Abstract
Tumor-infiltrating regulatory T cells (TI-Tregs) are characterized by their abnormal accumulation and heightened immunosuppressive activity. However, the biomechanical mechanisms that govern Treg identity and function through extracellular matrix (ECM) properties remain poorly understood. In three-dimensional culture systems and the tumor microenvironment (TME), increased matrix stiffness and viscoelasticity have been shown to promote Treg differentiation and expansion. Structural remodeling of the ECM, particularly the realignment of collagen fibers and the reduction in effective pore size, significantly enhances Treg migration. Moreover, biomechanical signals derived from the ECM strengthen the oxidative phosphorylation (OXPHOS) metabolic phenotype and immunosuppressive function of Tregs by modulating mitochondrial dynamics. This review provides a comprehensive analysis of the molecular events through which ECM mechanical properties—such as stiffness, viscoelasticity, and topological structure—regulate Treg identity and functionality, as well as the mechanical sensing and response mechanisms employed by Tregs. The potential for targeting Treg mechanosensors and mechanotransduction pathways to develop mechano-immunomodulatory strategies for cancer therapy is also discussed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-025-02380-z.
Keywords: Regulatory T cells, Extracellular matrix, Biomechanics, Stiffness, Viscoelasticity
Introduction
Tumors are complex entities, with their physical properties directly influencing growth and treatment outcomes. The tumor microenvironment (TME) functions as a sophisticated ecosystem composed of tumor cells, mesenchymal cells, immune cells, the extracellular matrix (ECM), and other constituents, all of which play pivotal roles in tumor progression [1]. The ECM is predominantly made up of structural proteins such as collagen and elastin, adhesive proteins including laminin and fibronectin (FN), and glycosaminoglycans like hyaluronic acid (HA) [2]. As a fundamental component of the TME, the ECM provides not only structural scaffolding and mechanical support but also modulates immune cell activities—proliferation, migration, differentiation, and survival—through the integration of biochemical and biomechanical signals [3].
Regulatory T cells (Tregs), a subset of CD4+ T cells defined by the expression of Foxp3, are categorized into thymus-derived natural Tregs (nTregs) and peripherally induced adaptive Tregs (iTregs) or peripheral Tregs (pTregs) based on their developmental origin [4, 5]. Acting as the immune system’s “brakes,” Tregs are essential for immune homeostasis and preventing autoimmune reactions. However, within the TME, the abnormal accumulation and functional enhancement of iTregs inhibit effector T cell activation and proliferation, thereby undermining anti-tumor immunity and fostering an immunosuppressive TME (ITME) [6, 7]. This phenomenon is a significant contributor to the failure of various cancer immunotherapies.
Recent research has revealed that the biomechanical properties of the ECM can directly or indirectly hinder immune cell migration and activation, promoting the establishment of an ITME that plays a critical role in the failure of immune checkpoint inhibitors (ICIs) [8, 9]. While the biochemical signaling of the ECM is crucial in modulating Treg function and tumor infiltration, these aspects have been extensively covered in other studies and are beyond the scope of this review [10, 11]. Increasing evidence emphasizes the centrality of biomechanical cues in driving the immunosuppressive adaptation of Tregs within the TME. This review aims to consolidate the regulatory effects of ECM mechanical properties on Tregs, with a focus on how biomechanical characteristics such as matrix stiffness, viscoelasticity, and topological structure influence Treg differentiation, proliferation, infiltration, migration, and metabolic reprogramming, while elucidating the underlying mechanisms. Moreover, the potential for targeting Treg mechanosensors and mechanotransduction pathways to develop mechano-immunomodulatory strategies for tumor therapy is explored.
The biomechanical properties of ECM and the immune suppressive microenvironment
The biomechanical properties of tumor ECM
The ECM, once considered a passive structural scaffold, is now acknowledged as a dynamic, non-cellular element within the complex TME, serving as a “mechanical signaling hub“ [12]. As the primary source of mechanical signals in tumor-associated ECM, its biomechanical properties are central to tumor progression, and this review highlights the key biomechanical characteristics of tumor-associated ECM along with its remodeling mechanisms.
Alterations in the stiffness of the tumor ECM
Stiffness, a key parameter defining a material’s resistance to deformation under external forces, quantifies its elastic properties, typically through the Elastic Modulus (Young’s modulus) [13]. Techniques like atomic force microscopy (AFM), nanoindentation, ultrasound elastography, magnetic resonance elastography (MRE), and optical coherence tomography elastography (OCT-E) provide multi-level, cross-scale stiffness measurements across molecular, cellular, and tissue levels [14, 15].
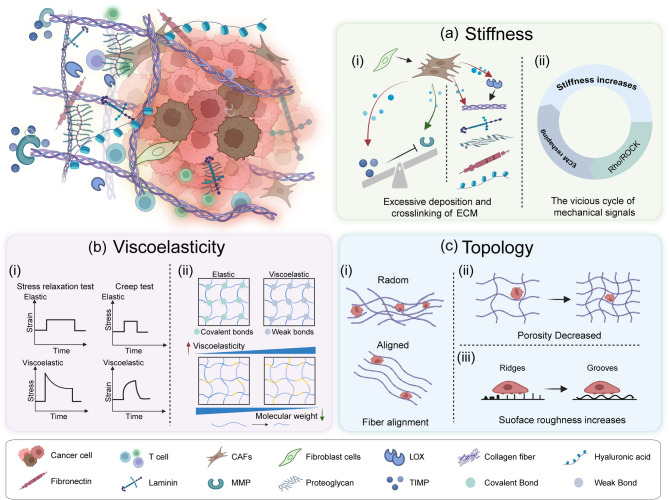
During glioblastoma (GBM) progression, stiffness and fluidity decrease [16], but the stiffness of tumor ECM is typically 8 to 10 times greater than that of corresponding healthy tissue [17]. This increase in matrix stiffness is primarily driven by augmented collagen synthesis and pathological cross-linking patterns [18, 19]. ECM components, including laminin [20], FN [21], and HA [22], also significantly contribute to ECM stiffening. Notably, cancer-associated fibroblasts (CAFs) play a pivotal role in increasing ECM stiffness (Fig. 1. a.i.). In healthy tissue, fibroblasts maintain ECM remodeling homeostasis by balancing matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [23]. However, aberrant CAF activation disrupts this balance within the TME. CAF-derived TIMP-1 inhibits MMP-mediated ECM degradation, fostering pathological matrix accumulation [24]. Simultaneously, CAFs overproduce collagen and FN, not only enhancing ECM deposition but also promoting abnormal collagen cross-linking and increased matrix rigidity via upregulation of lysyl oxidase (LOX) family enzymes [25]. Tumor cell proliferation and invasion further compact the ECM and exacerbate deposition, thus intensifying matrix rigidity [26]. Additionally, therapeutic interventions like chemotherapy and radiation therapy stimulate CAFs to secrete more ECM components, further contributing to matrix stiffening [27, 28].
Fig. 1.
The biomechanical properties of tumor ECM. a Stiffness: Aberrant CAF activation disrupts the balance between MMPs and TIMPs (i). The vicious cycle of mechanical signals critically drives the increase in matrix stiffness within the TME (ii). b Viscoelasticity: The two hallmark phenomena of viscoelastic materials: stress relaxation test, creep test (i). The viscoelasticity of biomaterials can be modified by crosslinking strength and polymer chain molecular weight(ii). c Topology, also referred to as matrix structure: Anisotropic alignment of collagen fibers (i). Reduced effective porosity (ii). Increased surface roughness (iii). CAFs, Cancer-associated fibroblasts; MMPs, Matrix metalloproteinases; TIMPs, Tissue inhibitors of metalloproteinases; LOX, Lysyl oxidase. Figures created with BioRender.com
The vicious cycle of mechanical signals is a pivotal mechanism driving the increased stiffness of the matrix within the TME. Rho-associated coiled-coil kinase (ROCK), a crucial mechanical sensor, detects changes in ECM stiffness through the integrin-focal adhesion complex. It then initiates a positive feedback loop, stimulating the synthesis of collagen, FN, and periostin, which further amplifies matrix stiffness [8, 29]. This creates a self-reinforcing cycle of “stiffness increase - ROCK signaling activation - exacerbated matrix remodeling.“(Fig. 1. a. ii) ECM prestress refers to the pre-existing tensional equilibrium within neoplastic tissues, arising from cellular contractility or external mechanical loading, which induces fiber network deformation through tensile or compressive strain [30]. Notably, prestress elevates ECM rigidity via direct mechanical reinforcement while orchestrating force-dependent fibrillar collagen alignment and enzymatic crosslinking, driving pathological matrix remodeling [30].
The rigid ECM influences malignant tumor cell phenotypes through various mechanotransduction pathways (Supplementary Table 1). A 2D in vitro study demonstrated that increased matrix stiffness enhances aerobic glycolysis in hepatocellular carcinoma (HCC) cells via the JNK/p38 pathway, thereby promoting migration [31]. Similarly, Wang et al. showed in 2D studies that heightened matrix stiffness activates the integrin β1/Plectin/F-actin axis, facilitating F-actin polymerization and accelerating HCC cell migration, with clinical validation in independent TCGA cohorts [32]. In addition to 2D studies, Machesky et al. further demonstrated that increased matrix stiffness accelerates pancreatic cancer (PC) invasion in 3D microenvironments, a process linked to the activation of the phosphocreatine-creatine kinase (PCr-CK) metabolic pathway [33]. However, Chang et al. found that within 3D basement membrane (BM) matrices, increased stiffness (ranging from 0.4 to 9.3 kPa) physically restricts invadopodia extension and reduces their lifespan, thereby inhibiting breast cancer cell migration [34]. These contrasting results underscore the complex role of matrix stiffness in cancer progression in vitro. Moreover, increased matrix stiffness sustains cancer stem cell (CSC) stemness and contributes to chemoresistance. For example, in a 3D environment, Xu et al. showed that a matrix stiffness of 45 kPa promotes breast cancer cell stemness through the integrin-cytoskeleton-AIRE axis [35]. A study using scanning electrochemical microscopy (SECM) revealed that ECM stiffness fosters drug resistance in breast cancer cells by enhancing the functional activity of multidrug resistance protein 1 (MRP1) [36]. Zhao et al. transplanted breast cancer cells into mouse mammary fat pads using hyaluronan-derived hydrogels of varying stiffness (0.5 vs. 9 kPa). Their findings demonstrated that stiffer niches facilitate TAZ nuclear translocation, maintaining CSC properties and chemoresistance [37]. Additionally, a study simulating tumor stiffness gradients using a fibrin gel system (0.5–5 mg/mL) showed that embedding metastasis-associated fibroblasts (MAFs) in the gel doubled endothelial cell sprouting length and increased sprout number by 1.8-fold. Targeting the renin-angiotensin system (RAS) reversed this matrix-mediated resistance to anti-angiogenic therapy [38].
While increased matrix stiffness is generally associated with poor prognosis across various cancers, relatively softer matrices in specific tumor types or disease stages may also promote invasion and metastasis [39]. For example, in breast cancer, ECM stiffness exhibits a gradient from the tumor core to the periphery, with the core region being relatively soft (< 2 kPa) and the invasive front reaching up to 20 kPa [40]. This gradient highlights the need for developing individualized mechano-targeted therapies based on tumor type and disease stage.
Biophysical cues of viscoelasticity in tumor ECM
Viscoelasticity describes the mechanical property of materials that exhibit both the instantaneous deformation typical of elastic solids and the time-dependent responses characteristic of viscous fluids [41]. This behavior is defined by two key phenomena: under constant strain, stress progressively decays over time (stress relaxation test), while under constant stress, strain develops gradually (creep test) (Fig. 1. b. i) [42]. The storage modulus (G’) represents the elastic component of viscoelastic materials, while the loss modulus (G’’) quantifies the viscous component [41].
Viscoelasticity has emerged as a crucial biomechanical feature of the tumor ECM. Tumor ECM demonstrates notably shorter stress relaxation times compared to healthy or physiological tissues [43]. For instance, Fan et al. employed AFM and rheometry on human liver specimens and found that, compared to healthy controls, patients with T2DM exhibited a marked increase in ECM viscoelasticity, characterized by a larger hysteresis area, higher loss tangent (tan δ), and a shortened stress relaxation half-time (τ1/2), independent of matrix stiffness. Through in vitro 3D hydrogel models, they further showed that AGE-mediated viscoelastic enhancement promotes HCC cell proliferation and invasion, accelerating tumor progression in pre-cirrhotic livers [44]. Tumor-associated remodeling of ECM viscoelasticity results from the coordinated regulation of ECM components. Collagen fibers, as the primary structural component of the ECM, influence its viscoelastic properties through their density, cross-linking, and spatial arrangement [45]. For example, in type 2 diabetes-associated nonalcoholic steatohepatitis (NASH) models, advanced glycation end products (AGEs) significantly enhance viscous dissipation and accelerate stress relaxation by shortening collagen fiber length and reducing network connectivity [44]. Additionally, hypoxia, induced by increased ECM stiffness, activates the reactive oxygen species (ROS) signaling pathway, which upregulates MMP-1 expression, promotes collagen degradation, and facilitates cell cluster formation, all of which contribute to ECM viscoelasticity regulation [46]. HA, another key ECM component, exhibits molecular weight-dependent bidirectional regulatory effects on viscoelastic properties. High-molecular-weight HA strengthens matrix viscosity through enhanced hydration interactions, while low-molecular-weight HA increases matrix plasticity by promoting network fluidity [47]. Notably, ECM viscoelasticity shows significant heterogeneity across different tumor types and even within distinct regions of the same tumor. Rubiano et al. quantified the viscoelastic properties of resected human pancreatic tissues using a custom cantilever-based indentation system. By fitting stress relaxation curves to the Standard Linear Solid (SLS) model, they extracted key parameters including the steady-state modulus (SSM), viscosity (η), and characteristic relaxation time (τ). Their analysis revealed that pancreatic ductal adenocarcinoma (PDAC) tumors exhibited elevated SSM and η, while chronic pancreatitis tissues, despite increased stiffness, showed significantly reduced viscosity [48]. Moreover, Onwudiwe et al., simulating brain physiological shear stress in a 2D environment, found no significant differences in viscoelastic properties (stiffness, viscosity, relaxation time) between normal human astrocytes (IHAs) and GBM cells [49]. The notable viscoelasticity and regional heterogeneity of tissues underscore the potential of viscoelasticity as a tissue-specific pathomechanical biomarker and therapeutic target.
Numerous static substrates and dynamic bioreactors have been developed to precisely tune the viscoelasticity or stiffness of materials, allowing the investigation of their impact on tumor cell biological behaviors (Supplementary Table 1). The viscoelasticity of biomaterials can be modulated by factors such as the type of molecular interactions (ion bonding, hydrogen bonding, hydrophobic interactions, and supramolecular interactions), crosslinking strength, and polymer chain molecular weight (Fig. 1. b. ii) [50, 51]. Current research indicates that the effect of rapidly relaxing viscoelastic matrices on tumors depends on matrix dimensionality and the specific tumor cell type under study. For instance, Najmina et al. demonstrated that controlling polymer molecular weight to regulate matrix viscoelasticity revealed that rapidly relaxing matrices inhibit breast cancer cell proliferation by inducing S-phase cell cycle arrest and activating senescence-associated β-galactosidase (SA-β-gal) expression [52]. In an in vitro 2D study using human osteosarcoma MG-63 cells, a low viscoelastic modulus (G’’ = 100 Pa) promoted cell survival and spreading, while a higher modulus (G’’ = 600 Pa) triggered mitochondrial apoptosis via the Hippo/NF-κB signaling pathway [53]. Conversely, other studies suggest that increased viscoelasticity typically promotes tumor progression. Nguyen et al. developed dynamic 3D biomimetic hydrogels with tunable stiffness and stress relaxation, finding that increased matrix stiffness (6 kPa vs. 2 kPa) is the primary driver of malignant phenotypes in PDAC cells, such as epithelial-mesenchymal transition (EMT) and activation of pro-tumorigenic pathways, while variations in stress relaxation rate had no significant effect [54]. Their subsequent study showed that rapidly stress-relaxing gelatin hydrogels, mimicking the mechanical properties of pancreatic tumors (G’ ≈ 6 kPa, τ1/2 ≈ 50 s), promoted PDAC cell proliferation, EMT, and invasiveness via integrin β1 signaling [55]. Zheng et al. developed a visible-light-responsive hydrogel platform for in vitro 3D models, allowing spatiotemporal dynamic regulation of stress relaxation times (500–3400 s) through Schiff base bonds and thiuram disulfide (TDS) moieties. Their findings revealed that substrates with longer relaxation times inhibited the spreading area of ovarian cancer cells, while shorter relaxation times promoted cell spreading [56]. Additionally, the impact of matrix viscoelasticity on chemotherapy efficacy varies with model dimensionality. Cieśluk et al. demonstrated, using a 2D model simulating the viscous dissipation properties of rat brain tissue (G’’ ≈ 334 Pa), that viscoelastic substrates reduced the chemotherapeutic efficacy of temozolomide (TMZ) against GBM cells by attenuating mitochondrial depolarization and DNA fragmentation [57]. In contrast, a 3D in vitro model that recapitulated brain-mimicking viscoelasticity (τ1/2 ≈ 133 s) showed that rapidly relaxing matrices enhanced tumor cell proliferation while simultaneously improving TMZ-mediated killing of GBM cells [58]. Notably, cellular responses to viscoelastic properties appear to be stiffness-dependent. In vitro 3D studies by Cebollada et al. showed that enhanced viscoelasticity promotes mesenchymal stem cell (MSC) spreading, focal adhesion formation, and Yes-associated protein (YAP) nuclear translocation on soft substrates (2.8 kPa), whereas increased viscoelasticity on stiff substrates (> 10 kPa) suppresses these cellular responses [59]. Recent work by Ciccon et al. has shown that micropatterned viscoelastic hydrogels with independently tunable Young’s modulus and stress relaxation reveal a stiffness-dependent regulatory role of matrix viscoelasticity in regulating breast epithelial cell spreading, adhesion, YAP nuclear translocation, and migration [60]. This indicates that viscous dissipation in viscoelastic materials can modulate the dominant role of matrix stiffness in regulating cellular behavior.
Abnormal topology remodeling of the tumor ECM
Topology, also termed matrix structure, refers to the spatial organization and connectivity of complex network structures within the ECM, including fiber alignment, porosity, and pore size distribution characteristics [61]. Currently, ECM topology is primarily characterized using high-resolution microscopy techniques such as Second Harmonic Generation (SHG) imaging, Scanning Electron Microscopy (SEM), and Transmission Electron Microscopy (TEM) [62]. In tumor-associated ECM, aberrant topological remodeling primarily involves the anisotropic alignment of collagen fibers, reduced effective porosity, and increased surface roughness (Fig. 1. c). These alterations not only serve as key pathological markers of tumor progression but also directly promote the malignant phenotype (Supplementary Table 1).
Unlike the isotropic, randomly oriented collagen distribution in healthy or physiological tissue, tumor ECM collagen fibers typically align anisotropically along the cellular longitudinal axis (Fig. 1. c. i) [63]. A 3D in vitro study demonstrated that the interaction between the ECM and human fibrosarcoma HT1080 cells induces collagen rearrangement, ultimately leading to strain-directed fiber reorganization. This process is mediated by Rho/ROCK kinase activation and stress fiber contraction driven by myosin light chain phosphorylation [64]. The discoidin domain receptor 1 (DDR1) on the cell surface, which binds directly to collagen, plays a critical role in promoting the spatial arrangement of collagen fiber anisotropy [65]. The anisotropic alignment of collagen fibers is not only strongly correlated with matrix stiffness but also widely associated with poor prognosis in various cancers. Analysis of clinical tissue specimens from 371 patients with invasive breast cancer revealed that aligned collagen bundles oriented perpendicular to the tumor boundary correlated with shorter survival duration [66]. Eliceiri et al. utilized SHG microscopy to examine pathological tissues from 114 patients with PDAC who underwent radical surgery, showing that a high degree of collagen fiber alignment served as an adverse prognostic factor after PDAC resection [67]. Similarly, another SHG microscopy study of PTEN-deficient murine breast cancer tissues demonstrated an increase in parallel collagen fiber alignment within tumor-bearing mice(fiber orientation defined as the angle relative to the ductal/tumor boundary) [68].The anisotropic alignment of collagen fibers profoundly promote tumor cell migration and invasion. For instance, Provenzano et al. developed a 3D collagen alignment methodology based on fibroblast-constrained contraction, demonstrating that highly aligned fibrous matrices specifically enhance the directional migration capacity of CSCs through contact guidance [69]. Furthermore, Kim et al. created a 3D breast cancer model featuring a radial-circumferential dual topological architecture through mechanically controlled collagen fiber alignment, showing that radially aligned fibers act as a critical biomechanical cue driving collective cancer invasion [70].
In healthy or physiological tissues, the ECM exhibits relatively uniform pore sizes and distributions. Soft tissues, such as mammary glands and subcutaneous fat, typically have pore diameters ranging from 1 to 5 μm, while denser tissues like skeletal muscle and liver maintain smaller pores (0.5–2 μm) [71]. In contrast, the tumor ECM is characterized by significantly reduced pore sizes (< 1 μm) and marked spatial heterogeneity in porosity (Fig. 1. c. ii) [72]. Dense cellular packing during tumor growth, coupled with increased ECM stiffness, substantially diminishes effective porosity [73]. Notably, excessive ECM component production and subsequent remodeling can paradoxically increase porosity in certain regions. For example, CAFs secrete MMPs that degrade the ECM to create new pores [74], while local HA degradation can further enlarge these pores [75]. These alterations collectively influence tumor cell migration, nutrient diffusion, and therapeutic efficacy. Cancer cell migration is governed by ECM pore confinement, which occurs in two distinct forms: protease-independent migration through micron-scale pores or channels that allow for cellular squeezing. This was demonstrated by Negrini et al. using 3D-printed polyurethane (PU) osteosarcoma models, where SaOS-2 cells infiltrated pores only in scaffolds that contained pre-deposited mineralized bone ECM from osteogenically differentiated human MSCs (hMSCs) and exhibited appropriate mechanical properties (55–67% porosity, 0.5-4.0 MPa elastic modulus) [76]. Similarly, tunable macroporous alginate hydrogel models showed that elevating porosity to 30% combined with rapid stress relaxation significantly enhanced migration and proliferation of encapsulated MDA-MB-231 breast cancer cells [77]. Protease-dependent migration followed within nanoporous restrictive matrices. Crucially, matrix mechanical plasticity, rather than initial pore size, determines the feasibility of migration through nanopores. Wisdom et al. demonstrated this using a nanoporous (< 40 nm) BM-mimetic IPN hydrogel, showing that breast cancer cells (MDA-MB-231, MCF7) and fibrosarcoma cells (HT-1080) could utilize invadopodia to exert localized forces, permanently expanding nanopores into 2–3 μm microchannels when the matrix exhibited high mechanical plasticity (30% permanent strain) [78]. This challenges the conventional paradigm that submicron pore migration depends solely on proteolytic degradation.
Surface roughness refers to the microscopic irregularities and variability in the morphology of the ECM surface [79]. In the TME, CAFs exert mechanical forces that drive collagen fiber realignment, crosslinking, and bundling, leading to a significant increase in ECM surface roughness [80]. Collagen fibers in the ECM exhibit textural patterns, such as spatially organized ridges and grooves (Fig. 1. c. iii) [60]. These features are commonly replicated in vitro using biomimetic substrates with micropatterned topographies at the micro- or nanoscale. Roughness is then quantified using parameters like average roughness (Ra) [79], which directly influences cell behaviors, including adhesion, migration, proliferation, and differentiation. For example, Domenech et al. engineered an ECM model with precisely controlled roughness using a customized pressure device, demonstrating that elevated roughness (Ra = 1.5 μm) significantly enhanced stemness properties and drug resistance in triple-negative breast cancer (TNBC) cells by modulating EGFR signaling and the YAP/TAZ mechanosensing pathway [81]. In contrast, King et al. found that variations in surface topographies (20 μm vs. 50 μm patterned polyacrylamide hydrogels fabricated via photolithography) had no significant impact on endothelial cell migration, with further investigation revealing substrate stiffness as the primary regulatory factor [82]. This finding highlights the complex interplay between ECM biomechanical properties. Notably, surface roughness exhibits a threshold effect on cell proliferation: growth slows when roughness exceeds a critical threshold (Ra = 1 μm), while cells cultured on surfaces with roughness between 0.5 and 1 μm show enhanced proliferation [83]. These seemingly contradictory results suggest that the biological impact of ECM surface roughness likely depends on cell type and microenvironmental context.
The biomechanical properties of ECM shape the immunosuppressive tumor microenvironment
The impact of the ECM on ITME was initially attributed to biochemical factors like metabolic abnormalities and hypoxia [84]; however, emerging evidence emphasizes that ECM biomechanical cues actively shape the ITME through mechanotransduction signaling pathways (Supplementary Table 2).
Increased matrix stiffness is a key biomechanical factor in modulating the ITME. Elevated ECM stiffness hinders immune cell migration toward tumor cores through two main mechanisms: physical barrier formation and mechanotransduction-mediated dysfunction. Stiffened ECM induces overexpression of tenascin-C, which forms dense structural barriers that obstruct T-cell trafficking and infiltration [85]. A 3D in vitro study revealed that high collagen density significantly inhibits the proliferation of CD4+ T cells. Further analysis of TNBC specimens showed reduced numbers of infiltrating CD8+ T cells in breast tumors with high collagen density [86]. Zhou et al. employed AFM to measure tissue stiffness in human HCC, finding that regions with high collagen expression led to increased expression of exhaustion markers PD-1 and TIM-3 in CD8+ T cells. This phenomenon was confirmed in mouse models of B16-OVA melanoma and MC38-OVA colon cancer [87]. Dendritic cells (DCs), which play a crucial role in mediating crosstalk between innate and adaptive immunity, exhibit impaired antigen-presentation abilities in response to aberrant ECM biomechanical cues. A 2D polyacrylamide substrate study showed that higher substrate stiffness (~ 12 kPa) significantly impaired DC antigen presentation, migration, and maturation [88]. A 3D hydrogel confinement study similarly demonstrated that higher matrix stiffness (≥ 8.0 kPa) impaired the migration speed and directional persistence of immature DCs. In contrast, under lower matrix stiffness (1.2 kPa) conditions, DC migration was rapid and highly persistent [89].
Increased ECM stiffness also actively promotes the recruitment and activation of immunosuppressive cells. High-stiffness ECM activates Piezo-type mechanosensitive ion channel component 1 (Piezo1). In a PDAC animal model, Piezo1 activation increased MDSC expansion and infiltration, accelerating tumor progression, while inhibition of PIEZO1 in patient-derived organotypic tumor spheroids (PDOTS) reduced MDSC infiltration and spheroid size [90]. ECM stiffness can also influence macrophage polarization through epigenetic regulation. For instance, matrix stiffness affects miRNA expression within macrophages [91]. Cai et al. demonstrated that macrophages sense mechanical signals through Piezo1, mediating calcium ion influx and promoting macrophage polarization toward the M2 phenotype [92]. A 2D polyacrylamide hydrogel study found that high matrix stiffness enhances the inflammatory polarization of macrophages by activating Piezo1 [93]. Additionally, the stiffened matrix increases tumor cell-derived colony-stimulating factor 1 (CSF-1) expression, promoting M2-polarized macrophage differentiation [94]. Furthermore, increased ECM stiffness also modulates chemokine secretion by CAFs and tumor-associated macrophages (TAMs), including CCL2, CCL17, and CCL22, which further promotes Treg infiltration and exacerbates the formation of the ITME [95].
The viscoelastic properties of the ECM significantly influence the function and phenotypes of various immune cells. For example, Berchie et al. engineered an ECM with independently tunable viscoelasticity, quantified by stress relaxation half-time, and found that slow-relaxing matrices enhanced the expression of activation and inhibitory markers (e.g., CD25, CD39) in T cells, while fast-relaxing matrices promoted the expression of memory markers (e.g., CD62L, CD45RA) [96]. Monocytes, a critical component of the immune system, also respond to ECM viscoelasticity. Mooney et al. created a 3D interpenetrating network hydrogel composed of collagen and alginate with tunable viscoelasticity. They demonstrated that monocytes encapsulated in viscous matrices (rapid stress relaxation) maintained an immature phenotype and exhibited suppressed differentiation into DCs, while elastic matrices (slow stress relaxation) promoted proinflammatory polarization [97]. Additionally, a study utilizing a 2D culture system based on tunable viscoelastic polyacrylamide hydrogels, showed that increased substrate viscoelasticity significantly suppressed the phagocytic function of THP-1-derived macrophages and induced a more rounded morphology [98].
The alignment of ECM fibers influences the adhesion and migration patterns of immune cells. Teo et al. placed a collagen solution on a 30-degree-tilted 3D-printed platform to form directionally aligned collagen fiber bundles. They found that the aligned fibers not only enhanced matrix stiffness but also reduced actin content, impaired cytoskeletal reorganization, and hindered immune synapse formation, which resulted in slower T cell migration and a loss of directionality over time [99]. Another study also demonstrated that this fiber alignment could disrupt integrin-mediated T-cell adhesion mechanisms, impairing immune cell migration patterns [100] and influencing the movement of resident CD8+ T cells within tumors [101]. DDR1, a collagen receptor tyrosine kinase, plays a crucial role in immune exclusion. Its extracellular domain (DDR1-ECD) binds collagen and facilitates the formation of aligned collagen fibrils, thereby restricting the infiltration of CD4+ and CD8+ T cells in transgenic mouse models of TNBC [102]. Furthermore, in vitro studies using a 3D biomimetic hydrogel model revealed that anisotropic fiber alignment directly reduced T cell proliferation and activation marker expression (CD25, CD69), as well as suppressed OXPHOS in immature DCs, impairing their ability to initiate immune responses [103]. Notably, therapies targeting cytotoxic T lymphocytes (CTLs) are less effective in collagen-rich environments, where low ECM porosity hinders the infiltration of ICIs and nanomedicines [104]. Tolentino et al. demonstrated that CTLs exhibit enhanced migration rates within low-crosslinking-density, large-pore (median pore size: 13.3 μm) hydrogels, developed as tunable 3D ECM mimetics with adjustable stiffness and porosity [105].
The elevation of hydrostatic pressure within the TME is closely linked to the biomechanical properties of the ECM. This increased pressure generates both static and dynamic mechanical signals, which not only directly promote tumor progression but also contribute to the development of the ITME. The tumor ECM, rich in densely packed proteins and molecules, exhibits reduced hydraulic conductivity, which underlies the abnormal elevation of interstitial fluid pressure (IFP) [106]. Tumors with high collagen deposition demonstrate significantly higher IFP compared to those with lower collagen content, due to the negative correlation between the collagen fiber network and ECM hydraulic conductivity [107]. Furthermore, the abnormal architecture and hyperpermeability of tumor-associated vasculature exacerbate the accumulation of intratumoral hydrostatic pressure [108]. In vitro studies reveal that elevated IFP activates specific mechanosignaling pathways that promote malignant behaviors. For instance, Papavassiliou et al., using devices to precisely control hydrostatic pressure (100 g/cm2) in vitro, demonstrated its ability to activate the mTOR/FAK/ERK signaling axis and the transcription factors YAP/TAZ, thereby promoting the proliferation, migration, and 3D invasion of GBM cells [109]. Additionally, PIEZO1 has been identified as a key mechanoreceptor sensing hydrostatic pressure [110]. Kuo et al. found that simulating high IFP (20 mmHg) significantly enhanced lung cancer cell migration and invasive capacity [111]. Within the core of solid tumors, hydrostatic pressure can reach 30–100 mmHg, creating a pressure gradient that radiates from the center towards the periphery. This gradient drives interstitial fluid efflux and subjects cells to fluid shear stress (FSS) [112], which has been shown to exert pro-migratory effects. In vitro studies by Sun et al. demonstrated that shear stress (2 dyne/cm2) reduced the stiffness of liver CSCs (LCSCs) through phosphorylation of FAK and ERK1/2 pathways and disruption of the F-actin structure, thereby enhancing LCSC migratory ability [113]. Low shear stress (1.8 dyn/cm2) promotes focal adhesion turnover and directional migration in human TNBC cells by activating the ROCK/HDAC6 pathway to accelerate Cav-1-dependent trafficking of integrin β1 [114]. Importantly, elevated IFP forms a physical barrier that severely impedes immune cell infiltration into the tumor core, significantly hindering the effective penetration of anti-tumor drugs [115, 116]. Mechanistic studies show that interstitial pressure (~ 3 μm/s) can polarize macrophages towards an M2 phenotype via an integrin/Src-mediated mechanotransduction pathway, promoting tumor cell invasion and progression [117].
The immune microenvironment not only responds to biomechanical signals from the ECM but also actively remodels its mechanical properties. Activated T cells secrete MMP-9, which degrades collagen and FN, reducing matrix stiffness [118, 119]. In contrast, immune cells release chemokines and cytokines, such as CXC, IFN-γ, and TNF-α, which stimulate collagen synthesis and fibroblast secretion, leading to matrix stiffening [120]. Additionally, T cell interactions with fibroblasts modulate collagen production and organization, influencing the ECM’s topological structure [121]. In summary, the biomechanical properties of the ECM and immune cells form a dynamic, interdependent network, with the biological outcome determined by the activation thresholds of distinct mechanical transduction pathways in specific immune cell subsets and the integration of these signals.
Effects of ECM biomechanical properties on Tregs and mechanotransduction mechanisms
The ECM intricately regulates Treg differentiation, functional phenotypes, and spatial distribution through both mechanical and biochemical signaling pathways. Mechanotransduction mechanisms [122], as the critical link between ECM mechanical properties and intracellular responses in Tregs, play a pivotal role. Therefore, understanding how ECM mechanical signals influence Treg behavior through mechanotransduction is crucial for the development of mechano-immunological anti-tumor strategies.
Effects of ECM mechanical properties on the differentiation and proliferation of Tregs
Stiffness, one of the most extensively studied ECM properties, has been shown to differentially affect Treg differentiation and proliferation across various experimental models (Table 1). In a 2D in vitro study, Lee et al. demonstrated that low-stiffness substrates (100 kPa) significantly enhanced the induction efficiency of CD4+CD25– Tconvs into Tregs compared to high-stiffness substrates (3 MPa). The inhibition of ROCK with Y-27,632 eliminated the differences in Treg induction efficiency across substrates of varying stiffness [123]. Y-27,632, a well-known ROCK family inhibitor, modulates actomyosin-mediated cellular contraction through both direct and indirect mechanisms [124]. These findings suggest that matrix stiffness influences naïve T cell differentiation into Tregs via the Rho/ROCK signaling pathway by dynamically regulating cytoskeletal tension. Shi et al.‘s 2D in vitro experiments further demonstrated that softer substrates (17 kPa) promoted Treg induction, with this effect persisting for several weeks. In contrast, iTregs induced on stiffer substrates (870 kPa) exhibited lower induction efficiency but enhanced suppressive capacity against conventional T cell proliferation [125], implying that substrate stiffness plays a critical role in the long-term regulation of Treg polarization. However, divergent trends have been observed across studies using different stiffness ranges. For instance, Bai et al. developed a 2D hydrogel model in vitro and observed a positive correlation between increased matrix stiffness (7.5 kPa − 140 kPa) and both the induction efficiency of Tregs and Foxp3 expression levels [126]. This mechanosensitive upregulation of Treg differentiation was also confirmed in another 2D study using PA gels, where Treg induction efficiency correlated positively with substrate rigidity (7.5–140 kPa) [127]. This discrepancy may arise from the activation of distinct mechanotransduction pathways within different stiffness ranges. Single-cell RNA sequencing has revealed that the p53-mediated pathway facilitates Treg induction on soft substrates but has minimal contribution on stiffer substrates [127].
Table 1.
In vitro effect of matrix stiffness on the directed differentiation and proliferation of Treg cells
| Matrix types and culture environments | The values of biomechanical Cues | Measurement method | Cells of interest | Polarization conditions of Treg | Findings | Refs. |
|---|---|---|---|---|---|---|
| 3D In vitro (Type I collagen gels) | Concentration collagen matrix: 1 mg/ml, 4 mg/ml | Directly control the concentration of collagen preparation | Human CD4⁺, CD8⁺ T cells | Cells were cultured in X-vivo media with 5% human serum at 37 °C/5% CO₂ | Increased collagen concentration upregulates the expression of Treg cell markers, including FOXP3 and CTLA-4. | [86] |
| 2D In vitro (PDMS) | E:1.3 kPa, 3.8 kPa and 100 kPa | Indentation apparatus | CD4+CD25− T cells | 1 mM sodium pyruvate, MEM non-essential amino acids solution, TGF-β, and IL-2. | Compared with higher stiffness levels (E ~ 3 MPa), Treg induction at lower substrate stiffness levels (E ~ 100 kPa) is more efficient | [128] |
| 2D In vitro (PDMS) | E:17 kPa, 870 kPa and 2600 kPa | Indentation | Human PBMC (CD4+CD25−T cells) | 10 ng/mL of IL-2 and TGF-β and 1 mM of sodium pyruvate and MEM non-essential amino acids for 72 h | On softer matrices, the proportion of FOXP3 + cells exhibited a significant increase, and this effect persisted for several weeks | [124] |
| 2D In vitro (Hydrogel) | E: 7.5 kPa, 140 kPa | Indentation apparatus | Mouse spleen (Naïve mouse CD4+T) | IL-2 (100 IU/mL), TGFβ (5 ng/mL) and anti-CD3/28 Dynabeads | With increasing matrix stiffness, the induction efficiency of Tregs demonstrates significant enhancement | [126] |
| 2D In vitro (PA-gels) | E: 7.5 kPa, 50 kPa and 140 kPa | Indentation apparatus | Human PBMC (CD4+CD25−T cells) | 10 ng/ml of IL-2 and TGF-β and 1 mM of sodium pyruvate and MEM non-essential amino acids for 72 h | FOXP3 expression increases with increasing matrix stiffness. high-stiffness matrices significantly upregulate the expression of genes associated with OXPHOS in Tregs | [127] |
| 3D In vitro (collagen fibers were harvested from rat tail tendons) | Concentration collagen matrix:1 mg/ml, 3 mg/ml and 6 mg/ml | Directly control the concentration of collagen preparation | Jurkat T | Supernatant of human breast cancer cell line (MB-231) | Increased collagen concentration promotes the expression of Tregs markers (FOXP3 and CD25) and enhances Tregs infiltration. | [129] |
| 3D in vitro (Collagen type I modified with norbornene) | 2 mg/mL(G’’: ≈20 Pa、60 Pa); 4 mg/mL(G’’: ≈40 Pa、100 Pa) | Rheometer + Nanoindentation | CD8⁺ T cells from healthy donors | T cells activated with synthetic CD3/CD28 dynabeads, then embedded in collagen matrices | Cells expressing slow-relaxing ECM show high expression of activation/exhaustion markers and low expression of memory markers. | [96] |
| 2D in vitro (PDMS) | E: 2 kPa, 50 kPa | Adjust PDMS cross-linking density to achieve without measurement | DCs | - | 50 kPa upregulates Piezo1 in DCs, promoting IL-12 secretion while suppressing TGF-β1 | [130] |
PDMS Polydimethylsiloxane, PA-gels polyacrylamide gels, PBMC Peripheral Blood Mononuclear Cell
These contradictory findings highlight the context-dependent nature of mechanoregulatory mechanisms, likely influenced by variations in stiffness ranges or cultivation environments. Previous studies have demonstrated a biphasic response of T cells to matrix stiffness, with proliferation peaking on substrates of 25 kPa and declining at both higher and lower rigidity levels [128]. This suggests the presence of an “optimal window” for in vitro Treg differentiation, where induction efficiency decreases when mechanical thresholds exceed or fall below this range. Additionally, the dimensionality of culture models (2D vs. 3D) likely plays a crucial role in explaining these discrepancies. There is broad consensus that 2D models fail to replicate the structural, mechanical, and biochemical cues of the tissue microenvironment, thus oversimplifying cell-cell and ECM interactions [131]. In contrast, 3D culture systems, which more closely mimic in vivo microenvironments, generally show that increased matrix stiffness enhances Treg differentiation and expansion. For example, Gao et al. encapsulated Jurkat T cells in 3D collagen matrices of varying concentrations, demonstrating that higher collagen density (6 mg/ml) increased the proportion of CD4+FOXP3+ T cells and upregulated Treg-associated markers, including FOXP3 and CD25 [129]. Subsequent studies by Kuczek et al. confirmed that high-density 3D collagen matrices not only increased Treg marker expression (e.g., FOXP3 and CTLA-4) but also suppressed cytotoxic T cell effectors like IFN-γ and granzyme B. Integrated Motif Activity Response Analysis (ISMARA) further revealed that the transcription factors SMAD4 and FOXO1 were significantly activated on high-collagen concentration matrices[86]. These transcription factors are key mediators of TGF-β-induced Treg differentiation, and given TGF-β’s dual role in inhibiting T cell cytotoxicity while promoting Treg commitment [132], it suggests that matrix stiffness enhances TGF-β signaling through biomechanical priming, thereby reinforcing Treg differentiation.
The viscoelasticity of the ECM may also influence Treg differentiation through the Activator Protein 1 (AP-1) signaling pathway. As a central regulator of T cell activation, differentiation, and exhaustion, the transcription factor AP-1 plays a crucial role in these processes [133]. Berchie et al. observed significant enrichment of AP-1 pathway-associated genes in TME-infiltrating T cells. They further constructed independently tunable viscoelastic type I collagen 3D matrices in vitro. Slow-relaxing matrices confirmed this association, significantly upregulating the expression of AP-1 pathway core genes (e.g., JUN, FOS, BATF, ATF3) in T cells, driving their differentiation toward an activated state that tends toward exhaustion [96]. Notably, JunB, a member of the AP-1 transcription factor family, promotes the differentiation of eTregs and sustains their immunosuppressive functions by regulating key molecules such as ICOS and CTLA-4 [134]. It is plausible that matrix viscoelasticity may indirectly promotes Treg differentiation by modulating JunB, further research is needed to confirm the direct effects of this mechanical property on Tregs (Fig. 2).
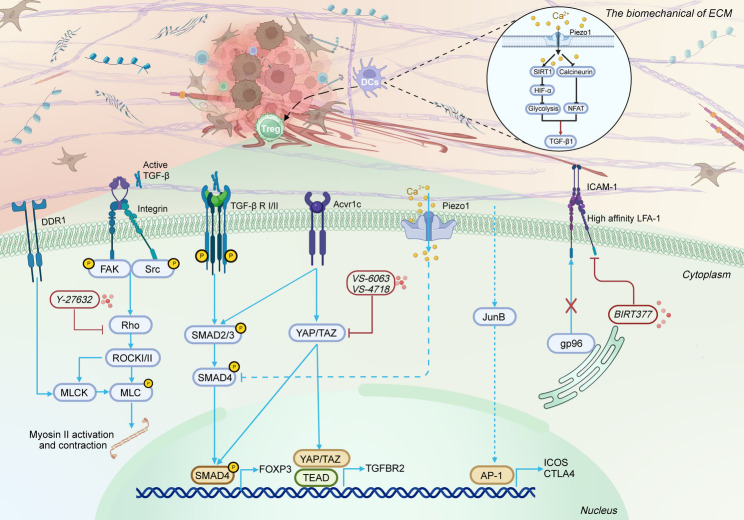
Fig. 2.
Effects of ECM mechanical properties on the differentiation, infiltration, and migration of Tregs. DDR1, Discoidin domain receptor 1; FAK, Focal adhesion kinase; Src, Src-family kinases; ICAM-1: Intercellular adhesion molecule-1; AP-1, Activator Protein 1; Figures were created with BioRender.com
Integrins, heterodimeric transmembrane receptors composed of α and β subunits, serve as critical mechanosensors [135]. Notably, the integrin αvβ8 subtype plays a distinctive role in regulating Treg differentiation and function. Under physiological conditions, integrin αvβ8 interacts with latent TGF-β complexes bound to Latent TGF-β Binding Protein-1 (LTBP-1), generating mechanical forces that activate TGF-β1. This process facilitates the differentiation of naïve T cells into Tregs and supports immune tolerance [135, 136]. In vivo studies using murine transplant tumor models (melanoma B16 and breast cancer E0771) demonstrated that Tregs specifically activate cancer cell-secreted latent TGF-β through integrin αvβ8, mediated by the Itgβ8 subunit. This mechanism not only promotes Treg differentiation but also suppresses the cytotoxic functionality of intratumoral CD8+ T cells, aiding immune evasion [137]. Mechanistically, this activation enhances Treg transcriptional regulatory networks through the TGF-β/SMAD signaling axis (Fig. 2) [138]. Additionally, mechanical activation of TGF-β1 is recognized as an acute process reliant on cellular contractile force. ECM-induced mechanical stress maintains Treg functional homeostasis and inhibits effector T cell activation via similar force-dependent mechanisms that govern TGF-β1 release [139]. For example, Klingberg et al. used highly extensible silicone membranes to apply controlled pre-strain (1.0–2.8 fold stretching) to the ECM. Their results showed that pre-strain significantly enhanced both the contractility of human dermal fibroblasts (hDMFs) and the activation efficiency of latent TGF-β1 [140]. These findings demonstrated that mechanical prestress within the ECM lowers the activation threshold for TGF-β1 by stretching LTBP-1 fibrils, highlighting the therapeutic potential of targeting integrin αvβ8.
YAP and Transcriptional coactivator with PDZ-binding motif (TAZ) are pivotal downstream effectors of the Hippo signaling pathway. Mechanical signals from the ECM promote the translocation of YAP/TAZ from the cytoplasm to the nucleus, where they bind to Transcriptional Enhanced Associate Domain (TEAD), activating the transcription of downstream target genes [141, 142]. Analysis of peripheral blood samples from 152 patients with HCC and 91 healthy controls revealed significant upregulation of YAP-1 expression in peripheral blood Tregs of patients with HCC, with elevated YAP-1 levels correlating with poor prognosis. Further in vitro experiments demonstrated that YAP-1, by forming a YAP-1/TEAD transcriptional complex, directly binds to the promoter region of the TGF-β type II receptor (TGFBR2) gene, promoting the differentiation of naïve T cells into Tregs [143]. Additionally, in vitro studies and experiments using YAP-deficient mice by Ni et al. demonstrated that YAP functions as a Treg-specific transcriptional coactivator. YAP upregulates the activin receptor (ACVR1C), thereby enhancing TGF-β/SMAD signaling efficiency and synergistically promoting both Treg differentiation and Treg-mediated tumor immune suppression [144]. This suggests the YAP/activin axis as a potential therapeutic target. However, conflicting evidence from Meng et al. showed no significant difference in the proportion of Foxp3+ CD4+ T cells (nTregs) within the spleens of YAP conditional knockout (YAPcKO) mice compared to wild-type (WT) controls. Furthermore, when WT and YAPcKO CD4+ T cells were stimulated in vitro with anti-CD3/CD28 antibodies plus TGF-β (2 ng/ml) for 3 days to induce iTreg differentiation, no difference in Foxp3+ iTreg differentiation efficiency was observed between the two groups [145]. This discrepancy suggests that YAP may regulate Treg differentiation through indirect mechanisms or crosstalk with alternative pathways, highlighting the complexity of its regulatory network and the need for further investigation.
Piezo1, a mechanosensitive non-selective cation channel, directly translates ECM-derived mechanical signals into conformational changes, triggering Ca2+ influx that activates various downstream signaling cascades [146]. Notably, Piezo1’s impact on Tregs appears to be selective. For instance, Jairaman et al., using a T cell-specific Piezo1 knockout mouse model, demonstrated that Piezo1 deficiency selectively enhanced the differentiation and expansion of Tregs, without significantly affecting the activation, migration, or proliferative capacity of effector CD4+ T cells (Th1, Th17) [147]. The functional dichotomy of Piezo1 is evident in different pathophysiological contexts. In conditions such as multiple sclerosis (MS) or experimental autoimmune encephalomyelitis (EAE), T cells navigating through stiff environments activate Piezo1 channels via membrane tension. Upon activation, Piezo1 inhibits the TGFβ/SMAD signaling pathway in CD4+ T cells, restraining Treg activity [147]. In contrast, Abiff et al. demonstrated that in T cell-specific Piezo1 knockout (P1KO) mice, the frequency and abundance of FoxP3+ Tregs in the TME and tumor-draining lymph nodes (tDLNs) showed no significant differences compared to WT controls across syngeneic murine rhabdomyosarcoma and medulloblastoma models, in stark contrast to observations in autoimmune models [148]. Notably, ECM mechanical cues can modulate Piezo1 signaling in non-T cells, indirectly influencing the differentiation, activation, and function of Tregs. For example, Wang et al. showed that matrix stiffness (50 kPa) upregulates Piezo1 expression in DCs, promoting IL-12 secretion while suppressing TGF-β1, These findings were further validated in colon carcinoma (MC38) and melanoma (B16.F10) models. Mechanistically, Piezo1 integrates the SIRT1-hypoxia-inducible factor-1α (HIF-1α)-dependent metabolic pathways with the calcium-calcineurin-NFAT signaling axis to coordinate DC-derived IL-12 and TGF-β1 secretion. This integration regulates the mutually exclusive differentiation of Th1 cells and Tregs (Fig. 2) [130].
Effect of the ECM mechanical properties on the infiltration and migration of Tregs
In addition to classical chemokine-mediated chemotaxis [95], ECM mechanical cues play a significant role in modulating Treg infiltration and migration dynamics. Under inflammatory conditions, Tregs undergo actin cytoskeletal reorganization that increases cellular stiffness. However, this mechanical adaptation reduces their deformability, limiting their ability to traverse narrow interstitial spaces and thereby restricting their migratory capacity and extravasation within inflammatory microenvironments, while simultaneously contributing to the formation of localized immunosuppressive niches [149]. Notably, Gao et al. observed through Sirius Red/Fast Green staining and Masson staining of metastatic TNBC samples (M1) that, compared to non-metastatic samples (M0), Treg infiltration in metastatic samples correlated with increased collagen fiber concentration and greater dispersion in orientation distribution [129], as previously reported [99]. This suggests that topological changes in the ECM may mechanically facilitate Treg accumulation. The collagen receptor DDR1, a mechanosensitive tyrosine kinase transmembrane protein, plays a pivotal role in regulating this process [150]. In lung adenocarcinoma models, DDR1 deficiency enhanced Treg infiltration and exacerbated tumor progression, establishing DDR1 as a critical mechanosensor that enables Tregs to perceive ECM biomechanical cues and modulate their migratory behavior [151].
Integrins and their downstream mechanosensors are key regulators of Treg tumor infiltration. Recent studies have shown that in non-tumor environments, activating transcription factor 4 (ATF4) enhances the migratory capacity and immunosuppressive function of Tregs by promoting integrin αvβ8-mediated TGF-β activation, thereby exacerbating obesity-associated liver fibrosis [152]. In the TME, integrin αvβ8 similarly promotes Treg tumor infiltration. Nishimura et al. discovered in lung carcinoma (Lewis) models that integrin αvβ8 activates TGF-β signaling through direct contact between tumor cells and T cells, inducing Treg differentiation and infiltration [153]. Moreover, the FAK-Src family kinase (Src) complex is a key mediator in promoting Treg migration to tumors through ECM mechanical properties. Increased ECM stiffness facilitates the nuclear translocation of the FAK-Src complex via multiple mechanotransduction pathways [154]. Using a subcutaneous squamous cell carcinoma (SCC) xenograft mouse model, Serrels et al. showed that intranuclear FAK binds chromatin in a kinase-dependent manner, driving the transcription of the chemokine CCL5, which recruits Tregs and suppresses CD8+ T cell function [155]. These findings offer novel insights into targeting the FAK-Src mechanotransduction pathways for tumor mechano-immunotherapy (Fig. 2).
Lymphocyte function-associated antigen-1 (LFA-1) is a critical integrin mediating Treg infiltration into the TME. As a core regulatory molecule for Treg function, LFA-1 plays a pivotal role in immune modulation. Klaus et al. investigated various β₂ integrin-deficient mouse models and found that the absence of LFA-1 impairs Treg differentiation, migration to local sites, and contact-dependent suppression, leading to a disruption of immune tolerance and the onset of autoimmune responses [156]. In vivo studies by Niu et al. demonstrated that subcutaneous growth of B16F10 melanoma was significantly suppressed in LFA-1 knockout mice, a phenomenon closely linked to a reduction in Treg numbers in the spleen, blood, and lymph nodes [157]. These findings underscore the essential role of LFA-1 in Treg recruitment and its potential therapeutic value. Mechanistically, conformational changes in integrins induced by increased ECM stiffness can enhance LFA-1’s affinity for intercellular adhesion molecule-1 (ICAM-1) by up to 10,000 times, thereby significantly promoting its binding to ICAM-1 and facilitating Treg infiltration into tumors [158]. Notably, Zhou et al. demonstrated in a Treg-specific gp96 knockout mouse model that the endoplasmic reticulum chaperone gp96 regulates Treg homing to the TME by facilitating LFA-1 folding [159]. These findings suggest that the gp96/LFA-1 complex on Tregs may serve as a promising target for reprogramming the ITME.
Effect of the ECM mechanical properties on the metabolism and function of Tregs
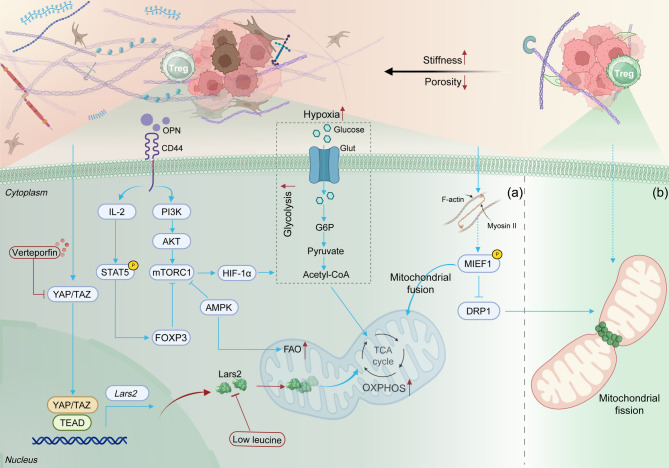
Tregs predominantly rely on metabolic pathways that favor mitochondrial OXPHOS and fatty acid oxidation (FAO), which are essential for sustaining their immunosuppressive functions [160]. The mechanical properties of the ECM play a critical role in regulating the metabolic preferences and functional stability of Tregs, particularly through modulation of their mitochondrial dynamics. Single-cell transcriptomic analysis by Shi et al. revealed significant upregulation of OXPHOS-related genes in Tregs induced on 2D stiff matrices (140 kPa). Notably, treatment with the AMP-activated protein kinase (AMPK) activator AICAR enhanced mitochondrial membrane potential and increased Treg induction efficiency on soft matrices (7.5 kPa). However, this treatment did not significantly enhance Treg induction on 140 kPa stiff matrices [127]. These findings suggest that a metabolically suppressed state exists in cells cultured on soft substrates. Moreover, Bai et al. demonstrated that increased ECM stiffness activates YAP/TAZ in Tregs, upregulating the transcription of leucyl-tRNA synthetase (Lars2), thereby enhancing mitochondrial OXPHOS and bolstering Treg immunosuppressive function (Fig. 3. a) [126]. Lars2 is a key enzyme in mitochondrial protein translation and is crucial for maintaining respiratory chain complex function [161]. High-stiffness ECM has also been shown to promote mitochondrial fusion by enhancing cytoskeletal tension, inducing the phosphorylation of mitochondrial elongation factor 1 (MIEF1), and restricting the mitochondrial localization of dynamin-related protein 1 (DRP1). Mitochondrial fusion further supports OXPHOS while inhibiting glycolysis (Fig. 3. b) [162]. Additionally, the absence of YAP leads to mitochondrial dysfunction, significantly impairing the immunosuppressive function of TI-Tregs [163]. These findings suggest that ECM stiffness plays a pivotal role in maintaining Treg metabolic preferences and functional homeostasis by regulating mitochondrial dynamics, offering a novel direction for immune regulation strategies based on the mechanical properties of the microenvironment.
Fig. 3.
Effect of ECM mechanical properties on the metabolism and function of Tregs. HIF-1α, Hypoxia-inducible factor-1 alpha; OXPHOS, Oxidative phosphorylation; FAO, Fatty acid oxidation; Lars2: Leucyl-tRNA synthetase; OPN, Osteopontin; MIEF1: Mitochondrial elongation factor 1; DRP1: Dynamin-related protein 1. Figures were created with BioRender.com
The relevant components of the ECM collaboratively regulate the functional and phenotypic characteristics of Tregs through mechanical and chemical signaling. Osteopontin (OPN), an ECM glycophosphoprotein, plays a key role in this regulation [164]. Vakrakou et al. demonstrated through adoptive transfer experiments that Opn-deficient Tregs exhibit impaired in vivo suppressive capacity in a colitis model. Further investigations revealed that these Opn-null Tregs enhance anti-tumor immunity and delay melanoma (B16-F10) progression, effects directly attributable to the loss of Treg stability [165]. Wu et al. further explored the mechanism by which collagen concentration gradients affect Treg phenotype. Specifically, high collagen concentrations activate the TGF-β/Smad signaling pathway, driving Tregs toward a pro-fibrotic phenotype [149]. Type 1 Tregs (TR1), a subset of Tregs [166], were found to be influenced by ECM components. In vitro studies by Bollyky et al. revealed that high molecular weight hyaluronan (HMW-HA) induces the differentiation of effector memory T cell precursors into TR1 cells via the CD44-p38/ERK1/2 signaling pathway [167]. Moreover, an in vitro study demonstrated that supplementing HMW-HA during the late stage of Treg induction maintains Foxp3 expression and promotes IL-2 secretion in induced Tregs through CD44 receptor cross-linking [168]. In addition, mechanical changes such as increased matrix stiffness and reduced porosity can exacerbate hypoxia in tumor tissues, upregulating glucose transporter (GLUT) via the HIF-1α signaling pathway, which enhances the glycolytic and migratory capabilities of Tregs (Fig. 3. a) [169–171]. Recent studies also suggest that ECM mechanical properties may influence the epigenetics of Tregs. In vitro 2D research has shown that soft viscoelastic substrates (with E: 2 kPa and τ1/2 ≈ 1000 s) promote an open chromatin state, characterized by H3 histone acetylation, in mouse fibroblasts by downregulating lamin A/C expression and enhancing chromatin dynamics [172]. Treg-specific genes such as Foxp3 and CTLA-4 exhibit significant hypomethylation on high-stiffness matrices [125]. This epigenetic reprogramming could open new avenues for the targeted regulation of Treg function.
The reaction of Tregs on the mechanical properties of ECM
The mechanical properties of the ECM significantly influence Treg function and phenotype, while Tregs themselves contribute to the remodeling of the ECM’s mechanical microenvironment. For instance, an in vivo study revealed that intrahepatic Tregs sustain liver fibrosis persistence by suppressing Kupffer cell (KC)-derived MMP secretion via a TGF-β-dependent mechanism. This suppression disrupts the MMP/TIMP balance, preventing ECM degradation [173]. Additionally, TGF-β secreted by Tregs activates hepatic stellate cells (HSCs), leading to the release of large quantities of ECM components, such as collagen, which accelerates matrix stiffening [174, 175]. In a bladder cancer model, Tregs were shown to selectively downregulate the expression of MMP2, inhibiting its matrix degradation function and thereby maintaining a high-stiffness matrix state [176]. Collectively, Treg-mediated bidirectional feedback between cellular dynamics and ECM biomechanics contributes to the persistence of pathological matrix stiffening.
Mechanical immunomodulatory strategies targeting the mechanotransduction pathways in Tregs
Current immunotherapeutic strategies targeting Tregs primarily focus on reducing their numbers or inhibiting their immunosuppressive functions by interfering with biochemical signaling pathways. These approaches include targeting immunosuppressive molecules on the Treg surface (e.g., anti-CD25 antibodies, anti-CTLA-4 antibodies, GITR agonists, and anti-OX40 antibodies) and blocking chemokine/chemokine receptor signals (e.g., anti-TGF-β antibodies, anti-CCR4 antibodies, and anti-CCR8 antibodies) [5–7, 177]. However, these interventions carry a significant risk of inducing systemic autoimmune diseases, posing a major limitation to their clinical application [178]. Therefore, strategies specifically targeting Treg responses to the mechanical cues within the TME represent a promising alternative. This review summarizes the current research landscape focused on targeting Treg mechanosensors and mechanotransduction pathways (Table 2).
Table 2.
Research on targeted Treg mechanics sensors and mechanical transduction pathways
| Targets | Drug | Cancer Types | Models | Findings | Ref. |
|---|---|---|---|---|---|
| Itgβ8 |
Anti-Itgβ8 antibody (ADWA-16) |
Melanoma (B16-F10); Breast cancer (E0771) | mice | Weakened the immunosuppressive function of Treg cells | [137] |
| YAP | VP | CRC(MC38); melanoma (B16-F10) | mice | Reducing mitochondrial OXPHOS in TI-Tregs weakens their immunosuppressive function | [126] |
| YAP | VP; Anti-Activin Antibody |
Melanoma (B16); CRC (MC38); Thymoma (EL4) |
mice | Reducing Treg proportion in the tumor microenvironment | [144] |
| LFA-1 | LFA-1 Antibody | CRC (MC38) | mice | Block the interaction between LFA-1 and ICAM-1 to inhibit Treg infiltration | [157] |
| LFA-1 | LFA-1 inhibitor (BIRT377) | Melanoma(B16F10) | mice | The number of Tregs decreases, inhibiting tumor growth | [159] |
| FAK | Defactinib (VS-4718) | Melanoma (YUMM3.2) | BRAF-mutant melanoma mouse | attenuates tumor growth in n EGFR-mutant mouse model | [179] |
| FAK | VS-4718 | PDAC | mice | Reduced the proportion of CD4 + FOXP3 + T cell in tumor tissue | [180] |
| FAK | Phellinus linteus (PL) | H22 liver cancer transplant tumor model | mice | PL inhibits tumor growth, promotes apoptosis, and reduces FAK, Treg, and M2 macrophages | [181] |
| FAK | Defactinib (VS-4718) | PDAC | Phase I clinical trial | Reduction in the number of Tregs within tumor tissue | [182] |
PDAC Pancreatic ductal adenocarcinoma, VP Verteporfin
Targeted integrin
As a critical molecule linking the mechanical properties of the ECM to intracellular mechanical signaling, integrins have emerged as key targets for mechano-immunomodulatory regulation. As previously discussed, the integrin αvβ8-mediated, mechanosensitive activation of TGF-β has been identified as a central regulator of Treg differentiation and tumor infiltration. Notably, αvβ8-specific antibodies have demonstrated significantly greater efficacy in inhibiting the TGF-β signaling pathway compared to TGF-βR2 antibodies [183]. This enhanced specificity underscores the therapeutic potential of targeting integrin-mediated mechanical signaling in Tregs. Supporting this, preclinical studies show that inhibition of Itgβ8 effectively blocks TGF-β activation by Tregs, attenuates their immunosuppressive function, and concurrently enhances the cytotoxicity of tumor-infiltrating CD8+ T cells, ultimately curbing tumor growth in murine models of melanoma (B16) and breast cancer (E0771) [137].
LFA-1, a crucial integrin mediating matrix mechanical signals, is regulated by the gp96 molecule. Research has shown that the LFA-1 inhibitor BIRT377 suppresses tumor growth and reduces Treg numbers in the murine B16F10 melanoma allograft model [157]. Further studies by Niu et al. revealed that subcutaneous growth of melanoma (B16F10) was significantly suppressed in LFA-1 knockout mice, an effect closely associated with reduced numbers of Tregs in the spleen, blood, and lymph nodes [157]. Additionally, Zhou et al. demonstrated that targeting the gp96/LFA-1 axis effectively blocked Treg tumor infiltration while concurrently enhancing CD8+ T cell function and inhibiting tumor progression in colon cancer (MC38), bladder cancer (MB49), and melanoma (B16-F10) models. Notably, this approach did not trigger autoimmunity or disrupt immune homeostasis [159]. These findings collectively suggest that Treg-specific targeting of the gp96/LFA-1 axis represents a valuable strategy for cancer immunotherapy without inducing autoinflammatory conditions.
Targeted YAP/TAZ
Verteporfin (VP), a specific inhibitor of YAP, attenuates tumor stroma mechanically-induced immunosuppression by inhibiting the formation of the YAP-TEAD complex [184]. Preclinical studies in GBM have demonstrated that VP not only decreases proliferation and induces glioma cell death in vitro [185, 186], but also reduces tumor growth in subcutaneous xenografts [187] and in an EGFR-mutant mouse model [179]. Beyond its direct anti-tumor effects, VP modulates the ITME. In T cell-specific YAP knockout mice, the proportion of FOXP3+ Tregs within the TME is reduced, and the growth of B16 melanoma is significantly suppressed. Furthermore, Ni et al. confirmed that the immunomodulatory effects of YAP deficiency synergize with checkpoint blockade therapy (anti-PD-1 antibody) or a tumor vaccine (GM-Vac), resulting in enhanced anti-tumor immune responses [144]. Combining VP with a leucine-restricted diet impairs mitochondrial function in TI-Tregs, severely weakening their immunosuppressive capabilities. However, given YAP’s physiological role in maintaining tissue homeostasis, long-term targeting of YAP may induce toxicity [126]. Consequently, researchers are exploring more precise regulatory strategies. Recent studies suggest that targeting the Activin/AcVR1C interaction may offer an ideal alternative, a strategy validated in mouse models [144]. This discovery opens new avenues for developing selective immunomodulatory approaches.
Targeted FAK/Src
The FAK/Src mechanical signaling pathway is essential in the mechanical response of Tregs. Preclinical studies have demonstrated the therapeutic potential of FAK inhibition in modulating the TME and improving anti-tumor immunity. Jiang et al. showed that the FAK inhibitor (VS-4718) significantly reduced the fibrotic stroma in PDAC, diminished the infiltration of immunosuppressive cells (including Tregs, TAMs, and MDSCs), and prolonged survival in KPC/KPPC transgenic mice [180]. Similarly, in a BRAF-mutant melanoma mouse model, dual inhibition of FAK and RAF/MEK significantly suppressed primary tumor growth, reduced lung and brain metastases, and prolonged survival in mice with brain metastases by cooperatively inducing apoptosis and suppressing metastatic signaling pathways [181]. Furthermore, a multicenter, open-label phase I study showed that a triple therapy combining the FAK inhibitor VS-6063 with pembrolizumab and gemcitabine reduced Treg numbers in tumor tissues and increased the infiltration of cytotoxic CD8+ T cells, providing clinical benefits for patients with advanced refractory PC [182]. Additionally, Chen et al. discovered through a C57BL/6-FAK–/– mouse model and a Treg-macrophage coculture system that FAK promotes Treg-mediated polarization of macrophages towards the M2 phenotype, thereby driving HCC growth. The Phellinus linteus can inhibit FAK and its downstream signaling pathway, reduce the Treg/M2 ratio, and remodel the anti-tumor immune microenvironment [188]. These findings highlight targeting FAK as a promising strategy to reprogram the ITME and enhance the efficacy of immunotherapy.
Emerging strategies focusing on the viscoelasticity and topological structure of the ECM have shown significant potential in modulating tumor progression and enhancing the efficacy of immunotherapy. For example, Fan et al. used pyridoxamine (PM) to inhibit AGE formation and alagebrium (ALT-711) to disrupt AGE-collagen cross-linking. These interventions reduced the viscoelasticity of liver tissue while inhibiting HCC progression in mice [44]. This approach not only underscores the therapeutic potential of ECM viscoelasticity modulation in metabolic disorder-associated cancers but also suggests broader applicability in stroma-rich malignancies. Advances in immune engineering have further leveraged ECM biomechanics to optimize adoptive cell therapies. Liu et al. developed synthetic cells (SynVACs) with viscoelastic properties similar to natural antigen-presenting cells (APCs), significantly reducing Treg numbers while enhancing CAR-T cell-mediated tumor attack [189]. Similarly, Lou et al. demonstrated that T-cell expansion was inhibited on viscoelastic microgels, compared to elastic microgels with identical surface ligand density, and there was an increased proportion of CD44–CD62L+ T cells, highlighting the potential of viscoelastic ECM modifications in boosting immune responses [190]. Notably, Schvartzman’s team engineered elastic PDMS microbrush platforms (1 μm diameter, 5 μm height) by integrating biochemical cues (antibody functionalization), mechanical signals (elastic modulus), and microscale topological features. This integrated approach significantly enhanced both the activation potency and proliferative capacity of CTLs [191]. These studies collectively suggest that regulating matrix viscoelasticity and topological structure can optimize the amplification efficiency of adoptive cell therapy products, such as CAR-T, providing crucial biomaterial design parameters for immune engineering.
Conclusion and challenges
This review systematically examines the impact and mechanisms of ECM mechanical properties—such as matrix stiffness, viscosity, and topological structure—on the differentiation, function, and migration of Tregs, alongside potential therapeutic strategies targeting the Treg mechanical transduction pathway. Overall, the research field targeting Treg mechanotransduction pathways to enhance anti-tumor immunity is still in its early exploratory stages, and current understanding is insufficient to support further clinical translation. Future research should focus on several key areas:
First, Complex interactions exist between different ECM mechanical properties, and manipulating a single physical cue often impacts other mechanical factors. For example, ECMs with high stress relaxation can dissipate energy, reducing cellular perception of stiffness [59]. It is essential for future studies to systematically decode how ECM mechanical cues dynamically regulate Treg immunosuppressive function, proliferation, and migration through specific mechanotransduction pathways (e.g., integrin, YAP/TAZ, Piezo1), providing critical insights into the development of tumor immunotherapies that specifically target Tregs while preserving effector T cell function. Secondly, ECM mechanical properties such as stiffness, viscoelasticity, and topology exhibit significant heterogeneity. This heterogeneity is not only observed across different tumor types and stages but also within various regions of the same tumor. Most current research focuses on a single mechanical parameter and fails to replicate the multiscale mechanical coupling of the ECM in vitro. Thus, future biomaterial designs should be based on a deep understanding of the mechanical heterogeneity present in different tumor ECMs, integrating multiple mechanical parameters to more accurately mimic the complex in vivo mechanical microenvironment. As noted earlier, both nuclear and cytoplasmic viscoelastic parameters (stiffness, viscosity, relaxation time) showed no significant differences between IHAs and GBM [49]. Recent studies combining AFM with electric cell-substrate impedance sensing (ECIS) revealed mechanical heterogeneity between T98G and U87 MG GBM cells: T98G cells exhibited a rougher surface (roughness RMS: 20.47 ± 7.04 nm), higher stiffness (E: 33.47 ± 1.67 kPa), and higher viscosity (η: 7.91 ± 1.17 kPa/s), whereas U87 MG cells had a smoother surface (roughness RMS: 13.71 ± 5.00 nm) and lower viscosity (η: 5.46 ± 2.45 kPa/s) [192]. Additionally, brain MRE showed that during the acute inflammatory phase of EAE, both the G’ and G’’ of brain tissue significantly decreased; tissue elasticity and viscosity returned to baseline levels post-inflammation resolution [193]. In thyroid cancer, compared to healthy thyroid cells, carcinoma cells exhibit reduced viscosity and elasticity (median E decreased from 1,190 Pa to 721 Pa; median η decreased from 496 Pa/s to 357 Pa/s). Conversely, at the tissue level, thyroid carcinoma tissue stiffness is significantly higher than healthy tissue, increasing approximately 2.6–14 fold [194]. These dynamic alterations in mechanical properties and their spatial heterogeneity, spanning from the cellular to the tissue level, profoundly influence tumor cell behavior and provide crucial design principles for constructing in vitro biomimetic platforms that more accurately simulate the complex in vivo mechanical environment.
In conclusion, the interdisciplinary integration of biomechanics, materials science, oncology, and immunology positions mechanical immunoregulation as a promising strategy to overcome drug resistance in immunotherapy. A deeper understanding of the interaction mechanisms between ECM mechanical properties and Tregs will significantly facilitate the development of tumor immune regulation strategies based on the mechanical microenvironment.
Supplementary Information
Supplementary Material 1: Supplementary Table 1. Effects of ECM mechanical cues on tumor cells.
Supplementary Material 2: Supplementary Table 2. Effects of ECM mechanical cues on immunosuppressive.
Acknowledgements
Thanks to ZYEdit for language help. Figures were created with BioRender.com.
Abbreviations
- TME
Tumor microenvironment
- TI-Tregs
Tumor-infiltrating regulatory T cells
- ICIs
Immune checkpoint inhibitors
- ECM
Extracellular matrix
- FN
Fibronectin
- HA
Hyaluronic acid
- ITME
Immunosuppressive tumor microenvironment
- CAFs
Cancer-associated fibroblasts
- MMPs
Matrix metalloproteinases
- TIMPs
Tissue inhibitors of metalloproteinases
- LOX
Lysyl oxidase
- ROCK
Rho-associated coiled-coil kinase
- CSC
Cancer stem cells
- HCC
Hepatocellular carcinoma
- SECM
scanning electrochemical microscopy
- MRP1
multidrug resistance protein 1
- NASH
Nonalcoholic steatohepatitis
- AGEs
Advanced glycation end products
- ROS
Reactive oxygen species
- PDAC
pancreatic ductal adenocarcinoma
- PAM
Polyacrylamide
- PDMS
Polydimethylsiloxane
- TAMs
Tumor-associated macrophages
- DDR1
Discoidin domain receptor 1
- CSF-1
Colony-stimulating factor 1
- MSC
mesenchymal stem cell
- DCs
Dendritic cells
- TNBC
Triple-negative breast cancer
- Piezo1
Piezo-type mechanosensitive ion channel component 1
- MDSCs
myeloid-derived suppressor cells
- CTLs
Cytotoxic T lymphocytes
- PA-gels
polyacrylamide gels
- ISMARA
Integrated Motif Activity Response Analysis
- AP-1
Activator Protein 1
- LTBP-1
Latent TGF-β Binding Protein-1
- HDFs
Human dermal fibroblasts
- YAP
Yes-associated protein
- TAZ
PDZ binding transcriptional activator of transcription
- TEAD
Transcriptional enhanced associate domain
- tDLNs
tumor-draining lymph nodes
- HIF-1α
Hypoxia-inducible factor-1 alpha
- ATF4
Activating transcription factor 4
- FAK
Focal adhesion kinase
- Src
Src-family kinases
- LFA-1
Lymphocyte function-associated antigen-1
- ICAM-1
Intercellular adhesion molecule-1
- OXPHOS
Oxidative phosphorylation
- FAO
Fatty acid oxidation
- Lars2
Leucyl-tRNA synthetase
- MIEF1
Mitochondrial elongation factor 1
- DRP1
Dynamin-related protein 1
- OPN
Osteopontin
- HSCs
Hepatic stellate cells
- VP
Verteporfin
- APCs
Antigen-presenting cells
- ECIS
Electric cell–substrate impedance sensing
Authors’ contributions
W.B.H. designed the review protocol, conducted the search, drafted the manuscript. W.B. H. and H.Z.L. prepared all Figures and tables. W.B.H. and H.Z.L. are contributed equally to this work and should be considered co-first authors. Z.L.D. and Q.M. screened potentially eligible studies, extracted and analyzed data, and updated reference lists. L.J., Q.M. and C.X. contributed to the design of the review protocol, arbitrating potentially eligible studies, and interpreting results. C.X. and F.M.Y. were responsible for the funding acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by the following funds:
1) National Natural Science Foundation of China (No. 82405359);
2) Chongqing Technology Innovation and Application Development Sichuan Chongqing Science and Technology Innovation Cooperation Plan Project (No. CSTB2024TIAD-CYKJCXX0037).
3) The “Xinglin Scholars” Discipline Talent Research Enhancement Plan of Chengdu University of TCM (QJRC2024009).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chong Xiao, Email: zyxcdoc@163.com.
Feng-Ming You, Email: yfmdoc@163.com.
References
- 1.Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41(3):404–20. 10.1016/j.ccell.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Peng H, Chao Z, Wang Z, Hao X, Xi Z, Ma S, et al. Biomechanics in the tumor microenvironment: from biological functions to potential clinical applications. Exp Hematol Oncol. 2025;14(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland TE, Dyer DP, Allen JE. The extracellular matrix and the immune system: A mutually dependent relationship. Science. 2023;379(6633):eabp8964. [DOI] [PubMed] [Google Scholar]
- 4.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–8. [DOI] [PubMed] [Google Scholar]
- 5.Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30(6):465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling MR, Kan A, Heinzel S, Marchingo JM, Hodgkin PD, Hawkins ED. Regulatory T cells suppress effector T cell proliferation by limiting division destiny. Front Immunol. 2018;9:2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Chen H, Zhao C, Li T, Zhang C, Ma L, et al. PRTN3 promotes IL33/Treg-mediated tumor immunosuppression by enhancing the M2 polarization of tumor-associated macrophages in lung adenocarcinoma. Cancer Lett. 2025;616:217584. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41(3):450–65. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai S, Itahashi K, Nishikawa H. Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine. Nat Rev Clin Oncol. 2024;21(5):337–53. [DOI] [PubMed] [Google Scholar]
- 12.Vogel V. Unraveling the mechanobiology of extracellular matrix. Annu Rev Physiol. 2018;80:353–87. [DOI] [PubMed] [Google Scholar]
- 13.Saraswathibhatla A, Indana D, Chaudhuri O. Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol. 2023;24(7):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efremov YM, Okajima T, Raman A. Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter. 2020;16(1):64–81. [DOI] [PubMed] [Google Scholar]
- 15.Chen SW, Teulon JM, Kaur H, Godon C, Pellequer JL. Nano-structural stiffness measure for soft biomaterials of heterogeneous elasticity. Nanoscale Horiz. 2022;8(1):75–82. [DOI] [PubMed] [Google Scholar]
- 16.Sauer F, Grosser S, Shahryari M, Hayn A, Guo J, Braun J, et al. Changes in tissue fluidity predict tumor aggressiveness in vivo. Adv Sci (Weinh). 2023;10(26):e2303523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, et al. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by Nidogens. J Biol Chem. 2012;287(22):18700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong W, Lyu C, Liao H, Du Y. Collagen crosslinking: effect on structure, mechanics and fibrosis progression. Biomed Mater. 2021;16(6):062005. [DOI] [PubMed]
- 20.Nonnast E, Mira E, Mañes S. Biomechanical properties of laminins and their impact on cancer progression. Biochim Biophys Acta Rev Cancer. 2024;1879(6):189181. [DOI] [PubMed] [Google Scholar]
- 21.Bradshaw MJ, Smith ML. Multiscale relationships between fibronectin structure and functional properties. Acta Biomater. 2014;10(4):1524–31. [DOI] [PubMed] [Google Scholar]
- 22.Roth J, Hoop CL, Williams JK, Hayes R, Baum J. Probing the effect of glycosaminoglycan depletion on integrin interactions with collagen I fibrils in the native extracellular matrix environment. Protein Sci. 2023;32(1):e4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. [DOI] [PubMed] [Google Scholar]
- 24.Nakai N, Hara M, Takahashi H, Shiga K, Hirokawa T, Maeda Y, et al. Cancer cellinduced tissue inhibitor of metalloproteinase-1 secretion by cancerassociated fibroblasts promotes cancer cell migration. Oncol Rep. 2022;47(6):112. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Li X, Wang L, Bi X, Zhong W, Yue J, et al. Lysine deacetylation is a key function of the Lysyl oxidase family of proteins in cancer. Cancer Res. 2024;84(5):652–8. [DOI] [PubMed] [Google Scholar]
- 26.Girigoswami K, Saini D, Girigoswami A. Extracellular matrix remodeling and development of cancer. Stem Cell Rev Rep. 2021;17(3):739–47. [DOI] [PubMed] [Google Scholar]
- 27.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatherree JP, Guarin JR, McGinn RA, Naber SP, Oudin MJ. Chemotherapy-Induced collagen IV drives cancer cell motility through activation of Src and focal adhesion kinase. Cancer Res. 2022;82(10):2031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19(6):776–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, et al. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol. 2014;207(2):283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu QP, Luo Q, Deng B, Ju Y, Song GB. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic Glycolysis via the MAPK-YAP signaling. Cancers (Basel). 2020;12(2):490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Wang W, Luo Q, Song G. High matrix stiffness accelerates migration of hepatocellular carcinoma cells through the integrin β1-Plectin-F-actin axis. BMC Biol. 2025;23(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papalazarou V, Zhang T, Paul NR, Juin A, Cantini M, Maddocks ODK, et al. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat Metab. 2020;2(1):62–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang J, Pang EM, Adebowale K, Wisdom KM, Chaudhuri O. Increased stiffness inhibits invadopodia formation and cell migration in 3D. Biophys J. 2020;119(4):726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy Choudhury A, Gupta S, Chaturvedi PK, Kumar N, Pandey D. Mechanobiology of cancer stem cells and their niche. Cancer Microenviron. 2019;12(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuermanbayi S, Yang Y, Zhao Y, Li Y, Wang L, Yang J, et al. In situ monitoring of functional activity of extracellular matrix stiffness-dependent multidrug resistance protein 1 using scanning electrochemical microscopy. Chem Sci. 2022;13(35):10349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Ye Y, Zhu L, Xiao X, Zhou B, Gu Y, et al. Niche stiffness sustains cancer stemness via TAZ and NANOG phase separation. Nat Commun. 2023;14(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Qiu S, Liu X, Guo F, Zhai J, Li Z, et al. Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling. Signal Transduct Target Ther. 2023;8(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cambria E, Coughlin MF, Floryan MA, Offeddu GS, Shelton SE, Kamm RD. Linking cell mechanical memory and cancer metastasis. Nat Rev Cancer. 2024;24(3):216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, et al. The Nanomechanical signature of breast cancer. Nat Nanotechnol. 2012;7(11):757–65. [DOI] [PubMed] [Google Scholar]
- 41.Mierke CT. Viscoelasticity acts as a marker for tumor extracellular matrix characteristics. Front Cell Dev Biol. 2021;9:785138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soliman BG, Nguyen AK, Gooding JJ, Kilian KA. Advancing synthetic hydrogels through Nature-Inspired materials chemistry. Adv Mater. 2024;36(42):e2404235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elosegui-Artola A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr Opin Cell Biol. 2021;72:10–8. [DOI] [PubMed] [Google Scholar]
- 44.Fan W, Adebowale K, Váncza L, Li Y, Rabbi MF, Kunimoto K, et al. Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver. Nature. 2024;626(7999):635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courbot O, Elosegui-Artola A. The role of extracellular matrix viscoelasticity in development and disease. NPJ Biol Phys Mech. 2025;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blatchley MR, Hall F, Wang S, Pruitt HC, Gerecht S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Sci Adv. 2019;5(3):eaau7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–22. [DOI] [PubMed] [Google Scholar]
- 48.Rubiano A, Delitto D, Han S, Gerber M, Galitz C, Trevino J, et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 2018;67:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onwudiwe K, Najera J, Holen L, Burchett AA, Rodriguez D, Zarodniuk M, et al. Single-cell mechanical assay unveils viscoelastic similarities in normal and neoplastic brain cells. Biophys J. 2024;123(9):1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khine YY, Nguyen H, Afolabi F, Lin CC. Fast-relaxing hydrogels with reversibly tunable mechanics for dynamic cancer cell culture. Biomater Adv. 2024;159:213829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seth P, Friedrichs J, Limasale YDP, Fertala N, Freudenberg U, Zhang Y, et al. Interpenetrating polymer network hydrogels with tunable viscoelasticity and proteolytic cleavability to direct stem cells in vitro. Adv Healthc Mater. 2025;14(9):e2402656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Najmina M, Ebara M, Ohmura T, Uto K. Viscoelastic liquid matrix with faster bulk relaxation time reinforces the cell cycle arrest induction of the breast cancer cells via oxidative stress. Int J Mol Sci. 2022;23(23):14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng H, Wang Y, Yin Y, Shu J, Zhang J, Shu X, et al. Effects of matrix viscoelasticity on cell-matrix interaction, actin cytoskeleton organization, and apoptosis of osteosarcoma MG-63 cells. J Mater Chem B. 2023;12(1):222–32. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen HD, Lin CC. Viscoelastic stiffening of gelatin hydrogels for dynamic culture of pancreatic cancer spheroids. Acta Biomater. 2024;177:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen H, Luong NH, Peil JK, Tong Y, Mitchell DK, Fishel ML, et al. Fast-Relaxing hydrogels promote pancreatic adenocarcinoma cell aggressiveness through integrin β1 signaling. Biomacromolecules. 2025;26(2):1098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Chen C, Li H, Zhao P, Zhao Y, Li B, et al. Visible light-responsive hydrogels for cellular dynamics and Spatiotemporal viscoelastic regulation. Nat Commun. 2025;16(1):1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cieśluk M, Piktel E, Wnorowska U, Skłodowski K, Kochanowicz J, Kułakowska A, et al. Substrate viscosity impairs temozolomide-mediated Inhibition of glioblastoma cells’ growth. Biochim Biophys Acta Mol Basis Dis. 2022;1868(11):166513. [DOI] [PubMed] [Google Scholar]
- 58.Sinha S, Ayushman M, Tong X, Yang F. Dynamically crosslinked Poly(ethylene-glycol) hydrogels reveal a critical role of viscoelasticity in modulating glioblastoma fates and drug responses in 3D. Adv Healthc Mater. 2023;12(1):e2202147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huerta-López C, Clemente-Manteca A, Velázquez-Carreras D, Espinosa FM, Sanchez JG, Martínez-Del-Pozo Á, et al. Cell response to extracellular matrix viscous energy dissipation outweighs high-rigidity sensing. Sci Adv. 2024;10(46):eadf9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciccone G, Azevedo Gonzalez-Oliva M, Versaevel M, Cantini M, Vassalli M, Salmeron-Sanchez M, et al. Epithelial cell mechanoresponse to matrix viscoelasticity and confinement within micropatterned viscoelastic hydrogels. Adv Sci (Weinh). 2025;12(18):e2408635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massey A, Stewart J, Smith C, Parvini C, McCormick M, Do K, et al. Mechanical properties of human tumour tissues and their implications for cancer development. Nat Rev Phys. 2024;6(4):269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naba A. Mechanisms of assembly and remodelling of the extracellular matrix. Nat Rev Mol Cell Biol. 2024;25(11):865–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beunk L, Bakker GJ, van Ens D, Bugter J, Gal F, Svoren M, et al. Actomyosin contractility requirements and reciprocal cell-tissue mechanics for cancer cell invasion through collagen-based channels. Eur Phys J E Soft Matter. 2022;45(5):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamauchi M, Gibbons DL, Zong C, Fradette JJ, Bota-Rabassedas N, Kurie JM, Kurie JM. Fibroblast heterogeneity and its impact on extracellular matrix and immune landscape remodeling in cancer. Matrix Biol. 2020;91–92:8–18. [DOI] [PubMed] [Google Scholar]
- 65.Esbona K, Yi Y, Saha S, Yu M, Van Doorn RR, Conklin MW, et al. The presence of cyclooxygenase 2, Tumor-Associated macrophages, and collagen alignment as prognostic markers for invasive breast carcinoma patients. Am J Pathol. 2018;188(3):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drifka CR, Loeffler AG, Mathewson K, Keikhosravi A, Eickhoff JC, Liu Y, et al. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget. 2016;7(46):76197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones CE, Hammer AM, Cho Y, Sizemore GM, Cukierman E, Yee LD, et al. Stromal PTEN regulates extracellular matrix organization in the mammary gland. Neoplasia. 2019;21(1):132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP. Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys J. 2017;112(5):1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su CY, Burchett A, Dunworth M, Choi JS, Ewald AJ, Ahn EH, et al. Engineering a 3D collective cancer invasion model with control over collagen fiber alignment. Biomaterials. 2021;275:120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasudevan J, Jiang K, Fernandez JG, Lim CT. Extracellular matrix mechanobiology in cancer cell migration. Acta Biomater. 2023;163:351–64. [DOI] [PubMed] [Google Scholar]
- 71.Blackmon RL, Sandhu R, Chapman BS, Casbas-Hernandez P, Tracy JB, Troester MA, et al. Imaging extracellular matrix remodeling in vitro by Diffusion-Sensitive optical coherence tomography. Biophys J. 2016;110(8):1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hadzipasic M, Zhang S, Huang Z, Passaro R, Sten MS, Shankar GM, et al. Emergence of nanoscale viscoelasticity from single cancer cells to established tumors. Biomaterials. 2024;305:122431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahr JC, Li XY, Feinberg TY, Jiang L, Weiss SJ. Divergent regulation of basement membrane trafficking by human macrophages and cancer cells. Nat Commun. 2022;13(1):6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sleeboom JJF, van Tienderen GS, Schenke-Layland K, van der Laan LJW, Khalil AA, Verstegen MMA. The extracellular matrix as hallmark of cancer and metastasis: from biomechanics to therapeutic targets. Sci Transl Med. 2024;16(728):eadg3840. [DOI] [PubMed] [Google Scholar]
- 75.Contessi Negrini N, Ricci C, Bongiorni F, Trombi L, D’Alessandro D, Danti S, et al. An osteosarcoma model by 3D printed polyurethane scaffold and in vitro generated bone extracellular matrix. Cancers (Basel). 2022;14(8):2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nerger BA, Kashyap K, Deveney BT, Lou J, Hanan BF, Liu Q, et al. Tuning porosity of macroporous hydrogels enables rapid rates of stress relaxation and promotes cell expansion and migration. Proc Natl Acad Sci U S A. 2024;121(45):e2410806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wisdom KM, Adebowale K, Chang J, Lee JY, Nam S, Desai R, et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat Commun. 2018;9(1):4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mierke CT. Extracellular matrix cues regulate mechanosensing and mechanotransduction of cancer cells. Cells. 2024;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Xiao Y, Liu C. The horizon of materiobiology: A perspective on Material-Guided cell behaviors and tissue engineering. Chem Rev. 2017;117(5):4376–421. [DOI] [PubMed] [Google Scholar]
- 80.Wendong Y, Jiali J, Qiaomei F, Yayun W, Xianze X, Zheng S, et al. Biomechanical forces and force-triggered drug delivery in tumor neovascularization. Biomed Pharmacother. 2024;171:116117. [DOI] [PubMed] [Google Scholar]
- 81.Rosado-Galindo H, Domenech M. Surface roughness modulates EGFR signaling and stemness of triple-negative breast cancer cells. Front Cell Dev Biol. 2023;11:1124250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kraning-Rush CM, Reinhart-King CA. Controlling matrix stiffness and topography for the study of tumor cell migration. Cell Adh Migr. 2012;6(3):274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu C, Chen M, Zheng T, Yang X. Effect of surface roughness on the initial response of MC3T3-E1 cells cultured on polished titanium alloy. Biomed Mater Eng. 2015;26(Suppl 1):S155–64. [DOI] [PubMed] [Google Scholar]
- 84.Peranzoni E, Rivas-Caicedo A, Bougherara H, Salmon H, Donnadieu E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70(23):4431–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang JY, Cheng YJ, Lin YP, Lin HC, Su CC, Juliano R, et al. Extracellular matrix of glioblastoma inhibits polarization and transmigration of T cells: the role of tenascin-C in immune suppression. J Immunol. 2010;185(3):1450–9. [DOI] [PubMed] [Google Scholar]
- 86.Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbæk MS, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Li J, Hou Y, Lin Y, Zhao H, Shi Y, et al. Osr2 functions as a Biomechanical checkpoint to aggravate CD8+ T cell exhaustion in tumor. Cell. 2024;187(13):3409–e342624. [DOI] [PubMed] [Google Scholar]
- 88.Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, van den Dries K. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci Rep. 2017;7(1):17511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi Y, Kwon JE, Cho YK. Dendritic cell migration is tuned by mechanical stiffness of the confining space. Cells. 2021;10(12):3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aykut B, Chen R, Kim JI, Wu D, Shadaloey SAA, Abengozar R, et al. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci Immunol. 2020;5(50):eabb5168. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Wang S, Li Y, Zhang N, Gribskov M, Zhang X, et al. miRNA-mediated macrophage behaviors responding to matrix stiffness and ox-LDL. J Cell Physiol. 2020;235(9):6139–53. [DOI] [PubMed] [Google Scholar]
- 92.Cai G, Lu Y, Zhong W, Wang T, Li Y, Ruan X, et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. 2023;56(9):e13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atcha H, Jairaman A, Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat Commun. 2021;12(1):3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chuang YC, Chang HM, Li CY, Cui Y, Lee CL, Chen CS. Reactive oxygen species and inflammatory responses of macrophages to substrates with physiological stiffness. ACS Appl Mater Interfaces. 2020;12(43):48432–41. [DOI] [PubMed] [Google Scholar]
- 95.Talarico G, Lecchi M, Costanza M, Chiodoni C, Cappelletti V, Verderio P, et al. Extracellular matrix drives high-grade breast cancer immune suppression down-modulating PD-1 on Treg cells via the IL-23/SATB1 axis. Cancer Res. 2023;83:3637–7. [Google Scholar]
- 96.Adu-Berchie K, Liu Y, Zhang DKY, Freedman BR, Brockman JM, Vining KH, et al. Generation of functionally distinct T-cell populations by altering the viscoelasticity of their extracellular matrix. Nat Biomed Eng. 2023;7(11):1374–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vining KH, Marneth AE, Adu-Berchie K, Grolman JM, Tringides CM, Liu Y, et al. Mechanical checkpoint regulates monocyte differentiation in fibrotic niches. Nat Mater. 2022;21(8):939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalashnikov N, Moraes C. Substrate viscoelasticity affects human macrophage morphology and phagocytosis. Soft Matter. 2023;19(13):2438–45. [DOI] [PubMed] [Google Scholar]
- 99.Sapudom J, Alatoom A, Tipay PS, Teo JC. Matrix stiffening from collagen fibril density and alignment modulates YAP-mediated T-cell immune suppression. Biomaterials. 2025;315:122900. [DOI] [PubMed] [Google Scholar]
- 100.Hedde PN, Staaf E, Singh SB, Johansson S, Gratton E. Pair correlation analysis maps the dynamic Two-Dimensional organization of natural killer cell receptors at the synapse. ACS Nano. 2019;13(12):14274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bougherara H, Mansuet-Lupo A, Alifano M, Ngô C, Damotte D, Le Frère-Belda MA, et al. Real-Time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol. 2015;6:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun X, Wu B, Chiang HC, Deng H, Zhang X, Xiong W, et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature. 2021;599(7886):673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quartey BC, Sapudom J, Tipay PS, Hunashal Y, Alshehhi S, Arnoux M, et al. Hydrogel-Based tumor tissue microarchitecture reshapes dendritic cell metabolic profile and functions. Adv Healthc Mater. 2025;14(12):e2500681. [DOI] [PubMed] [Google Scholar]
- 104.Zhao R, Zhou X, Khan ES, Alansary D, Friedmann KS, Yang W, et al. Targeting the Microtubule-Network rescues CTL killing efficiency in dense 3D matrices. Front Immunol. 2021;12:729820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tolentino MAK, Seyedzadeh MH, Peres NG, Du EY, Zhu L, Gaus K, et al. Polyethylene Glycol-Based hydrogel as a 3D extracellular matrix mimic for cytotoxic T lymphocytes. J Biomed Mater Res A. 2025;113(1):e37811. [DOI] [PubMed] [Google Scholar]
- 106.Alamer M, Yun Xu X. The influence of tumour vasculature on fluid flow in solid tumours: a mathematical modelling study. Biophys Rep. 2021;7(1):35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poli A, Pennacchio FA, Ghisleni A, di Gennaro M, Lecacheur M, Nastały P, et al. PIP4K2B is mechanoresponsive and controls heterochromatin-driven nuclear softening through UHRF1. Nat Commun. 2023;14(1):1432. [DOI] [PMC free article] [PubMed]
- 108.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62(18):5381–5. [PubMed] [Google Scholar]
- 109.Zoi I, Gargalionis AN, Papavassiliou KA, Nasiri-Ansari N, Piperi C, Basdra EK, et al. Polycystin-1 and hydrostatic pressure are implicated in glioblastoma pathogenesis in vitro. J Cell Mol Med. 2022;26(5):1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7(1):17696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kao YC, Jheng JR, Pan HJ, Liao WY, Lee CH, Kuo PL. Elevated hydrostatic pressure enhances the motility and enlarges the size of the lung cancer cells through Aquaporin upregulation mediated by caveolin-1 and ERK1/2 signaling. Oncogene. 2017;36(6):863–74. [DOI] [PubMed] [Google Scholar]
- 112.Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, Jain RK. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 2013;73(13):3833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun J, Luo Q, Liu L, Song G. Low-level shear stress promotes migration of liver cancer stem cells via the FAK-ERK1/2 signalling pathway. Cancer Lett. 2018;427:1–8. [DOI] [PubMed] [Google Scholar]
- 114.Tang K, Li S, Li P, Xia Q, Yang R, Li T, et al. Shear stress stimulates integrin β1 trafficking and increases directional migration of cancer cells via promoting deacetylation of microtubules. Biochim Biophys Acta Mol Cell Res. 2020;1867(5):11867. [DOI] [PubMed] [Google Scholar]
- 115.Heine M, Freund B, Nielsen P, Jung C, Reimer R, Hohenberg H, et al. High interstitial fluid pressure is associated with low tumour penetration of diagnostic monoclonal antibodies applied for molecular imaging purposes. PLoS ONE. 2012;7(5):e36258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miao L, Lin CM, Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J Control Release. 2015;219:192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li R, Serrano JC, Xing H, Lee TA, Azizgolshani H, Zaman M, et al. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol Biol Cell. 2018;29(16):1927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeng Y, Gao M, Lin D, Du G, Cai Y. Prognostic and immunological roles of MMP-9 in Pan-Cancer. Biomed Res Int. 2022;2022:2592962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niland S, Riscanevo AX, Eble JA. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int J Mol Sci. 2021;23(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. [DOI] [PubMed] [Google Scholar]
- 121.Arpinati L, Carradori G, Scherz-Shouval R. CAF-induced physical constraints controlling T cell state and localization in solid tumours. Nat Rev Cancer. 2024;24(10):676–93. [DOI] [PubMed] [Google Scholar]
- 122.Cinier J, Hubert M, Besson L, Di Roio A, Rodriguez C, Lombardi V, et al. Recruitment and expansion of Tregs cells in the tumor Environment-How to target them?? Cancers (Basel). 2021;13(8):1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nataraj NM, Dang AP, Kam LC, Lee JH. Ex vivo induction of regulatory T cells from conventional CD4+ T cells is sensitive to substrate rigidity. J Biomed Mater Res A. 2018;106(12):3001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Li C, Gao H, Nabeka H, Shimokawa T, Wakisaka H, et al. Rho kinase inhibitors stimulate the migration of human cultured osteoblastic cells by regulating actomyosin activity. Cell Mol Biol Lett. 2011;16(2):279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shi L, Lim JY, Kam LC. Improving regulatory T cell production through mechanosensing. J Biomed Mater Res A. 2024;112(7):1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bai J, Yan M, Xu Y, Wang Y, Yao Y, Jin P, et al. YAP enhances mitochondrial OXPHOS in tumor-infiltrating Treg through upregulating Lars2 on stiff matrix. J Immunother Cancer. 2024;12(11):e010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi L, Lim JY, Kam LC. Substrate stiffness enhances human regulatory T cell induction and metabolism. Biomaterials. 2023;292:121928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan DJ, Shi L, Kam LC. Biphasic response of T cell activation to substrate stiffness. Biomaterials. 2021;273:120797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao H, Tian Q, Zhou Y, Zhu L, Lu Y, Ma Y, et al. 3D collagen fiber concentration regulates Treg cell infiltration in triple negative breast cancer. Front Immunol. 2022;13:904418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Yang H, Jia A, Wang Y, Yang Q, Dong Y, et al. Dendritic cell Piezo1 directs the differentiation of Th1 and Treg cells in cancer. Elife. 2022;11:e79957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Batlle E, Massagué J. Transforming growth Factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yukawa M, Jagannathan S, Vallabh S, Kartashov AV, Chen X, Weirauch MT, et al. AP-1 activity induced by co-stimulation is required for chromatin opening during T cell activation. J Exp Med. 2020;217(1):e20182009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koizumi SI, Sasaki D, Hsieh TH, Taira N, Arakaki N, Yamasaki S, et al. JunB regulates homeostasis and suppressive functions of effector regulatory T cells. Nat Commun. 2018;9(1):5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klann JE, Kim SH, Remedios KA, He Z, Metz PJ, Lopez J, et al. Integrin activation controls regulatory T Cell-Mediated peripheral tolerance. J Immunol. 2018;200(12):4012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Worthington JJ, Kelly A, Smedley C, Bauché D, Campbell S, Marie JC, et al. Integrin αvβ8-Mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-Cell-Mediated inflammation. Immunity. 2015;42(5):903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lainé A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Léon S, et al. Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat Commun. 2021;12(1):6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4(1):29–37. [DOI] [PubMed] [Google Scholar]
- 138.Hinz B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. [DOI] [PubMed] [Google Scholar]
- 139.Klingberg F, Chau G, Walraven M, Boo S, Koehler A, Chow ML, et al. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci. 2018;131(5):jcs201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20(8):888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fan Y, Gao Y, Rao J, Wang K, Zhang F, Zhang C. YAP-1 promotes Tregs differentiation in hepatocellular carcinoma by enhancing TGFBR2 transcription. Cell Physiol Biochem. 2017;41(3):1189–98. [DOI] [PubMed] [Google Scholar]
- 143.Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, et al. YAP is essential for Treg-Mediated suppression of antitumor immunity. Cancer Discov. 2018;8(8):1026–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meng KP, Majedi FS, Thauland TJ, Butte MJ. Mechanosensing through YAP controls T cell activation and metabolism. J Exp Med. 2020;217(8):e20200053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019;573(7773):230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jairaman A, Othy S, Dynes JL, Yeromin AV, Zavala A, Greenberg ML, et al. Piezo1 channels restrain regulatory T cells but are dispensable for effector CD4+ T cell responses. Sci Adv. 2021;7(28):eabg5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abiff M, Alshebremi M, Bonner M, Myers JT, Kim BG, Tomchuck SL, et al. Piezo1 facilitates optimal T cell activation during tumor challenge. Oncoimmunology. 2023;12(1):2281179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang C, Tang Z, Zhao Y, Yao R, Li L, Sun W. Three-dimensional in vitro cancer models: a short review. Biofabrication. 2014;6(2):022001. [DOI] [PubMed] [Google Scholar]
- 149.Ullrich KA, Derdau J, Baltes C, Battistella A, Rosso G, Uderhardt S, et al. IL-3 receptor signalling suppresses chronic intestinal inflammation by controlling mechanobiology and tissue egress of regulatory T cells. Gut. 2023;72(11):2081–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J, Xie SA, Li N, Zhang T, Yao W, Zhao H, et al. Matrix stiffness exacerbates the Proinflammatory responses of vascular smooth muscle cell through the DDR1-DNMT1 mechanotransduction axis. Bioact Mater. 2022;17:406–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maitz K, Valadez-Cosmes P, Raftopoulou S, Kindler O, Kienzl M, Bolouri H, et al. Altered Treg infiltration after discoidin domain receptor 1 (DDR1) Inhibition and knockout promotes tumor growth in lung adenocarcinoma. Cancers (Basel). 2023;15(24):5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang K, Farrell A, Zhou E, Qin H, Zeng Z, Zhou K, et al. ATF4 drives regulatory T cell functional specification in homeostasis and obesity. Sci Immunol. 2025;10(105):eadp7193. [DOI] [PubMed] [Google Scholar]
- 153.Seed RI, Kobayashi K, Ito S, Takasaka N, Cormier A, Jespersen JM, et al. A tumor-specific mechanism of Treg enrichment mediated by the integrin αvβ8. Sci Immunol. 2021;6(57):eabf0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang W, Lollis EM, Bordeleau F, Reinhart-King CA. Matrix stiffness regulates vascular integrity through focal adhesion kinase activity. FASEB J. 2019;33(1):1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, et al. Nuclear FAK controls chemokine transcription, tregs, and evasion of anti-tumor immunity. Cell. 2015;163(1):160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klaus T, Wilson A, Fichter M, Bros M, Bopp T, Grabbe S, et al. The role of LFA-1 for the differentiation and function of regulatory T Cells-Lessons learned from different Transgenic mouse models. Int J Mol Sci. 2023;24(7):6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Niu T, Li Z, Huang Y, Ye Y, Liu Y, Ye Z, et al. LFA-1 knockout inhibited the tumor growth and is correlated with Treg cells. Cell Commun Signal. 2023;21(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walling BL, Kim M. LFA-1 in T cell migration and differentiation. Front Immunol. 2018;9:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou L, Velegraki M, Wang Y, Mandula JK, Chang Y, Liu W, et al. Spatial and functional targeting of intratumoral Tregs reverses CD8 + T cell exhaustion and promotes cancer immunotherapy. J Clin Invest. 2024;134(14):e180080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. 2022;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.López-Soldado I, Torres AG, Ventura R, Martínez-Ruiz I, Díaz-Ramos A, Planet E, et al. Decreased expression of mitochondrial aminoacyl-tRNA synthetases causes downregulation of OXPHOS subunits in type 2 diabetic muscle. Redox Biol. 2023;61:102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Romani P, Benedetti G, Cusan M, Arboit M, Cirillo C, Wu X, et al. Mitochondrial mechanotransduction through MIEF1 coordinates the nuclear response to forces. Nat Cell Biol. 2024;26(12):2046–60. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Gershoni A, Hassin O, Nataraj NB, Baruch S, Avioz-Seligman A, Pirona AC, et al. TAZ facilitates breast tumor growth by promoting an immune-suppressive tumor microenvironment. Mol Oncol. 2023;17(12):2675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumari A, Kashyap D, Garg VK. Osteopontin in cancer. Adv Clin Chem. 2024;118:87–110. [DOI] [PubMed] [Google Scholar]
- 165.Vakrakou AG, Kourepini E, Skordos I, Nieto N, Panoutsakopoulou V, Paschalidis N. Osteopontin regulates Treg cell stability and function with implications for Anti-Tumor immunity and autoimmunity. Cancers (Basel). 2024;16(17):2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The biology of T regulatory type 1 cells and their therapeutic application in Immune-Mediated diseases. Immunity. 2018;49(6):1004–19. [DOI] [PubMed] [Google Scholar]
- 167.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108(19):7938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4 + CD25 + regulatory T cells. J Leukoc Biol. 2009;86(3):567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu W, Zhang W, Zhao D, Wang Q, Zhang M, Li Q, et al. Unveiling the role of regulatory T cells in the tumor microenvironment of pancreatic cancer through single-cell transcriptomics and in vitro experiments. Front Immunol. 2023;14:1242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 and Treg cells. J Exp Med. 2011;208(7):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wu Y, Song Y, Soto J, Hoffman T, Lin X, Zhang A, et al. Viscoelastic extracellular matrix enhances epigenetic remodeling and cellular plasticity. Nat Commun. 2025;16(1):4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang X, Feng M, Liu X, et al. Persistence of cirrhosis is maintained by intrahepatic regulatory T cells that inhibit fibrosis resolution by regulating the balance of tissue inhibitors of metalloproteinases and matrix metalloproteinases. Transl Res. 2016;169:67–79. e1-2. [DOI] [PubMed] [Google Scholar]
- 174.Zhao W, Su W, Kuang P, Zhang L, Liu J, Yin Z, et al. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol. 2012;41(2):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu KJ, Qian QF, Zhou JR, Sun DL, Duan YF, Zhu X, et al. Regulatory T cells (Tregs) in liver fibrosis. Cell Death Discov. 2023;9(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Winerdal ME, Krantz D, Hartana CA, Zirakzadeh AA, Linton L, Bergman EA, et al. Urinary bladder cancer Tregs suppress MMP2 and potentially regulate invasiveness. Cancer Immunol Res. 2018;6(5):528–38. [DOI] [PubMed] [Google Scholar]
- 177.Shan F, Somasundaram A, Bruno TC, Workman CJ, Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. 2022;8(11):944–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kumar P, Saini S, Prabhakar BS. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. 2020;64:29–35. [DOI] [PubMed] [Google Scholar]
- 179.Vigneswaran K, Boyd NH, Oh SY, Lallani S, Boucher A, Neill SG, et al. YAP/TAZ transcriptional coactivators create therapeutic vulnerability to verteporfin in EGFR-mutant glioblastoma. Clin Cancer Res. 2021;27(5):1553–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Almazan J, Turapov T, Kircher DA, Stanley KA, Culver K, Medellin AP, et al. Combined Inhibition of focal adhesion kinase and RAF/MEK elicits synergistic Inhibition of melanoma growth and reduces metastases. Cell Rep Med. 2025;6(2):101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang-Gillam A, Lim KH, McWilliams R, Suresh R, Lockhart AC, Brown A, et al. Defactinib, pembrolizumab, and gemcitabine in patients with advanced treatment refractory pancreatic cancer: A phase I dose escalation and expansion study. Clin Cancer Res. 2022;28(24):5254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shah SR, Kim J, Schiapparelli P, Vazquez-Ramos CA, Martinez-Gutierrez JC, Ruiz-Valls A, et al. Verteporfin-Loaded polymeric microparticles for intratumoral treatment of brain cancer. Mol Pharm. 2019;16(4):1433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang Y, Fu L, Liu B, Li F. YAP induces FAK phosphorylation to inhibit gastric cancer cell proliferation via upregulation of HMGB1. Int J Biol Macromol. 2024;262(Pt 1):130037. [DOI] [PubMed] [Google Scholar]
- 185.Barrette AM, Ronk H, Joshi T, Mussa Z, Mehrotra M, Bouras A, et al. Anti-invasive efficacy and survival benefit of the YAP-TEAD inhibitor verteporfin in preclinical glioblastoma models. Neuro Oncol. 2022;24(5):694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kuramoto K, Yamamoto M, Suzuki S, Sanomachi T, Togashi K, Seino S, et al. Verteporfin inhibits oxidative phosphorylation and induces cell death specifically in glioma stem cells. FEBS J. 2020;287(10):2023–36. [DOI] [PubMed] [Google Scholar]
- 187.Stockis J, Liénart S, Colau D, Collignon A, Nishimura SL, Sheppard D, et al. Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8. Proc Natl Acad Sci U S A. 2017;114(47):E10161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen F, Gong M, Weng D, Jin Z, Han G, Yang Z, et al. Phellinus Linteus activates Treg cells via FAK to promote M2 macrophage polarization in hepatocellular carcinoma. Cancer Immunol Immunother. 2024;73(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Z, Li YR, Yang Y, Zhu Y, Yuan W, Hoffman T, et al. Viscoelastic synthetic antigen-presenting cells for augmenting the potency of cancer therapies. Nat Biomed Eng. 2024;8(12):1615–33. [DOI] [PubMed] [Google Scholar]
- 190.Lou J, Meyer C, Vitner EB, Adu-Berchie K, Dacus MT, Bovone G, et al. Surface-Functionalized microgels as artificial Antigen-Presenting cells to regulate expansion of T cells. Adv Mater. 2024;36(31):e2309860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pandey A, Iraqi M, Toledo E, Al-Kader Yassin A, Podvalni E, Naaz S, et al. Elastic microstructures: combining biochemical, mechanical, and topographical cues for the effective activation and proliferation of cytotoxic T cells. ACS Appl Mater Interfaces. 2023;15(26):31103–13. [DOI] [PubMed] [Google Scholar]
- 192.Masud N, Hasib MHH, Ibironke B, Block C, Hughes J, Ekpenyong A, et al. Exploring the heterogeneity in glioblastoma cellular mechanics using in-vitro assays and atomic force microscopy. Sci Rep. 2025;15(1):19302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Riek K, Millward JM, Hamann I, Mueller S, Pfueller CF, Paul F, et al. Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. Neuroimage Clin. 2012;1(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rianna C, Radmacher M. Comparison of viscoelastic properties of cancer and normal thyroid cells on different stiffness substrates. Eur Biophys J. 2017;46(4):309–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Table 1. Effects of ECM mechanical cues on tumor cells.
Supplementary Material 2: Supplementary Table 2. Effects of ECM mechanical cues on immunosuppressive.
Data Availability Statement
No datasets were generated or analysed during the current study.