 2023). Therefore, the modulation of dopaminergic pathways following acute exercise may contribute to the reduction in SICI observed with HIIT, and particularly the sustained reduction following MIIT, as observed in the current study. The greater reduction in SICI following MIIT compared to HIIT could be related to MIIT striking a balance in exercise-related neuromodulatory signals supporting reduced SICI. While both intensities can increase catecholamines like dopamine and neurotrophic factors like BDNF, HIIT may also trigger elevated cortisol levels, which can transiently dampen cortical excitability and interfere with GABAergic modulation (Sale et al. 2008a; Mang et al. 2013; Mang et al. 2017; Neva 2024). MIIT may avoid these excitability-dampening effects by inducing lower levels of cortisol compared to HIIT, allowing for a sustained reduction in M1 inhibition (Ekblom et al. 2022).
Decreased SICI following LIIT suggests that the physiological mechanisms activated by light-intensity exercise are effective at modulating GABAergic functioning in M1 but may not be as potent as those triggered by higher intensities (MIIT and HIIT). Future work is needed to directly investigate the effect of AEX intensity on these underlying neural mechanisms and their contribution to exercise-induced decrease in SICI.
Effect of acute exercise intensity on specific M1 interneurons
We found a dose–response effect of exercise on SICI as measured by different TMS currents (AP versus PA). Specifically, we found a greater decrease in SICI measured with AP TMS current 20 min post-MIIT compared to rest, which was not observed with PA TMS current. This suggests that our MIIT exercise uniquely impacted M1 interneurons sensitive to AP TMS current while measuring SICI, like previous findings (Neva et al. 2021). Interestingly, this result has been demonstrated in the current study while using interval exercise at a moderate intensity, while not showing the same effects for HIIT and LIIT. This suggests a potential unique association between M1 interneurons sensitive to AP TMS current interacting with GABAA-receptor-related activity that is impacted by MIIT. Interestingly, these same M1 interneurons sensitive to AP TMS current have been impacted by other interventions as demonstrated using paired-pulse paradigms like SICI (Mirdamadi et al. 2017; Spampinato 2020). It is also possible that use of AP current while measuring SICI may be more reliable than PA current and thus may be more sensitive to changes to due interventions or specific behaviors (Lazzaro et al. 1998; Di Lazzaro et al. 2001; Sale et al. 2016; Cirillo et al. 2018). This suggests that SICI measured with AP TMS current may reveal the AEX effects more sensitively and reliably compared to SICI with PA TMS current. However, this finding should be interpreted with caution since it was revealed following exploratory analysis.
Contrary to our hypothesis, corticospinal excitability increased following HIIT and MIIT regardless of TMS current direction. We previously found that an acute session of moderate-intensity continuous exercise increased corticospinal excitability as measured with AP TMS current but not with posterior-to-anterior TMS current (Neva et al. 2021). Therefore, we did not confirm our previous results (Neva et al. 2021). It is possible that the exercise type played an important role in these effects, as the current study employed interval training exercise (ie MIIT), whereas our previous study used continuous cycling exercise at a moderate intensity. The alternating periods of moderate intensity to lower intensity periods of active recovery may have led to a net positive neural environment that shifted the release of neurotrophic factors and neurotransmitters (Sale et al. 2008a; Mang et al. 2013; Skriver et al. 2014) in the motor cortex to increase the excitable corticospinal output of interneurons sensitive to both PA and AP TMS currents. However, this remains highly speculative since we did not measure any specific neurotrophic factors and neurotransmitters.
Limitations
This study has limitations that should be considered when interpreting the results. First, while our primary focus was on the effect of AEX intensity on M1 excitability, we did not control for the total work across different exercise conditions. It is entirely possible that the total work completed during AEX may impact brain excitability. Instead, we controlled for exercise duration to ensure a consistent time frame for comparing different exercise intensity levels (light, moderate, high). Controlling AEX duration attempts to control for differences in outcomes to be related to intensity rather than accumulated fatigue or total energy expenditure (Tschakert and Hofmann 2013). Second, we chose interval exercise over continuous exercise to ensure that participants could complete all AEX conditions. Particularly during high-intensity cycling, maintaining continuous pedaling for 20 min at such intensity could be highly challenging. Yet, it is important to acknowledge that continuous exercise may uniquely influence TMS-based neurophysiological measures across the different AEX intensities. Future studies should investigate the impact of exercise type (interval vs. continuous) on M1 excitability to comprehensively understand the effects of AEX intensity. Third, we used HRR to prescribe exercise intensity. We are aware that this method is less precise and more variable compared to using oxygen uptake (VO2) reserve when prescribing exercise intensity. Indeed, each method has advantages and limitations (Mann et al. 2013). We decided to prescribe the exercise at a percentage of HRR for two reasons. Firstly, this approach accounts for individual differences and potential inaccuracies associated with HR-dependent methods or oxygen uptake methods such as percent of peak HR (%HRpeak) or peak oxygen uptake (%VO2peak). Therefore, %HRR provides less risk of overestimating or underestimating exercise intensity (ACSM 2018). Secondly, %HRR allows for easy translation of exercise prescriptions into practical use, as individuals can monitor their HR during exercise without the need for complex equipment or measurements. Further, we did not normalize MEP amplitude to maximal M-wave (M-max) amplitude in the present study. We continuously monitored EMG to ensure that the hand muscles remained inactive during cycling for the purpose of controlling muscle fatigue or other potential changes in muscle excitability, which may impact postexercise assessment of corticospinal excitability. Importantly, M-max is typically recorded to account for post-exercise changes within the active muscle(s) during exercise protocols (Davranche et al. 2015). Although some work has shown reductions in M-max amplitude postexercise (McDonnell et al. 2013; Ostadan et al. 2016), the majority of previous research has shown a lack of change in M-max for nonexercised upper-limb muscles following lower-limb cycling exercise (Motl and Dishman 2003; Mang et al. 2014; Mang et al. 2016; Neva et al. 2017; Yamazaki et al. 2019; Brown et al. 2020; Hendy et al. 2022). Despite this, it remains a possibility that acute exercise could alter muscle excitability, which may impact measures of corticospinal and spinal excitability. Thus, we encourage future studies to measure M-max amplitude before and after acute exercise for MEP normalization and replicate our results (Davranche et al. 2015; Roig et al. 2016). Finally, TMS measures during the Pre, Post0, and Post20 time points took 10 to 20 min, which could be considered a long period of time. Since exercise-induced M1 excitability modulation can be time-sensitive, it is possible that some subtle changes were not captured. Prior research has shown that the timing of TMS assessments is crucial for detecting short-lived changes in M1 excitable output (Ridding and Ziemann 2010). Future studies could consider reducing the number of TMS measures collected postexercise to further understand the time-sensitive effects following AEX.
Conclusion
This study investigated the impact of AEX intensity on distinct motor cortical circuits. Our main findings indicate that HIIT increased M1 excitable output to the greatest extent, and MIIT reduced GABAergic inhibition as measured by SICI to the greatest extent. Importantly, MIIT also transiently increased M1 excitable output, and LIIT, MIIT, and HIIT reduced GABAergic inhibition transiently. There is also evidence for preferential modulation of interneurons sensitive to AP TMS current interacting with SICI, particularly following MIIT. Our findings provide evidence for a nuanced dose–response effect of exercise intensity on the modulation of distinct motor cortical circuits.
Supplementary Material
Acknowledgments
We thank Callum O’Malley and Jonathan Tremblay for their valuable advice during the conception of this project. We also thank Maxime Bergevin for contributing to data collection and statistical analysis. Finally, we thank Yasmine Mahrez for her contribution to data processing.
Contributor Information
Nesrine Harroum, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada; Centre interdisciplinaire de recherche sur le cerveau et l'apprentissage (CIRCA), Montreal, QC, Canada.
Amanda O’Farrell, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada.
Layale Youssef, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada; Centre interdisciplinaire de recherche sur le cerveau et l'apprentissage (CIRCA), Montreal, QC, Canada.
Louna Bohbot, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada.
Hajar Maati, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada.
Marie Joubert, Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada.
Benjamin Pageaux, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada; Centre interdisciplinaire de recherche sur le cerveau et l'apprentissage (CIRCA), Montreal, QC, Canada.
Jason L Neva, École de kinésiologie et des sciences de l'activité physique (EKSAP), Faculté de médecine, Université de Montréal, 2100, boul Édouard-Montpetit, Montréal, QC H3T 1J4, Canada; Centre de recherche de l'Institut universitaire de gériatrie de Montréal (CRIUGM), 4545, Chemin Queen-Mary Montréal (Québec) H3W 1W4, Canada; Centre interdisciplinaire de recherche sur le cerveau et l'apprentissage (CIRCA), Montreal, QC, Canada.
Author contributions
Nesrine Harroum (Data curation, Formal analysis, Methodology, Project administration, Writing—original draft), Amanda O'Farrell (Data curation, Writing—review & editing), Layale Youssef (Data curation, Writing—review & editing), Louna Bohbot (Data curation, Writing—review & editing), Hajar Maati (Data curation, Writing—review & editing), Marie Joubert (Data curation, Writing—review & editing), Benjamin Pageaux (Conceptualization, Writing—review & editing, Funding acquisition, Methodology, Supervision), Jason L. Neva (Conceptualization, Writing—review & editing, Funding acquisition, Methodology, Supervision).
Supplementary material
Supplementary material is available at Cerebral Cortex online.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2020-05263 to J.L.N.). J.L.N. and B.P. received support from the Chercheur Boursier Junior 1 award of the Fonds de Recherche du Québec—Santé (FRQS). N.H. and L.Y. received support from both the Centre de Recherche de L’Institut Universitaire de Gériatrie de Montréal (CRIUGM) and the Faculty of Medicine at Université de Montréal. Additionally, L.Y. is supported by the Centre Interdisciplinaire de Recherche sur le Cerveau et l’Apprentissage (CIRCA) and by Fonds de Recherche du Quebec—Nature et Technologies (FRQ-NT).
Conflict of interest statement. None declared.
Data availability
All main data presented in this manuscript are included within the figures. The datasets produced and analyzed in this study are available from the corresponding author upon reasonable request.
References
- ACSM . 2018. ACSM's guidelines for exercise testing and prescription. Wolters Kluwer, 2400 BA Alphen aan den Rijn, Netherlands. [Google Scholar]
- Ammann C et al. 2020. A framework to assess the impact of number of trials on the amplitude of motor evoked potentials. Sci Rep. 10:21422. 10.1038/s41598-020-77383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SC et al. 2020. Intensity matters: high-intensity interval exercise enhances motor cortex plasticity more than moderate exercise. Cereb Cortex. 30:101–112. 10.1093/cercor/bhz075. [DOI] [PubMed] [Google Scholar]
- Booth M. 2000. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 71:114–120. 10.1080/02701367.2000.11082794. [DOI] [PubMed] [Google Scholar]
- Borg E, Kaijser L. 2006. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 16:57–69. 10.1111/j.1600-0838.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Brown KE et al. 2020. The influence of an acute bout of moderate-intensity cycling exercise on sensorimotor integration. Eur J Neurosci. 52:4779–4790. 10.1111/ejn.14909. [DOI] [PubMed] [Google Scholar]
- Cavaleri R, Schabrun SM, Chipchase LS. 2017. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis. Syst Rev. 6:48. 10.1186/s13643-017-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Semmler JG, Mooney RA, Byblow WD. 2018. Conventional or threshold-hunting TMS? A tale of two SICIs. Brain Stimulation. 11:1296–1305. 10.1016/j.brs.2018.07.047. [DOI] [PubMed] [Google Scholar]
- Coco M et al. 2010. Elevated blood lactate is associated with increased motor cortex excitability. Somatosens Mot Res. 27:1–8. 10.3109/08990220903471765. [DOI] [PubMed] [Google Scholar]
- Coco M et al. 2014. Changes in cortical excitability and blood lactate after a fatiguing hand-grip exercise. Somatosens Mot Res. 31:35–39. 10.3109/08990220.2013.834816. [DOI] [PubMed] [Google Scholar]
- Coco M et al. 2020. Influences of blood lactate levels on cognitive domains and physical health during a sports stress. Brief review. Int J Environ Res Public Health. 17:9043. 10.3390/ijerph17239043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2013. Statistical power analysis for the behavioral sciences. Routledge, Oxfordshire, United Kingdom. [Google Scholar]
- Coxon JP et al. 2018. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol. 596:691–702. 10.1113/JP274660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL et al. 2003. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 35:1381–1395. 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Curtin D, Taylor EM, Bellgrove MA, Chong TTJ, Coxon JP. 2023. D2 receptor blockade eliminates exercise-induced changes in cortical inhibition and excitation. Brain Stimulation. 16:727–733. 10.1016/j.brs.2023.04.019. [DOI] [PubMed] [Google Scholar]
- Davranche K, Temesi J, Verges S, Hasbroucq T. 2015. Transcranial magnetic stimulation probes the excitability of the primary motor cortex: a framework to account for the facilitating effects of acute whole-body exercise on motor processes. J Sport Health Sci. 4:24–29. 10.1016/j.jshs.2014.09.001. [DOI] [Google Scholar]
- Day B et al. 1989. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 412:449–473. 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V et al. 2001. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 138:268–273. 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V et al. 2012. I-wave origin and modulation. Brain Stimul. 5:512–525. 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Ekblom MM et al. 2022. Acute effects of physical activity patterns on plasma cortisol and brain-derived neurotrophic factor in relation to corticospinal excitability. Behav Brain Res. 430:113926. 10.1016/j.bbr.2022.113926. [DOI] [PubMed] [Google Scholar]
- El-Sayes D, Harasym CV, Turco MBL, Nelson AJ. 2019a. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 25:65–85. 10.1177/1073858418771538. [DOI] [PubMed] [Google Scholar]
- El-Sayes J et al. 2019b. The effects of biological sex and ovarian hormones on exercise-induced neuroplasticity. Neuroscience. 410:29–40. 10.1016/j.neuroscience.2019.04.054. [DOI] [PubMed] [Google Scholar]
- El-Sayes J et al. 2020. Acute high-intensity and moderate-intensity interval exercise do not change corticospinal excitability in low fit, young adults. PLoS One. 15:e0227581. 10.1371/journal.pone.0227581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J et al. 2000. D(1) dopamine receptor activation reduces GABA(a) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol. 83:2996–3004. 10.1152/jn.2000.83.5.2996. [DOI] [PubMed] [Google Scholar]
- Garnier YM, Lepers R, Dubau Q, Pageaux B, Paizis C. 2018. Neuromuscular and perceptual responses to moderate-intensity incline, level and decline treadmill exercise. Eur J Appl Physiol. 118:2039–2053. 10.1007/s00421-018-3934-8. [DOI] [PubMed] [Google Scholar]
- Grossl T, de Lucas RD, de Souza KM, Guglielmo LGA. 2012. Time to exhaustion at intermittent maximal lactate steady state is longer than continuous cycling exercise. Appl Physiol Nutr Metab. 37:1047–1053. 10.1139/h2012-088. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. 2013. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 23:1593–1605. 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hamada M et al. 2014. Two distinct interneuron circuits in human motor cortex are linked to different subsets of physiological and behavioral plasticity. J Neurosci. 34:12837–12849. 10.1523/JNEUROSCI.1960-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R et al. 1998. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 509:607–618. 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Cavanagh SE, Tremblay S, Simeoni S, Rothwell JC. 2018. Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J Neurosci. 38:1264–1276. 10.1523/JNEUROSCI.2869-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJ, Rejeski WJ. 1989. Not what, but how one feels: the measurement of affect during exercise. Journal of sport and exercise psychology. 11:304–317. 10.1123/jsep.11.3.304. [DOI] [Google Scholar]
- Hendy AM, Andrushko JW, Della Gatta PA, Teo W-P. 2022. Acute effects of high-intensity aerobic exercise on motor cortical excitability and inhibition in sedentary adults. Front Psychol. 13:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera SGR, Leon-Rojas JE, Herrera SGR. 2024. The effect of aerobic exercise in neuroplasticity, learning, and cognition: a systematic review. Cureus. 16:e54021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O. 2024. Lactate as a supplemental fuel for synaptic transmission and neuronal network oscillations: potentials and limitations. J Neurochem. 168:608–631. 10.1111/jnc.15867. [DOI] [PubMed] [Google Scholar]
- Kanthack TFD, Guillot A, Clémençon M, Debarnot U, Di Rienzo F. 2020. Effect of physical fatigue elicited by continuous and intermittent exercise on motor imagery ability. Res Q Exerc Sport. 91:525–538. 10.1080/02701367.2019.1691709. [DOI] [PubMed] [Google Scholar]
- Karvonen J, Vuorimaa T. 1988. Heart rate and exercise intensity during sports activities. Practical application. Sports Med. 5:303–312. 10.2165/00007256-198805050-00002. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Hsieh YT, Lin MF, Kuo MF, Nitsche MA. 2023. A single bout of aerobic exercise modulates motor learning performance and cortical excitability in humans. Int J Clin Health Psychol. 23:100333. 10.1016/j.ijchp.2022.100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. 2022. Sample size justification. Collabra: psychology. 8:33267. 10.1525/collabra.33267. [DOI] [Google Scholar]
- Lazzaro VD et al. 1998. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 119:265–268. 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Li H et al. 2022. The role of MRS-assessed GABA in human behavioral performance. Prog Neurobiol. 212:102247. 10.1016/j.pneurobio.2022.102247. [DOI] [PubMed] [Google Scholar]
- Lulic T, El-Sayes J, Fassett HJ, Nelson AJ. 2017. Physical activity levels determine exercise-induced changes in brain excitability. PLoS One. 12:e0173672. 10.1371/journal.pone.0173672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MA et al. 2019. Intensity of acute aerobic exercise but not aerobic fitness impacts on corticospinal excitability. Applied Physiology, Nutrition, & Metabolism = Physiologie Appliquee, Nutrition et Metabolisme. 44:869–878. 10.1139/apnm-2018-0643. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. 2016. Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci. 36:2449–2457. 10.1523/JNEUROSCI.3455-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Campbell KL, Ross CJ, Boyd LA. 2013. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 93:1707–1716. 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. 2014. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol. 117:1325–1336. 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS et al. 2016. Promoting motor cortical plasticity with acute aerobic exercise: a role for cerebellar circuits. Neural Plasticity. 2016:6797928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS et al. 2017. Exploring genetic influences underlying acute aerobic exercise effects on motor learning. Sci Rep. 7:12123. 10.1038/s41598-017-12422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T, Lamberts RP, Lambert MI. 2013. Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Med. 43:613–625. 10.1007/s40279-013-0045-x. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG. 2013. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J Appl Physiol. 114:1174–1182. 10.1152/japplphysiol.01378.2012. [DOI] [PubMed] [Google Scholar]
- McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. 2010. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 45:671–678. 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell LH et al. 2024. Myths and methodologies: standardisation in human physiology research-should we control the controllables? Exp Physiol. 109:1099–1108. 10.1113/EP091557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdamadi JL, Suzuki LY, Meehan SK. 2017. Attention modulates specific motor cortical circuits recruited by transcranial magnetic stimulation. Neuroscience. 359:151–158. 10.1016/j.neuroscience.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA et al. 2016. Acute aerobic exercise modulates primary motor cortex inhibition. Exp Brain Res. 234:3669–3676. 10.1007/s00221-016-4767-5. [DOI] [PubMed] [Google Scholar]
- Morris TP et al. 2020. Light aerobic exercise modulates executive function and cortical excitability. Eur J Neurosci. 51:1723–1734. 10.1111/ejn.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, Dishman RK. 2003. Acute leg-cycling exercise attenuates the H-reflex recorded in soleus but not flexor carpi radialis. Muscle Nerve. 28:609–614. 10.1002/mus.10479. [DOI] [PubMed] [Google Scholar]
- Myers J et al. 2009. Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation. 119:3144–3161. 10.1161/CIRCULATIONAHA.109.192520. [DOI] [PubMed] [Google Scholar]
- Neva J. 2025. Exercise-induced neuroplasticity. Encyclopedia of the Human Brain (Second Edition). Grafman JH. (ed), Oxford, Elsevier. 706–729.
- Neva JL, Brown KE, Mang CS, Francisco BA, Boyd LA. 2017. An acute bout of exercise modulates both intracortical and interhemispheric excitability. Eur J Neurosci. 45:1343–1355. 10.1111/ejn.13569. [DOI] [PubMed] [Google Scholar]
- Neva JL et al. 2021. Acute exercise modulates the excitability of specific interneurons in human motor cortex. Neuroscience. 475:103–116. 10.1016/j.neuroscience.2021.08032. [DOI] [PubMed] [Google Scholar]
- Nicolini C et al. 2020. A single bout of high-intensity interval exercise increases corticospinal excitability, brain-derived neurotrophic factor, and Uncarboxylated Osteolcalcin in sedentary, healthy males. Neuroscience. 437:242–255. 10.1016/j.neuroscience.2020.03.042. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opie GM, Semmler JG. 2019. Acute exercise at different intensities influences corticomotor excitability and performance of a ballistic thumb training task. Neuroscience. 412:29–39. 10.1016/j.neuroscience.2019.05.049. [DOI] [PubMed] [Google Scholar]
- Ostadan F et al. 2016. Changes in corticospinal excitability during consolidation predict acute exercise-induced off-line gains in procedural memory. Neurobiology of Learning & Memory. 136:196–203. 10.1016/j.nlm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Paulus W et al. 2008. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 1:151–163. 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JF, Arai N, Ziemann U. 2008. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol. 119:2291–2297. 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Piercy KL et al. 2018. The physical activity guidelines for Americans. Jama. 320:2020–2028. 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–304. 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye-Jensen J, Kiens B, Nielsen JB. 2012. A single bout of exercise improves motor memory. PLoS One. 7:e44594. 10.1371/journal.pone.0044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M et al. 2016. Time-dependent effects of cardiovascular exercise on memory. Exerc Sport Sci Rev. 44:81–88. 10.1249/JES.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Ryberg M, Boraxbekk C-J, Kjaer M, Demnitz N. 2023. Effects of acute physical activity on brain metabolites as measured by magnetic resonance spectroscopy (1H-MRS) in humans: a systematic review. Heliyon. 9:e20534. 10.1016/j.heliyon.2023.e20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli MA et al. 2019. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Mov Disord. 34:1891–1900. 10.1002/mds.27865. [DOI] [PubMed] [Google Scholar]
- Safikhani S et al. 2018. Response scale selection in adult pain measures: results from a literature review. Journal of patient-reported outcomes. 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom MA. 2008. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 28:8285–8293. 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale AP, Lavender GM, Opie MA, Nordstrom MA, Semmler JG. 2016. Increased intracortical inhibition in elderly adults with anterior–posterior current flow: a TMS study. Clin Neurophysiol. 127:635–640. 10.1016/j.clinph.2015.04.062. [DOI] [PubMed] [Google Scholar]
- Singh RE, Duncan JLN, Staines WR. 2014. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC sports science, medicine and rehabilitation. 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AM, Neva JL, Staines WR. 2016. Aerobic exercise enhances neural correlates of motor skill learning. Behav Brain Res. 301:19–26. 10.1016/j.bbr.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Skriver K et al. 2014. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem. 116:46–58. 10.1016/j.nlm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Smith AE, Goldsworthy MR, Garside T, Wood FM, Ridding MC. 2014. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp Brain Res. 232:1875–1882. 10.1007/s00221-014-3879-z. [DOI] [PubMed] [Google Scholar]
- Smith AE et al. 2018. High-intensity aerobic exercise blocks the facilitation of iTBS-induced plasticity in the human motor cortex. Neuroscience. 373:1–6. 10.1016/j.neuroscience.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Spampinato D. 2020. Dissecting two distinct interneuronal networks in M1 with transcranial magnetic stimulation. Exp Brain Res. 238:1693–1700. 10.1007/s00221-020-05875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato DA, Celnik PA, Rothwell JC. 2020. Cerebellar–motor cortex connectivity: one or two different networks? J Neurosci. 40:4230–4239. 10.1523/JNEUROSCI.2397-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrinos EL, Coxon JP. 2017. High-intensity interval exercise promotes motor cortex disinhibition and early motor skill consolidation. J Cogn Neurosci. 29:593–604. 10.1162/jocn_a_01078. [DOI] [PubMed] [Google Scholar]
- Tamm AS, Lagerquist O, Ley AL, Collins DF. 2009. Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythm. 24:211–224. 10.1177/0748730409334135. [DOI] [PubMed] [Google Scholar]
- Thomas R et al. 2016. Acute exercise and motor memory consolidation: the role of exercise intensity. PLoS One. 11:e0159589. 10.1371/journal.pone.0159589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakert G, Hofmann P. 2013. High-intensity intermittent exercise: methodological and physiological aspects. Int J Sports Physiol Perform. 8:600–610. 10.1123/ijspp.8.6.600. [DOI] [PubMed] [Google Scholar]
- Turco CV, Nelson AJ. 2021. Transcranial magnetic stimulation to assess exercise-induced neuroplasticity. Frontiers in Neuroergonomics. 2:679033. 10.3389/fnrgo.2021.679033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y et al. 2019. Acute low-intensity aerobic exercise modulates intracortical inhibitory and excitatory circuits in an exercised and a non-exercised muscle in the primary motor cortex. Front Physiol. 10:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y et al. 2020. Modulation of inhibitory function in the primary somatosensory cortex and temporal discrimination threshold induced by acute aerobic exercise. Behav Brain Res. 377:112253. 10.1016/j.bbr.2019.112253. [DOI] [PubMed] [Google Scholar]
- Youssef L et al. 2024. Neurophysiological effects of acute aerobic exercise in young adults: a systematic review and meta-analysis. Neurosci Biobehav Rev. 164:105811. 10.1016/j.neubiorev.2024.105811. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. 2000. I-waves in motor cortex. J Clin Neurophysiol. 17:397–405. 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. 1998. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 109:321–330. 10.1016/S0924-980X(98)00023-X. [DOI] [PubMed] [Google Scholar]
- Ziemann U et al. 2015. TMS and drugs revisited 2014. Clin Neurophysiol. 126:1847–1868. 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All main data presented in this manuscript are included within the figures. The datasets produced and analyzed in this study are available from the corresponding author upon reasonable request.





