Highlights
-
•
Cold stress increased survival of B6 cells frozen in liquid nitrogen to 53 %.
-
•
Cold stress increased cell membrane UFA content, aiding its fluidity in freezing.
-
•
Proteins in fatty acid synthesis were up regulated in cold stressed B6 cells.
-
•
Cold tolerance of B6 cells might link to its capsule, acting as a cryoprotectant.
Keywords: Lacticaseibacillus, Cold stress, Fatty acids, Cryotolerance, Proteomics
Abstract
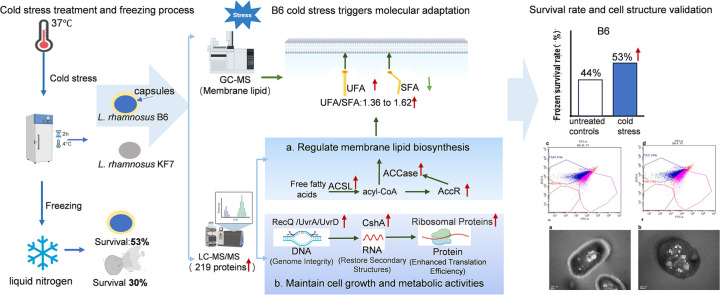
Understanding the molecular mechanisms underlying the cryotolerance of lactic acid bacteria is critical for preserving their viability in food processing. In this study, Lacticaseibacillus rhamnosus B6 and KF7 with different phenotypic properties were pretreated at 4 °C for 2 h before liquid nitrogen freezing. Cold-stressed B6 exhibited significantly higher survival (53 %) than KF7 (30 %) and untreated controls (44 % vs. 10 %, p < 0.05), with few disrupted cells observed under SEM. Cold stress altered the membrane fluidity in B6 by increasing unsaturated fatty acids (UFA) and the ratio of UFA to saturated fatty acids (UFA/SFA) from 1.36 to 1.62 (p < 0.05). 219 proteins, primarily those involved in fatty acid biosynthesis, translation and transport processes were up regulated in cold-stressed B6, compared to untreated counterparts. Noteworthy, capsules of B6 likely contributed to its higher cryotolerance. Our findings revealed a dual cryotolerance mechanism in L. rhamnosus B6, i.e., dynamic lipidome remodeling and coordinated protein expression regulation. This study highlighted the importance of the cell surface features of individual strain of L. rhamnosus and cold stress treatment in preparing probiotics as well as direct to vat starter cultures with high cell vitality employing deep freezing process.
Graphical abstract
1. Introduction
Lactic acid bacteria (LAB) are gram-positive and non-spore-forming bacteria that mainly produce lactic acid by fermenting carbohydrate (Abedin et al., 2024). Numerous studies reported LAB could adjust the balance of intestinal flora, enhance the immunity and defense of the body, and promote the development of intestinal tract (Leeuwendaal et al., 2022). LAB are widely applied as starter cultures in food, pharmaceutical and chemical industry. Meanwhile, some members in LAB have been successfully launched as commercial probiotics to regulate host immunity and health (Chourasia et al., 2021). However, their efficacy as either starter cultures or probiotics is fundamentally dependent on high cell viability, which can be significantly compromised by processing parameters including temperature fluctuations, acidic environments, and osmotic pressure (Gao et al., 2021). Lyophilization is widely utilized for manufacturing starter cultures or probiotics powders with high levels of viable cells, while deep-freeze pelleting is a newly developed technology in preparing direct to vat starter cultures. Besides, long-term preservation of LAB relies heavily on freeze-drying and frozen storage. LAB cells in both of the two processes would be challenged with extremely low temperatures, which might damage the integrity of bacterial cells for the formation of ice crystals or imbalance of osmic pressure outer versus inner cells (Fonseca et al., 2016). Exogenous cryoprotectants or cold adaption might improve the survival of LAB during the processing (Gautier et al., 2013; Song et al., 2014).
Current research on LAB cryotolerance has primarily focused on species such as Lactobacillus plantarum and Streptococcus thermophilus, revealing adaptive strategies like membrane lipid modulation, stress protein upregulation, and genetic regulation (Gautier et al., 2013; Song et al., 2014) . However, the cryotolerance mechanisms of important industrial strains remain underexplored, especially for those lactobacilli strains in the same species but with distinct cell phenotypes.
Lacticaseibacillus rhamnosus, previously known as Lactobacillus rhamnosus or L. casei subsp. rhamnosus, is well studied for its beneficial effects in promoting host health (Petrova et al., 2021). In our previous study, L. rhamnosus B6 and KF7 exhibited varied bioactivities, including strain-specific extracellular polysaccharides (EPS) with immunomodulatory activity (Han et al., 2024; Shao et al., 2014) . Notably, L. rhamnosus B6 demonstrates exceptional industrial relevance, validated through its ability to generate bioactive peptides and enhance flavor in probiotics co-fermented cheese (Zhang et al., 2025; Zhang et al., 2024), and its mercury (II) ion adsorption capacity (Yu et al., 2025).
L. rhamnosus B6 formed slimy colonies on de MAN, ROGOSA and SHARP (MRS) agar plates (Han et al., 2024) and exhibited higher survival ratio during lyophilization than that of strain KF7 (in press). Therefore, we hypothesized the difference in cell surface features between these two strains might influence their cryotolerance, and cold adaptation might enhance their cryotolerance. Our study tried to unveil the underlying strategies adopted by some LAB to cope extremely low environmental temperature and provide guidelines for industries in manufacturing high viability probiotic formulations and starter cultures in selecting specific strain and cold stress protocol.
2. Materials and methods
2.1. Bacteria and culture medium
L. rhamnosus B6 (= CGMCC 13,310) was isolated from homemade sour milk collected in Bulgaria, and L. rhamnosus KF7 (= CGMCC 6430) was isolated from Kefir granules collected in Yunnan, China. The bacterial stains were routinely propagated on MRS agar (Merck KGaA, Germany) and stored in MRS broth supplemented with 20 % (w/v) glycerol at −80 °C for long term storage.
2.2. Growth curve determination
The bacterial strain was first activated on MRS agar plate and incubated at 37 °C for 24 h. A loop of the freshly activated bacterial cells was inoculated into sterile MRS broth and cultivated at 37 °C for 20 h. The bacterial cells were collected by centrifugation, washed with sterile deionized water and resuspended into fresh MRS broth to prepare the inoculum. The inoculum was transferred at a ratio of 2 % (v/v) into fresh sterile MRS broth and cultivated at 37 °C for 24 h. The Growth curve of the bacterial strain was recorded by a microbial automatic growth curve analyzer (Bioscreen C, OY Growth Curves, Finland) with measurement of the OD600 of the culture at an interval of 1 h.
2.3. Cold stress treatment and liquid nitrogen freezing of L. rhamnosus B6 and KF7
The bacterial inoculum was transferred into fresh sterilized MRS broth at a ratio of 2 % and cultivated to logarithmic phase (OD600=0.8). After treating at 30 °C or 4 °C respectively for 2 h, they were immediately placed into liquid nitrogen for 24 h.
2.4. Determination of survival rate
The frozen culture was thawed at room temperature. The number of viable cell counts before or after freezing in liquid nitrogen was determined by plate counting. 0.5 mL of bacterial culture was mixed with 4.5 mL of sterile deionized water and diluted in 10-fold series. Then, 100 μL of diluted culture was spread on MRS agar plate. The plates were cultivated at 37 °C for 48 h and numerated.
Survival rate = counts of viable cells per milliliters after liquid nitrogen freezing/counts of viable cells per milliliters before liquid nitrogen freezing × 100 %
2.5. Flow cytometry
Frozen with liquid nitrogen on the viability of bacterial cells treated in Section 2.3 was also checked by flow cytometry. The double staining of the cells was carried out according to the protocol published (Lebeer et al., 2012). The working solutions of carboxyfluorescein diacetate (cFDA) and propidium iodide (PI) were prepared with dimethyl sulfoxide at concentrations of 2.5 mM and 0.8 mM respectively. Firstly, the sample was diluted with PBS buffer with pH7.0 to adjust the cell concentration to 106 cells/mL. For double staining, 2 μL cFDA was added to 1 mL diluted cell suspension and incubated for 15 min in a 30 °C incubator. After adding 2 μL PI, the mixture was incubated at 30 °C for another 15 min. All samples were incubated in the dark and checked by flow cytometry immediately.
2.6. Scanning electron microscope (SEM)
After the cells frozen in liquid nitrogen were thawed at room temperature, the cells were collected by centrifugation (5000 × g, 10 min). The cells were fixed with 2.5 % (v/v) glutaraldehyde at 4 °C overnight, washed 4 times with PBS, and then fixed with OsO4. The fixed cell sample was washed twice with PBS and twice with pure water. Then, the fixed cell sample was dehydrated by 50 % ethanol, 70 % ethanol, 90 % ethanol and 100 % ethanol for 10 min in turn, dried at the critical point, plated with gold by ion sputtering, and observed under high vacuum. Cell integrity was quantified by counting cells with visible envelope rupture, structural collapse, or cytoplasmic leakage in SEM images using ImageJ (National Institutes of Health, v1.53).
2.7. Membrane lipid extraction and fatty acid methylation analysis by gas chromatography-mass spectrometry (GC–MS)
The cold-stressed (4 °C) and control (30 °C) cells of L. rhamnosus B6 were collected by centrifugation. Total lipids were extracted following a modified Folch method (Folch et al., 1957). Briefly, 1 g of wet cell biomass was homogenized with 20 vol of chloroform: methanol (2:1, v/v) using a magnetic stirrer for 2 h at room temperature. The mixture was stored at 4 °C for 24 h to ensure complete lipid extraction, followed by centrifugation (5000 × g, 10 min). The upper phase containing non-lipid contaminants was removed. The lower chloroform phase was washed twice with 0.2 vol of upper phase solvent (chloroform: methanol: water = 3:48:47, v/v) containing 0.88 % KCl to minimize lipid loss. Each wash step included gentle agitation followed by phase separation via centrifugation. The lipid-containing chloroform phase was dried under a nitrogen stream.
To analyze fatty acid composition, lipids were hydrolyzed and methylated (Segura-Campos et al., 2014). Briefly, the dried lipids were saponified with 0.5 M NaOH in methanol at 70 °C for 30 min, followed by methylation with 14 % BF₃ in methanol at 70 °C for 10 min. Fatty acid methyl esters (FAMEs) were extracted with hexane and analyzed by GC–MS (Agilent 7890B-7000D) equipped with a DB-23 capillary column (60 m × 0.25 mm × 0.25 μm). The oven temperature was programmed from 50 °C (2 min) to 240 °C at 4 °C/min, with helium as the carrier gas. Peaks were identified using the NIST 17 library and quantified against external standards.
2.8. Protein extraction and quantification
The inoculum of strain B6 was transferred to sterile MRS broth at a ratio of 2 % (v/v), cultivated at 37 °C for 8 h. The culture was then further incubated at 4 °C or 30 °C for 2 h. Bacterial cells were collected at 9000 × g at 4 °C for 5 min. The cells were frozen in liquid nitrogen for 30 min and thawed at room temperature for 4 cycles. The thawed cells were then added the pyrolysis liquid and disrupted by ultrasonic treatment. The supernatant was collected by centrifuging at 12,000 × g, 4 °C for 10 min. The protein concentration was quantified by BCA method.
2.9. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
100 μg of protein was precipitated from the supernatant of the lysed cells by five volumes of pre-chilled acetone overnight at −20 °C. The pellet was washed twice with 80 % acetone, dried, and resuspended in 100 μL of 50 mM ammonium bicarbonate buffer. Proteins were reduced with 10 mM dithiothreitol (DTT) at 55 °C for 30 min, alkylated with 20 mM iodoacetamide (IAA) in the dark for 30 min, and digested with sequencing-grade trypsin (1:50 w/w) at 37 °C overnight.
Peptides were separated on a nano-UPLC system (EASY-nLC1200, Thermo Scientific) using a reversed-phase C18 column (Reprosil-Pur 120 C18-AQ, 1.9 μm, 100 μm × 15 cm). The mobile phase consisted of phase A (0.1 % formic acid in water) and phase B (0.1 % formic acid in 80 % acetonitrile). A linear gradient was applied from 5 % to 45 % phase B over 88 min at a flow rate of 300 nL/min, followed by column equilibration.
Eluted peptides were analyzed on Q Exactive HF-X mass spectrometer (Thermo Scientific) in positive ion mode. Full MS scans (350–1600 m/z) were acquired at 120,000 resolutions, with automatic gain control (AGC) set to 3 × 10⁶ and maximum injection time (max IT) of 50 ms. The top 20 most intense ions were selected for higher-energy collisional dissociation (HCD) fragmentation (NCE 27 %, resolution 15,000). Dynamic exclusion was set to 45 s to prevent repeated sequencing.
Raw data were searched against the L. rhamnosus B6 UniProt database using MaxQuant (v2.0.3) with a false discovery rate (FDR) <1 %. Oxidation of methionine and acetylation of protein N-termini were set as variable modifications, while carbamidomethylation of cysteine was fixed.
2.10. Statistical analysis
All tests were performed in triplicate. Data visualization was performed using Graphpad Prism 8 software (Graphpad Software. La Jolla. CA. USA). Statistical significance was tested using a one-way analysis of variance (ANOVA).
3. Results
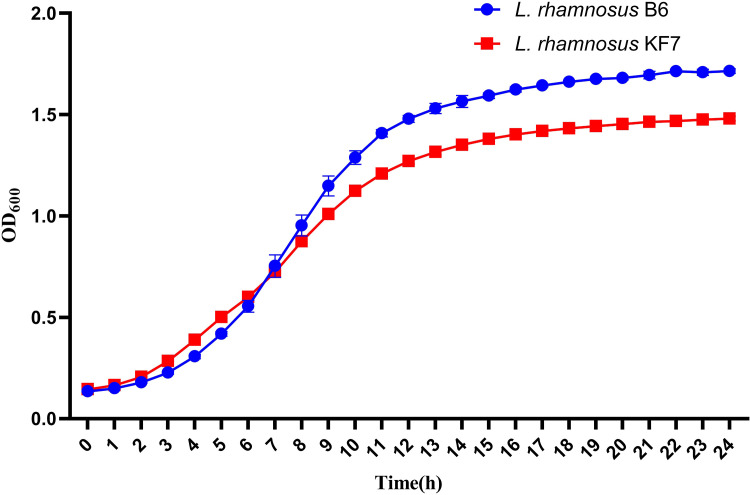
3.1. Strain B6 and KF7 showed a similar growth curve
The growth curves of L. rhamnosus B6 and KF7 were shown in Fig 1. Both strains exhibited similar growth kinetics in MRS broth. The density of bacterial cells in the culture reflected by the optical absorbance increased slowly in 0–2 h, indicating the cells were in the growth lag period. The density of bacterial cells increased rapidly in 2–12 h, meaning the cells entered logarithmic growth phase. After 12 h, the cells reached the stable phase. Cells in the early logarithmic phase (OD600 = 0.8) was selected for assay of L. rhamnosus B6 protein differentially expressed under subsequent cold stress.
Fig. 1.
Growth curve of L. rhamnosus B6 and KF7 in MRS broth at 37 °C.
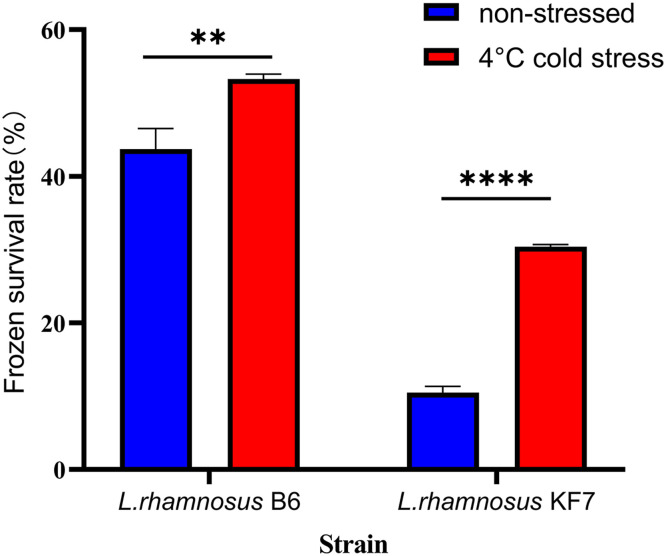
Fig. 2.
Survival rate of frozen cell of L. rhamnosus B6 and KF7 in liquid nitrogen. Results of three independent experiments were expressed as mean ± SD (n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.2. Cold stress enhanced the survival of cells in deep frozen
To analyze the effect of cold stress on the survival rate of frozen cells, L. rhamnosus B6 and KF7 were subjected to cold stress at 4 °C for 2 h before freezing in liquid nitrogen. The survival rates of untreated L. rhamnosus B6 and KF7 cells frozen in liquid nitrogen were 44 % and 10 %, respectively, whereas cold stress (4 °C, 2 h) significantly increased survival to 53 % and 30 % (p < 0.05). Notably, L. rhamnosus KF7 exhibited lower cryotolerance than L. rhamnosus B6 under both conditions. These results confirmed that cold stress enhanced post-freezing viability in both strains. Therefore, L. rhamnosus B6 showed a higher cryoresistance, an expectable characteristic in starter manufacturing.
3.3. Viability of frozen cells determined by flow cytometry
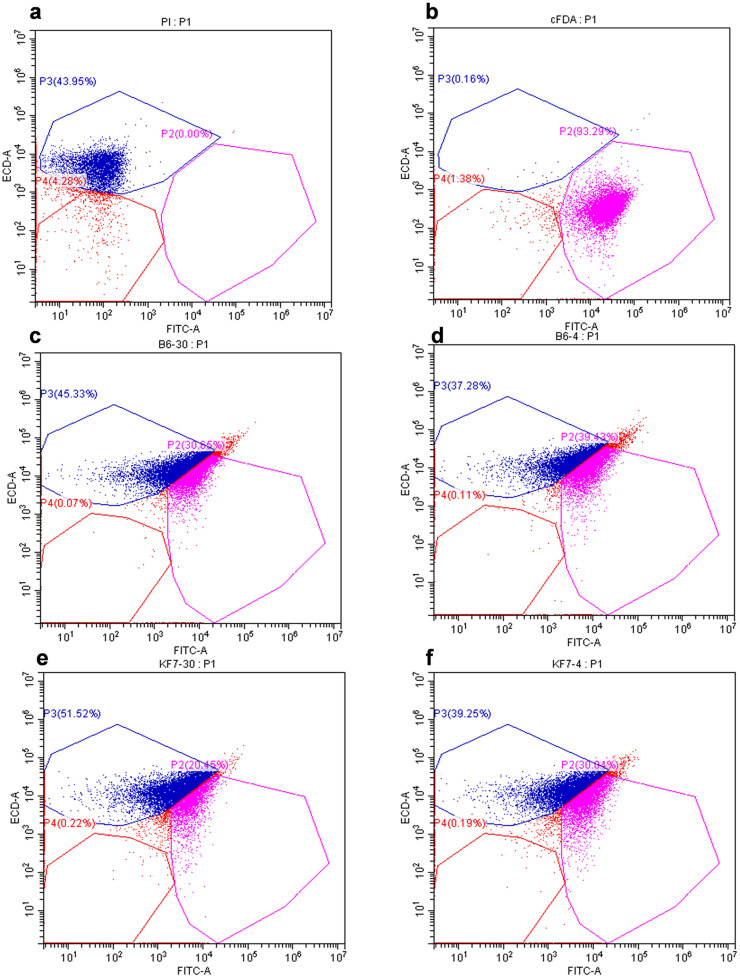
To evaluate the ratio of living/dead cells, bacterial cells with different treatment were double stained and distinguished by flow cytometry with living cells labeled with purple fluorescence whilst dead cells with blue fluorescence. Fig. 3a and Fig. 3b were flow cytometric charts of PI and cFDA staining respectively. The populations labeled by PI and cFDA showed different distributions in space. There are three subgroups with different staining characteristics in the fluorescence bitmap. The first area was located in the lower left quadrant (P4). The fluorescence intensity was <103, which was <1 % corresponding to stained cells. PI-labeled cells were located in the upper left area (P3) (Fig. 3a), representing dead cells. The cFDA labeled cells were located in the lower right area (P2) (Fig. 3b), corresponding to living cells.
Fig. 3.
Flow cytometry of L. rhamnosus B6 and KF7 after freezing in liquid nitrogen before and after cold stress. (a) Flow cytometry histogram of PI staining. (b) Flow cytometry histogram of cFDA staining. (c) Flow cytometry histogram of strain B6 frozen by liquid nitrogen control (non-stressed) condition. (d) Flow Cytogram of strain B6 frozen by liquid nitrogen after cold stress. (e) Flow cytometry histogram of strain KF7 frozen by liquid nitrogen control (non-stressed) condition. (f) Flow cytometry histogram of strain KF7 frozen by liquid nitrogen after cold stress.
The proportion of live cells and dead cells of strain B6 without prior cold stress after freezing were 30.65 % and 45.33 % (Fig. 3c). The calculated rate of live/dead cells was 40.34 %. However, after cold stress at 4 °C, the survival calculated rate of live/dead cells of strain B6 increased to 51.40 % (Fig. 3d). For strain KF7, cold stress treatment also improved the calculated rate of live/dead cells in liquid nitrogen freezing, and the indexes increased from 28.41 % (Fig. 3e) to 43.35 % (Fig. 3f). This result was consistent with the survival rate obtained by cultivation. It provided further support that cold stress could improve the survival of strains B6 and KF7.This result indicated that cold stress ahead would be beneficial to trigger the defense mechanism of L. rhamnosus and thus enhance its cold adaptability.
3.4. Cold stress protected the cell integrity post liquid nitrogen freezing
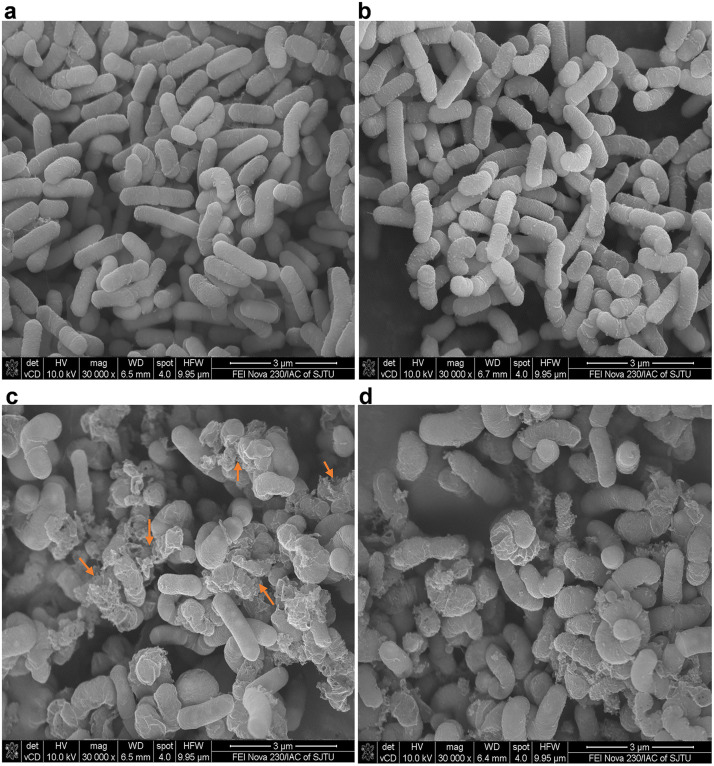
The growth of ice crystals during freezing might damage the integrity of bacterial cells, especially the membrane. Therefore, the morphological changes of microorganisms are visible indicators of their adaptability to environmental stress, which could be visualized under scanning electron microscope.
The results showed that after liquid nitrogen freezing, the integrity either of cold stressed or non-stressed L. rhamnosus B6 cells maintained well, with smooth surfaces and neat edges (Fig. 4a and Fig.4b). On the contrary, for cells of strain KF7 frozen in liquid nitrogen without cold stress ahead, ruptures and surface collapses could be easily observed, as indicated by the arrows (Fig. 4c), whist these damages were greatly decreased in the cells cold stressed ahead (Fig. 4d). Quantitative SEM analysis confirmed cold stress significantly reduced structural damage in KF7 from 69.35 % to 55.32 % (p < 0.05), while B6 maintained minimal damage (<4 %) under both conditions. Compared to cells of strain B6, freezing in liquid nitrogen caused serious damages to those of KF7, no matter of cold stress ahead. This result was consistent with the above-mentioned flow cytometry analysis of the proportion of living cells after cold stress.
Fig. 4.
Cell morphological changes of strains B6 and KF7 were observed by scanning electron microscope after liquid nitrogen freezing. (a) Morphology of strain B6 control (non-stressed) condition. (b) Morphology of strain B6 with cold stress. (c) Morphology of strain KF7 control (non-stressed) condition. (d) Morphology of strain KF7 with cold stress. The arrows indicate cells with ruptures or surface collapses.
Based on the above results, L. rhamnosus B6 showed a superior survival ability in extremely low temperature, indicating strain B6 might resist adverse environment, an important feature required for the strain to be a candidate of starter and intestinal probiotics. Therefore, L. rhamnosus B6 was selected to further explore the underlying antifreeze mechanism.
3.5. Cold stress changed the fatty acid composition in membrane lipids
To explore the mechanism by which cold stress enhances the freeze survival rate of strains, the changes in membrane fatty acid composition after cold stress were analyzed. The cell membrane lipids of L. rhamnosus B6 were mainly composed of seven fatty acids (C12:0, C14:0, C16:0, C16:1, C18:0, C18:1, C19:1), accounting for >80 % of the total fatty acids (Table 1). They were divided into two groups. Saturated fatty acids (SFA) include dodecanoic acid (C12:0), tetradecanoic acid (C14:0), hexadecanoic acid (C16:0) and octadecanoic acid (C18:0). The unsaturated fatty acids (UFA) were represented by hexadecenoic acid (C16:1), octadecenoic acid (C18:1) and trinonadecenoin (C19:1).
Table 1.
Composition of fatty acids in the membrane lipids of the cells of strains B6 with or without 4 °C cold stress treatment.
| Fatty acid | No cold stress | Cold stress |
|---|---|---|
| C12:0 | 10.61±2.91a | 10.56±1.25 a |
| C14:0 | 56.86±6.10 a | 40.61±3.94 b |
| C16:0 | 119.70±3.15 a | 106.73±5.65b |
| C16:1 | 16.46±2.10 a | 28.14±1.80 b |
| C18:0 | 19.49±2.43 a | 21.94±2.92 a |
| C18:1 | 137.76±3.50 a | 172.99±3.11 b |
| C19:1 | 127.03±4.00 a | 89.57±2.45 b |
| Total SFA | 206.66±1.9 a | 179.84±1.77 b |
| Total UFA | 281.25±0.76 a | 290.70±3.91 b |
| UFA/SFA | 1.36±0.12 a | 1.62±0.18 b |
Note: Results of three independent experiments were expressed as mean ± SD (n = 3). Values with different superscripts (a and b) within a row differ significantly at the 0.05 significance level (p < 0.05). SFA: saturated fatty acid; UFA: unsaturated fatty acid. Unit: μg/g.
After cold stress, the content of SFA in the membrane lipids of strain B6 decreased significantly from 206.66 ± 1.9 to 179.84 ± 1.77 μg/g dry cell biomass (p < 0.05), while the UFA increased from 281.25 ± 0.76 to 290.70 ± 3.91 μg/g (p < 0.05) (Table 1). Notably, UFA/SFA ratio increased from 1.36 ± 0.12 to 1.62 ± 0.18 (p < 0.05), indicating that the strain B6 could regulate the composition of fatty acids in the cell membrane lipids in response to cold stress. Among individual fatty acids, C14:0 (56.86 ± 6.10 vs. 40.61 ± 3.94 μg/g, p < 0.05) and C19:1 (127.03 ± 4.00 vs. 89.57 ± 2.45 μg/g, p < 0.05) showed the most pronounced changes. In contrast, C18:1 exhibited a significant increase (137.76 ± 3.50 vs. 172.99 ± 3.11 μg/g, p < 0.05). These results suggested that cold stress triggered a dynamic remodeling of membrane lipids, favoring the accumulation of UFA to enhance membrane fluidity.
3.6. Cold stress leads to proteins differentially expressed in cells of strain B6
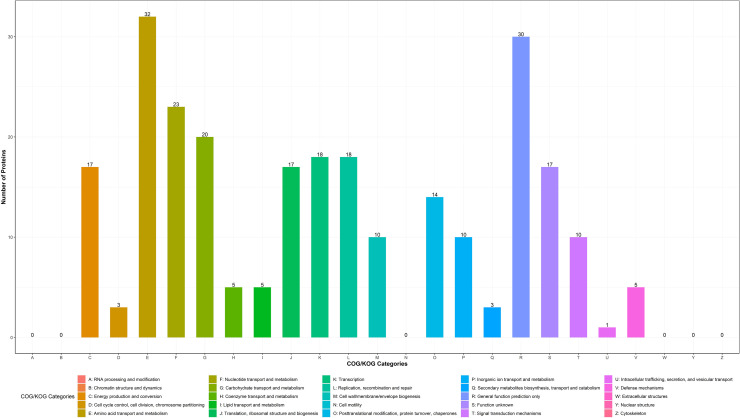
The differentially expressed proteins were screened by statistical method, and the threshold of differentially expressed proteins was FOLD CHANGE>1.5. After cold stress, 325 differentially expressed proteins were identified in cells of L. rhamnosus B6 among which 219 kinds of protein were significantly up regulated. To clarify the possible functional relationship among these 325 proteins, they were categorized into different groups according to their functions. The proteins up-regulated were mainly involved in amino acid transport and metabolism (E), nucleotide transport and metabolism (F), carbohydrate transport and metabolism (G), transcription (K), cell wall/membrane/envelope biogenesis (M) and lipid transport and metabolism (I) (Fig.5). The results showed that the metabolic pathways of B6 cells changed after cold stress treatment.
Fig. 5.
Pathway classification based on GOG/KOG analysis of differentially expressed protein of L. rhamnosus B6 in response to cold stress. The ordinate was the number of differentially expressed genes in this pathway. Proteins with a variation of at least 1.5 folds were selected.
Table2 listed part of the proteins significantly up-regulated including proteins related to transcription, translation and ribosome in the cold stressed cells of strain B6, such as recQ (11.73),rpoD (1.90), rpoB (2.13), dnaB (1.73), rpsT (2.12), rpsZ (1.76), rpsS (1.59) and infB (1.50). Overall, cold stress induced cells of strain B6 preferentially expressed proteins related to cell growth and division. Proteins involved in fatty acid biosynthesis and metabolism, such as LysR (5.68), ACCase (3.40), long-chain fatty acid-CoA ligase (1.59), 2-diacylglycerol 3-glucosyltransferase (1.75), ornithine decarboxylase (4.38) and acetyl-CoA carboxylase biotin carboxylase (3.40) and citrate lyase acyl carrier protein (2.66) were also up regulated. Interestingly, the influence of cold stress on expression of proteins involved in amino acid metabolism and biosynthesis, such as metF (2.65), lysA (2.05) and trpA (178.18) seemed more obvious. In addition, some proteins involved in two-component systems, ABC transporter and carbohydrate metabolism were also upregulated. The results indicated that cold stress might cause a domino effect in L. rhamnosus B6 cells in coping with low temperature, and thus to maintain the vitality of frozen cells.
Table 2.
Differentially expressed proteins in cold stressed cells of L.rhamnosus B6.
| GeneName | Accession | Description | Fold Change |
|---|---|---|---|
| secE | A0A0D6U4V1 | Protein translocase subunit | 1.58 |
| pyrR | A0A0D6UDR4 | Bifunctional protein | 1.76 |
| greA | A0A0E3CRS6 | Transcription elongation factor | 1.77 |
| dnaB | A0A5P5Z8W8 | Replicative DNA helicase | 1.73 |
| recQ | A0A5P5ZB43 | DNA helicase | 11.73 |
| parC | A0A5P5ZBG5 | DNA topoisomerase (ATP-hydrolyzing) | 1.83 |
| rpoD | A0A5P5ZBM9 | RNA polymerase sigma factor | 2.04 |
| cshA | A0A0D6U556 | DEAD-box ATP-dependent RNA helicase | 2.55 |
| rpsT | A0A2A5L9I8 | 30S ribosomal protein S20 | 2.12 |
| F8M46_08210 | A0A5P5ZDD5 | 50S ribosomal protein L11 methyltransferase | 1.92 |
| rpsZ | J7MG19 | 30S ribosomal protein S14 type Z | 1.76 |
| rpsS | J7M420 | 30S ribosomal protein S19 | 1.59 |
| infB | A0A5P5ZCN3 | Translation initiation factor IF-2 | 1.51 |
| rpoB | A0A4Q1TPZ4 | DNA-directed RNA polymerase subunit | 2.13 |
| F8M46_05965 | A0A5P5ZAP6 | DNA/RNA endonuclease | 1.59 |
| uvrC | A0A2A5L9F5 | UvrABC system protein C | 1.82 |
| uvrA | V5XRD4 | UvrABC system protein A | 1.75 |
| F8277_04640 | A0A6H9XZ73 | Long-chain fatty acid–CoA ligase | 3.06 |
| F8M46_13,845 | A0A4Z0GR89 | LysR family transcriptional regulator | 5.69 |
| F8M46_10,995 | A0A5P5ZD75 | Acetyl-CoA carboxylase biotin carboxylase subunit (ACCase) | 3.4 |
| BGK71_03645 | A0A179YCV6 | 1,2-diacylglycerol 3-glucosyltransferase | 1.75 |
| citX | A0A2A5L4E0 | Citrate lyase holo-[acyl-carrier protein] synthase | 17.65 |
| citD | A0A1Z2F3M8 | Citrate lyase acyl carrier protein | 2.66 |
| A0R58_04045 | A0A199Q9Z7 | Ornithine decarboxylase OS=Lactobacillus paraplantarum OX=60,520 GN=A0R58_04045 PE=3 SV=1 | 4.38 |
| F8M46_10,995 | A0A5P5ZD75 | Acetyl-CoA carboxylase biotin carboxylase subunit OS=Lactobacillus rhamnosus OX=47,715 GN=F8M46_10,995 PE=4 SV=1 | 3.40 |
| dltA | A5XEG0 | D-alanine–d-alanyl carrier protein ligase | 3.10 |
| cydA | A0A2A5L7T8 | Cytochrome D ubiquinol oxidase subunit I | 1.63 |
| F8M46_11,160 | A0A0J7A1U4 | Glycine betaine/L-proline ABC transporter ATP-binding protein | 1.66 |
| F8277_05460 | A0A6H9XVW5 | ABC transporter substrate-binding protein | 4.99 |
| F8M46_13,675 | A0A5P5ZEH3 | ABC transporter ATP-binding protein | 3.43 |
| oppB_2 | A0A171J7Q1 | ABC transporter permease | 2.53 |
4. Discussions
When bacteria faced a stress in natural environment, a series of strategies might be adopted to cope with these challenges, including certain anti-freezing pathway to mitigate the extremely low temperature on the damages to cells (Bustos et al., 2024). Unraveling the mechanism of anti-freezing would be helpful for overcome the loss of vitality of important microorganisms during manufacturing process. So far, our knowledge on the response to cold stress of L. rhamnosus is still poor. In the present study, two strains of L. rhamnosus B6 and KF7 with different cell surface features were employed to explore the underlying mechanisms in coping with extremely low temperature to maintain the cell viability, which might provide glimmer on the strategies adopted by LAB during deep freezing.
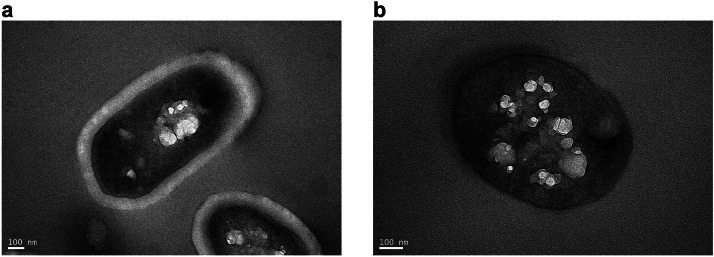
Cells of strain B6 exhibited a stronger resistance to deep freezing compared to those of strain KF7. To explore the intrinsic for the differences in their resistance to freezing, we used transmission electron microscopy to observe their cellular structures. Cells of L. rhamnosus B6 were wrapped with an obvious capsule, while cells of strain KF7 lack of such a capsular envelope (Fig. 6). Notably, SEM analysis further corroborated these structural advantages: B6 cells retained smooth surfaces and intact morphology post-freezing regardless of cold stress treatment (Fig .4a, 4b), whereas KF7 cells exhibited severe ruptures and surface collapses without prior cold adaptation (Fig. 4c). Psychrophilic bacteria usually produce such a capsule structure composed predominantly of exopolysaccharides (EPS) in low temperature niches (Tribelli and Lopez, 2018). The capsule or a thinner slime layer outside the cell may also serve as a cryoprotectant to decrease the freezing point of water and ice nuclei formation below freezing point. Besides, the cell envelope (capsule or slime layer) can lock moisture, nutrients, ions, and avoid of the phagocytosis of immune cells in vivo, promote cell aggregation and the formation of biofilms (Jiang et al., 2018). For example, the capsule of Colwellia psychrerythraea 34H (Vessella et al., 2019) prevented the fixed ice crystal boundary to inhibit ice recrystallization. Moreover, cell envelope could create a relatively favorable microenvironment for the producer in low-temperature conditions, preventing microbial cells from damage caused during the freezing process (Qin et al., 2007). As cells of strain B6 exhibited a superior anti-freezing ability over those of strain KF7, strain B6 was selected to study further for its molecular mechanisms of low-temperature adaptation.
Fig. 6.
The cell structures of L. rhamnosus B6 (a) and KF7 (b) were observed by transmission electron microscope.
Cold stress of cells of stain B6 at 4 °C resulted in 325 proteins differentially expressed, among which 219 proteins were significantly up-regulated. The differentially expressed proteins in cold-stressed cells of strain B6 formed a complex biological network, covering the metabolism and synthesis of fatty acids, amino acids and nucleotide. Thus, the cold resistance mechanism of this strain involved of enhancing the fluidity of cell membranes, maintaining transcription and translation efficiency, and regulating osmotic pressure of cells.
As the main components of cell membrane lipids, fatty acids were very important to maintain the integrity, fluidity and permeability of cell membrane under stress conditions(Gao et al., 2022).Unsaturated fatty acid content was postulated to be important for improving freeze-drying survival rate (E et al., 2020). Increase of unsaturated fatty acid content could improve the fluidity of cell membrane of E.coli, which was the pivotal factor in maintaining the survival rate of bacterial cells in freeze-drying (Scherber et al., 2009). In this study, the unsaturated fatty acid content and UFA/SFA ratio of cell membrane lipids of L. rhamnosus B6 increased significantly after cold stress, which could effectively maintain the integrity and fluidity of cell membrane during freezing. Cold stress could significantly up-regulate the expression of proteins/enzymes related to fatty acid synthesis and desaturation in cells of strain B6. For example, long-chain fatty acid-CoA ligase is the key enzyme that catalyzes the conversion of long-chain free fatty acids into their active acyl-CoA forms. This activation step provides essential substrates for desaturases, thereby facilitating the biosynthesis of UFA (Hao et al., 2022). Additionally, long-chain fatty acid-CoA ligase might also indirectly regulate the fluidity of the cell membrane by affecting the synthesis and metabolism of phospholipids(H. H. Zhang et al., 2024). Therefore, the significant up-regulation of long-chain fatty acid-CoA ligase by gene F8277_04640 was beneficial for the synthesis of UFA and the regulation of cell membrane fluidity. Citrate lyase acyl carrier protein plays a crucial role in fatty acid metabolism. It participated in the initiation and elongation processes of fatty acid synthesis, thereby regulating the composition and fluidity of the cell membrane (Chan and Vogel, 2010). Thus, the upregulation of this protein by 2.66 times indicated its participation in the synthesis of unsaturated fatty acids. Acetyl-CoA carboxylase (ACCase) catalyzes the conversion of acetyl-CoA (acetyl-CoA) to malonyl-CoA (malonyl-CoA), which is the first key rate-limiting step in the de novo synthesis of fatty acids (FAS) (Cronan, 2021). This step is strictly regulated, but the regulatory factors that specifically control the transcription of ACCase genes remain elusive (Xu et al., 2024).The quantity and activity of ACCase directly affect the lipid homeostasis and a series of physiological functions of cells. Simultaneously, AccR, an LysR-type transcriptional regulator, could respond directly to acetyl-CoA levels and specifically activate the expression of the ACCase gene thereby promote the synthesis of downstream fatty acids (Xu et al., 2024). In this study we found that the LysR family transcriptional regulator encoded by the E4665_02625 gene and the ACCase encoded by the F8M46_10,995 gene exhibited upregulated expression (Table 2). Therefore, we speculate that the LysR identified in this study may behave as a transcriptional regulator of ACCase. This could broaden our knowledge of the regulation involved in de novo fatty acid synthesis. In addition, 1,2-diacylglycerol 3-glucosyltransferase was mainly responsible for synthesizing α-monoglucosyl diacylglycerol, which was irreversiblyc combined to anionic bilayer structures (Li et al., 2003). This lipid is essential for the characteristics of bacterial cell membranes and the surface charge density of lipids. Studies have shown that ACCase could regulate the composition and properties of the cell membrane, assisting bacteria in adapting to extreme environmental conditions (Li et al., 2003). Based on these results, these genes may facilitate the cells of strain B6 to combat the stress in low-temperature environments by promoting fatty acid synthesis and regulating the flexibility of the cell membrane.
Low temperatures may lead to the instability of secondary structures in RNA and DNA, thereby causing a decrease in the efficiency of translation and transcription (Mangiagalli et al., 2018). Therefore, maintaining appropriate and necessary transcription, translation, and ribosomal processes in bacteria under low-temperature conditions is crucial for keeping the cell alive. After cold stress, the expression of a scaffold of proteins involved in the aforementioned processes in cold stressed cells of strain B6 were significantly upregulated, including several ribosomal proteins, ribosome biogenesis GTPase A, translation initiation factor IF-2, DNA replication related protein (rpoD, rpoB, recQ) and RNA helicase CshA. The proteins encoded by the genes recQ, rpoD, rpoB, and infB played crucial roles in the processes of DNA unwinding, transcription, replication, and translation (Park et al., 2024; Silva et al., 2024). RecQ helicase is a molecular engine, which plays a vital role in all organisms (Pike et al., 2015), maintains genome integrity through DNA replication, (Gupta and Schmidt, 2020) recombination and repair, and makes significant contributions to transcription (Dutta et al., 2024). The expression level of RecQ helicase is 11 folds higher than that of the non-cold-stressed state, indicating its significant role in maintaining active translation under low-temperature conditions. CshA is a multifunctional ATP-dependent enzyme (Derunets et al., 2024). In another study, CshA could bind to nucleic acids and restore their secondary structures, which was a common feature of cold growth in many cold-adapted bacteria (Duru et al., 2021). Ribosomal protein subunits participate in the assembly of the ribosome, thus playing an important role in maintaining an active translation process under low-temperature conditions. Studies have found that initiation factor IF2 also plays a crucial role in the assembly and maturation of ribosomes in cold-shocked cells. For example, in Escherichia coli, cold stress triggered post-transcriptional events of the translation initiation factor IF2 and increased the expression levels of IF2 (Brandi et al., 2019). In addition, bacteria may suffer from DNA damage after cold stress and need DNA repair. The proteins UvrA and UvrD in bacterial nucleotide excision repair system increased significantly after B6 cold stress (Table 2). These repair proteins could identify and remove a wide range of DNA damages with different structures and properties (Chen et al., 2023). After cold stress, the significant upregulation of the aforementioned ribosomal, transcriptional, and translational proteins ultimately made strain B6adapt and maintain translation at low temperatures, thus sustaining cellular growth and metabolic activities.
Some proteins potentially involved in intercellular or intracellular signaling systems showed increased abundance after cold stress, including two-component systems (TCS) and ATP-binding cassette (ABC) transporters. The TCS system would be activated upon exposure to environmental signals such as nutrient deprivation, temperature changes, and oxidative stress (Steele et al., 2012). Once activated, it modulated various cellular processes, including cell membrane surface modifications, changes in permeability, and biofilm formation, thereby enhancing the bacteria's resistance to adverse conditions (Alvarez and Georgellis, 2023). After cold stress, the upregulation of enzymes and proteins associated with the TCS system (such as dltA, cydA, citX, citD) might also contribute to the enhanced the cryotolerance of the strain B6. ABC transporters, responsible for transporting inorganic salt ions, sugars, amino acids, and metal ions, played important roles in microbial stress resistance (Wang et al., 2024). The expression of ABC transporters in L. plantarum K25 was upregulated after cold stress, which could protect cellular machinery from oxidative damage (Liu et al., 2020). Similarly, the expression of ABC transporter permease (F8M46_10,370), ABC transporter substrate-binding protein (F8277_05460) and ABC transporter ATP-binding protein (F8M46_13,675) were up-regulated (Table 2) in cold stressed cells of strain B6, which might presumably contribute to the cold resistance of strain B6. Additionally, the gene F8M46_12,340 encoded a transmembrane transport protein that could export threonine/serine from within the cell to the outside (Gao et al., 2021). These two hydroxylated amino acids can act as compatible solutes, reducing the freezing point around the cells during the freezing process. Overall, the enhancement of these transport systems was beneficial for bacteria to regulate the osmotic pressure caused by temperature changes, and thus overcome the deficiency of low diffusion rates at low temperatures, and ensured effective nutrient absorption.
The expression of MetF in cold stressed cell of strain B6, a protein involved in the biosynthesis of methionine, was 2.6 folds higher than that of the non-cold-stressed cells. Methionine is mainly the precursor of cell methyl donor (S-methionine adenosine). Our result regarding the enhanced expression of MetF in cold stressed cells of strain B6 was in consistent with the increase of methyltransferases in a broad of psychrophiles living in low temperature environments (Raymond-Bouchard et al., 2018). The expression of diaminopimelate decarboxylase (lysA), an enzyme involved in the synthesis of lysine in cold stressed cells of strain B6 was significantly increased. Lysine, essential for the biosynthesis of peptidoglycan, helps to ensure the integrity of the cell wall (Ayu Eka Pitaloka et al., 2023). Furthermore, the expression of Tryptophan synthase (trpA) increased by 177 folds (Table 2). Tryptophan is a basic component of proteins and various secondary metabolites. The upregulation of tryptophan synthesis contributed to the enhanced resistance of Saccharomyces cerevisiae to SDS (Schroeder and Ikui, 2019).
In summary, under cold stress, the remodeling of membrane lipids and upregulated expression of a scaffold of proteins correlated to fatty acid metabolism altered the fluidity of the cell membrane of strain B6, thereby improving their adaptability to low-temperature environments. At the same time, the up-regulation of ribosomal protein, helicase and translation initiation factor improved the efficacy of transcription and translation, which could repair the DNA/RNA damages, facilitate the synthesize protein to resist cold stress and maintain cell vitality.
5. Conclusion
Cold stress enhanced the frozen survival rate of L. rhamnosus B6 and KF7 frozen in liquid nitrogen, while strain B6 exhibited a superior anti-freezing ability over strain KF7. Cold stress improved the fluidity of the cell membrane of strain B6, as reflected by the elevated UFA/SFA as well as the content of UFA in cell membrane lipids. A total of 325 proteins were differentially expressed in cold stressed cells of strain B6, with 219 proteins significantly up-regulated. Therefore, it was inferred that L. rhamnosus B6 primarily utilized a cold adaptation strategy by regulating the composition of membrane lipids via the differential expression of proteins correlated to fatty acid metabolism and DNA/RNA damage repairing. However, the role of capsule of the cells of strain B6 should be further verified by mutants knocking off the core genes involved in EPS synthesis and comparison the difference of freezing resistance between the mutant deficient in capsule formation and the wild strain.
CRediT authorship contribution statement
Ting Yang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Funding acquisition. Jia Zhang: Investigation, Validation, Formal analysis. Ranjin Zhang: Validation, Methodology, Formal analysis. Juan Zhang: Resources, Supervision, Project administration. Zhenmin Liu: Resources, Visualization. Zhengjun Wu: Resources, Supervision, Project administration, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2022YFD2100704) and the Enterprise Innovation Development and Energy Upgrading Project of Municipal State-owned Assets Supervision and Administration Commission (2022013).
Data availability
Data will be made available on request.
References
- Abedin M.M., Chourasia R., Phukon L.C., Sarkar P., Ray R.C., Singh S.P., Rai A.K. Lactic acid bacteria in the functional food industry: biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2024;64(29):10730–10748. doi: 10.1080/10408398.2023.2227896. [DOI] [PubMed] [Google Scholar]
- Alvarez A.F., Georgellis D. Environmental adaptation and diversification of bacterial two-component systems. Curr. Opin. Microbiol. 2023;76 doi: 10.1016/j.mib.2023.102399. [DOI] [PubMed] [Google Scholar]
- Ayu Eka Pitaloka D., Izzati A., Rafa Amirah S., Abdan Syakuran L., Muhammad Irham L., Darumas Putri A., Adikusuma W. Bioinformatics analysis to uncover the potential drug targets responsible for mycobacterium tuberculosis peptidoglycan and lysine biosynthesis. Bioinform. Biol. Insights. 2023;17 doi: 10.1177/11779322231171774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi A., Giangrossi M., Paoloni S., Spurio R., Giuliodori A.M., Pon C.L., Gualerzi C.O. Transcriptional and post-transcriptional events trigger de novo infB expression in cold stressed Escherichia coli. Nucleic. Acids. Res. 2019;47(9):4638–4651. doi: 10.1093/nar/gkz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos A.Y., Taranto M.P., Gerez C.L., Agriopoulou S., Smaoui S., Varzakas T., Enshasy H.A.E. Recent advances in the understanding of stress resistance mechanisms in probiotics: relevance for the design of functional food systems. Probiotics. Antimicrob. Proteins. 2024 doi: 10.1007/s12602-024-10273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.I., Vogel H.J. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 2010;430(1):1–19. doi: 10.1042/BJ20100462. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu R., Li S., Wu M., Yu H., Ge Q. Metabolism of hydrogen peroxide by Lactobacillus plantarum NJAU-01: a proteomics study. Food Microbiol. 2023;112 doi: 10.1016/j.fm.2023.104246. [DOI] [PubMed] [Google Scholar]
- Chourasia R., Abedin M.M., Chiring Phukon L., Sahoo D., Singh S.P., Rai A.K. Biotechnological approaches for the production of designer cheese with improved functionality. Compr. Rev. Food Sci. Food Saf. 2021;20(1):960–979. doi: 10.1111/1541-4337.12680. [DOI] [PubMed] [Google Scholar]
- Cronan J.E. The classical, yet controversial, first enzyme of lipid synthesis: escherichia coli acetyl-CoA carboxylase. Microbiol. Mol. Biol. Rev. 2021;85(3) doi: 10.1128/MMBR.00032-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derunets A.S., Selimzyanova A.I., Rykov S.V., Kuznetsov A.E., Berezina O.V. Strategies to enhance stress tolerance in lactic acid bacteria across diverse stress conditions. World J. Microbiol. Biotechnol. 2024;40(4):126. doi: 10.1007/s11274-024-03905-3. [DOI] [PubMed] [Google Scholar]
- Duru I.C., Ylinen A., Belanov S., Pulido A.A., Paulin L., Auvinen P. Transcriptomic time-series analysis of cold- and heat-shock response in psychrotrophic lactic acid bacteria. BMC Genom. 2021;22(1):28. doi: 10.1186/s12864-020-07338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A.K., Hossain M.F., Sultana M.M., Hachiya T., Nakagawa T. Expression patterns of Arabidopsis thaliana RecQ-like (AtRecQl) genes and the roles of AtRecQl2 and AtRecQl3 in response to abiotic stress. Biosci. Biotechnol. Biochem. 2024 doi: 10.1093/bbb/zbae136. [DOI] [PubMed] [Google Scholar]
- E J.J., Ma L.L., Chen Z.C., Ma R.Z., Zhang Q.L., Sun R.Y., He Z.B., Wang J.G. Effects of buffer salts on the freeze-drying survival rate of Lactobacillus plantarum LIP-1 based on transcriptome and proteome analyses. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126849. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Fonseca F., Meneghel J., Cenard S., Passot S., Morris G.J. Determination of intracellular vitrification temperatures for unicellular micro organisms under conditions relevant for cryopreservation. PLoS. One. 2016;11(4) doi: 10.1371/journal.pone.0152939. ARTN e0152939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Lv Q., Mao R., Sun Y., Chen Y., Qiu Y., Chang Q., Wang C. Integrative transcriptomics and proteomics analyses to reveal the developmental regulation of metorchis orientalis: a neglected trematode with potential carcinogenic implications. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.783662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Kong J., Zhu H., Mao B., Cui S., Zhao J.Lactobacillus. Bifidobacterium and lactococcus response to environmental stress: mechanisms and application of cross-protection to improve resistance against freeze-drying. J. Appl. Microbiol. 2022;132(2):802–821. doi: 10.1111/jam.15251. [DOI] [PubMed] [Google Scholar]
- Gautier J., Passot S., Penicaud C., Guillemin H., Cenard S., Lieben P., Fonseca F. A low membrane lipid phase transition temperature is associated with a high cryotolerance of Lactobacillus delbrueckii subspecies bulgaricus CFL1. J. Dairy. Sci. 2013;96(9):5591–5602. doi: 10.3168/jds.2013-6802. [DOI] [PubMed] [Google Scholar]
- Gupta S.V., Schmidt K.H. Maintenance of yeast genome integrity by RecQ Family DNA helicases. Genes. (Basel) 2020;11(2) doi: 10.3390/genes11020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Xia W., Wang D., Wang Y., Liu Z., Wu Z. Characterization of an exopolysaccharide synthesized by lacticaseibacillus rhamnosus B6 and its immunomodulatory activity in vitro. Int. J. Biol. Macromol. 2024;264(Pt 1) doi: 10.1016/j.ijbiomac.2024.130576. [DOI] [PubMed] [Google Scholar]
- Hao X.H., Chen W.C., Amato A., Jouhet J., Maréchal E., Moog D., Hu H.H., Jin H., You L.J., Huang F.H., Moosburner M., Allen A.E., Gong Y.M. Multiplexed CRISPR/Cas9 editing of the long-chain acyl-CoA synthetase family in the diatom reveals that mitochondrial ptACSL3 is involved in the synthesis of storage lipids. New. Phytol. 2022;233(4):1797–1812. doi: 10.1111/nph.17911. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang J., Zhao X., Zhao W., Yu Z., Chen C., Yang Z. Complete genome sequencing of exopolysaccharide-producing Lactobacillus plantarum K25 provides genetic evidence for the probiotic functionality and cold endurance capacity of the strain. Biosci. Biotechnol. Biochem. 2018;82(7):1225–1233. doi: 10.1080/09168451.2018.1453293. [DOI] [PubMed] [Google Scholar]
- Lebeer S., Claes I., Tytgat H.L., Verhoeven T.L., Marien E., von Ossowski I., Reunanen J., Palva A., Vos W.M., Keersmaecker S.C., Vanderleyden J. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Env. Microbiol. 2012;78(1):185–193. doi: 10.1128/AEM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwendaal N.K., Stanton C., O'Toole P.W., Beresford T.P. Fermented foods, health and the gut microbiome. Nutrients. 2022;14(7) doi: 10.3390/nu14071527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Storm P., Karlsson O.P., Berg S., Wieslander A. Irreversible binding and activity control of the 1,2-diacylglycerol 3-glucosyltransferase from Acholeplasma laidlawii at an anionic lipid bilayer surface. Biochemistry. 2003;42(32):9677–9686. doi: 10.1021/bi034360l. [DOI] [PubMed] [Google Scholar]
- Liu S., Ma Y., Zheng Y., Zhao W., Zhao X., Luo T., Zhang J., Yang Z. Cold-stress response of probiotic Lactobacillus plantarum K25 by iTRAQ Proteomic analysis. J. Microbiol. Biotechnol. 2020;30(2):187–195. doi: 10.4014/jmb.1909.09021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiagalli M., Sarusi G., Kaleda A., Bar Dolev M., Nardone V., Vena V.F., Braslavsky I., Lotti M., Nardini M. Structure of a bacterial ice binding protein with two faces of interaction with ice. FEBS. J. 2018;285(9):1653–1666. doi: 10.1111/febs.14434. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Jang M., Lee S.M., Woo J., Lee E.J., Kim D. Unveiling the novel regulatory roles of RpoD-family sigma factors in Salmonella typhimurium heat shock response through systems biology approaches. PLoS. Genet. 2024;20(10) doi: 10.1371/journal.pgen.1011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova M.I., Reid G., Ter Haar J.A. Lacticaseibacillus rhamnosus GR-1, a.k.a. Lactobacillus rhamnosus GR-1: past and future perspectives. Trends. Microbiol. 2021;29(8):747–761. doi: 10.1016/j.tim.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Pike A.C., Gomathinayagam S., Swuec P., Berti M., Zhang Y., Schnecke C., Marino F., von Delft F., Renault L., Costa A., Gileadi O., Vindigni A. Human RECQ1 helicase-driven DNA unwinding, annealing, and branch migration: insights from DNA complex structures. Proc. Natl. Acad. Sci. U S A. 2015;112(14):4286–4291. doi: 10.1073/pnas.1417594112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Zhu L., Chen X., Wang P.G., Zhang Y. Structural characterization and ecological roles of a novel exopolysaccharide from the deep-sea psychrotolerant bacterium pseudoalteromonas sp. SM9913. Microbiology. 2007;153(Pt 5):1566–1572. doi: 10.1099/mic.0.2006/003327-0. [DOI] [PubMed] [Google Scholar]
- Raymond-Bouchard I., Tremblay J., Altshuler I., Greer C.W., Whyte L.G. Comparative transcriptomics of cold growth and adaptive features of a eury- and steno-psychrophile. Front. Microbiol. 2018;9:1565. doi: 10.3389/fmicb.2018.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherber C.M., Schottel J.L., Aksan A. Membrane phase behavior of Escherichia coli during desiccation, rehydration, and growth recovery. Biochim. Biophys. Acta. 2009;1788(11):2427–2435. doi: 10.1016/j.bbamem.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Schroeder L., Ikui A.E. Tryptophan confers resistance to SDS-associated cell membrane stress in Saccharomyces cerevisiae. PLoS. One. 2019;14(3) doi: 10.1371/journal.pone.0199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Campos M.R., Ciau-Solís N., Rosado-Rubio G., Chel-Guerrero L., Betancur-Ancona D. Physicochemical characterization of chia (Salvia hispanica) seed oil from Yucatán. Méx. Agric. Sci. 2014;05(03):220–226. doi: 10.4236/as.2014.53025. [DOI] [Google Scholar]
- Shao L., Wu Z., Zhang H., Chen W., Ai L., Guo B. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014;107:51–56. doi: 10.1016/j.carbpol.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Silva J.P.L., Donaires F.S., Gutierrez-Rodrigues F., Martins D.J., Carvalho V.S., Santana B.A., Cunha R.L.G., Kajigaya S., Menck C.F.M., Young N.S., Kjeldsen E., Calado R.T. RecQ helicase expression in patients with telomeropathies. Mol. Biol. Rep. 2024;51(1):754. doi: 10.1007/s11033-024-09678-0. [DOI] [PubMed] [Google Scholar]
- Song S., Bae D.W., Lim K., Griffiths M.W., Oh S. Cold stress improves the ability of Lactobacillus plantarum L67 to survive freezing. Int. J. Food Microbiol. 2014;191:135–143. doi: 10.1016/j.ijfoodmicro.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Steele K.H., O'Connor L.H., Burpo N., Kohler K., Johnston J.W. Characterization of a ferrous iron-responsive two-component system in nontypeable Haemophilus influenzae. J. Bacteriol. 2012;194(22):6162–6173. doi: 10.1128/JB.01465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribelli P.M., Lopez N.I. Reporting key features in cold-adapted bacteria. Life (Basel) 2018;8(1) doi: 10.3390/life8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessella G., Casillo A., Fabozzi A., Traboni S., Iadonisi A., Corsaro M.M., Bedini E. Synthesis of the tetrasaccharide repeating unit of the cryoprotectant capsular polysaccharide from Colwellia psychrerythraea 34H. Org. Biomol. Chem. 2019;17(12):3129–3140. doi: 10.1039/c9ob00104b. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Yan J., Xie J. Coculture of Acinetobacter johnsonii and Shewanella putrefaciens contributes to the ABC transporter that impacts cold adaption in the aquatic food storage environment. J. Agric. Food Chem. 2024;72(18):10605–10615. doi: 10.1021/acs.jafc.4c00885. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lin Z., Hou J., Ye K., Han S., Liang Y., Liang H., Wu S., Tao Y.J., Gao H. A bacterial transcription activator dedicated to the expression of the enzyme catalyzing the first committed step in fatty acid biosynthesis. Nucleic. Acids. Res. 2024 doi: 10.1093/nar/gkae960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhang Y., Zhao Y., Lv K., Ai L., Wu Z., Song Z., Zhang J. Corrigendum to "probiotic bacterial adsorption coupled with CRISPR/Cas12a system for mercury (II) ions detection" [Biosens. Bioelectron. 263 (2024) 116627] Biosens. Bioelectron. 2025;267 doi: 10.1016/j.bios.2024.116831. [DOI] [PubMed] [Google Scholar]
- Zhang H., Sun Q., Dong H., Jin Z., Li M., Jin S., Zeng X., Fan J., Kong Y. Long-chain acyl-CoA synthetase-4 regulates endometrial decidualization through a fatty acid beta-oxidation pathway rather than lipid droplet accumulation. Mol. Metab. 2024;84 doi: 10.1016/j.molmet.2024.101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zheng Y., Liu Z., Su M., Wu Z., Xu X. Integrated analysis of characteristic volatile flavor formation mechanisms in probiotic co-fermented cheese by untargeted metabolomics and sensory predictive modeling. Food Res. Int. 2025;211 doi: 10.1016/j.foodres.2025.116379. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zheng Y., Liu Z., Su M., Wu Z., Zhang H., Zhang C., Xu X. Insights into characteristic metabolites and potential bioactive peptides profiles of fresh cheese fermented with three novel probiotics based metabolomics and peptidomics. Food Chem. X. 2024;21 doi: 10.1016/j.fochx.2024.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.