Abstract
Accurately predicting the therapeutic response of non-small cell lung cancer (NSCLC) patients to tyrosine kinase inhibitors (TKIs) is of significant clinical importance. The use of TKIs in clinical is primarily guided by the detection of EGFR gene mutations. However, the current EGFR mutation assays face challenges such as inconsistent correlation with therapeutic outcomes, inconvenient sample availability and limited sensitivity. To address these, we have designed and synthesized a novel theranostic agent, OSA-Cy7, by conjugating the third-generation EGFR-TKI osimertinib with the near-infrared (NIR) fluorophore Cy7. This conjugate aims to enable fluorescence-based detection of mutant EGFR and targeted therapy of NSCLC. Our studies demonstrated that OSA-Cy7 selectively accumulates in EGFR-mutant NSCLC cell lines, such as PC9 (exon 19 deletion) and H1975 (L858R/T790M), exhibiting enhanced fluorescence signals, while showing minimal uptake in wild-type EGFR A549 cells. Western blot analysis confirmed that OSA-Cy7 effectively inhibits EGFR phosphorylation in mutant cell lines, with negligible effects on wild-type EGFR phosphorylation. Furthermore, OSA-Cy7 treatment resulted in significant suppression on cell proliferation and colony formation in EGFR-mutant cells, indicating its potent anticancer activity. These findings suggest that OSA-Cy7 holds promise as a theranostic agent for the selective imaging and treatment of EGFR-mutant NSCLC, potentially improving patient stratification and therapeutic monitoring.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12896-025-01025-w.
Keywords: TKI response evaluation, EGFR mutation dectection, Osimertinib, Fluorescence-based assay, NSCLC, Theranostic agent
Introduction
Epidermal growth factor receptor (EGFR) is one of the major driver genes in non-small cell lung cancer (NSCLC) [1]. EGFR mutations, particularly those in the tyrosine kinase domain, are commonly observed in NSCLC and have been implicated in both the development of cancer and its response to targeted therapies [2–4]. Currently, EGFR tyrosine kinase inhibitors (EGFR-TKIs) have emerged as an important treatment strategy for EGFR-sensitive NSCLC [5]. The EGFR exon 21 L858R substitution and in-frame deletions in exon 19 (19del) are the most frequently identified activating mutations in the EGFR tyrosine kinase domain, which represent ∼90% of all EGFR mutations [2, 3]. First- and second-generation EGFR-TKIs, including gefitinib, erlotinib, afatinib, and dacotinib, have become the standard first-line treatment for patients with EGFR mutant NSCLC [6]. However, the development of resistance to these drugs occurred after about a median time of 9–15 months of treatment [7]. It is now well established that the EGFR secondary T790M mutation is the dominant cause of failure in first- and second-generation EGFR-TKI therapies [8]. In response, third-generation EGFR-TKI, such as AZD9291 (osimertinib), have been developed [9]. It irreversibly binds to mutant EGFR, particularly the T790M mutation, while sparing wild-type EGFR. Moreover, osimertinib was also shown to have superior efficacy over standard first and second-generation EGFR TKIs in the treatment of NSCLC patients with the common activating mutations and was recently approved for first-line therapy [10].
Testing for EGFR mutations is essential for predicting TKI response and informing clinical treatment decisions [11]. Currently, biopsy-based gene sequencing is considered the gold standard for identifying patients who may benefit from third-generation EGFR-TKI therapy. However, this gene-based approach often faces challenges such as poor reproducibility and limited sensitivity, particularly when detecting EGFR mutant tumor cells against a background of nonmutant cells. Additionally, genetic method-based predictions may not always align with clinical outcomes from EGFR-TKI treatment. For example, it has been found that about 10% of NSCLC patients with sensitive EGFR mutations do not respond to EGFR-TKI therapy, while a small subset of patients who do not exhibit mutations also benefit from TKI therapy [12, 13]. Therefore, from a therapeutic perspective, using probes based on the structural properties of EGFR-TKI drugs to directly assess EGFR drug interaction may provide a more reliable approach for predicting TKI treatment response.
Several efforts have been made to develop molecular imaging methods using EGFR TKIs to predict responses to EGFR-TKI therapy [14–18]. For example, gefitinb (second-generation TKI) was conjugated with the fluorophore SBD (4-sulfonamidebenzoxadiazole) to create a fluorogenic probe that enhances fluorescence upon binding to mutant EGFR, thereby enabling the prediction of treatment response in NSCLC [14]. Similarly, erlotinib, a first-generation TKI, was conjugated with hemicyanine to create a reactive oxygen species (ROS)-responsive near-infrared (NIR) fluorescent probe for eligible patient screening and drug resistance monitoring [15]. However, a fluorescent probe specifically designed to predict therapy response to third-generation EGFR-TKIs has not yet been developed. Third-generation EGFR-TKIs, such as osimertinib, are designed to irreversibly bind to both common activating EGFR mutations and the secondary T790M resistance mutation, while sparing wild-type EGFR. Therefore, developing imaging probes based on third-generation EGFR-TKIs is of significant importance for predicting therapeutic responses and monitoring resistance in NSCLC patients.
In this study, we have developed a near-infrared (NIR) probe, OSA-Cy7, by conjugating osimertinib with the Cy7 fluorophore. Our study demonstrated that OSA-Cy7 could selectively binds to cells harboring the common active mutant EGFR, including 19del, and L858R/T790M, compared with wild-type EGFR. Furthermore, OSA-Cy7 showed significant inhibitory activity against these mutant NSCLC cells. These results suggest that OSA-Cy7 could serve as a promising tool for screening and treating patients sensitive to third-generation EGFR-TKI therapy.
Materials and methods
Instruments
UV–vis absorption and fluorescence spectra were recorded with Spectrofluorometer FS5 (Edinburgh Instruments). Fluorescence imaging of cells was carried out by a ImageXpress Micro Confocal High-Content Imaging System (San Jose, USA). 1H NMR and 13C NMR spectra were collected on Bruker AV-600. High-resolution mass spectra (HRMS) were obtained using Q-Exactive Orbitrap (Thermo-Fisher Scientific, USA). Compounds were purified using semi-preparative HPLC (High Performance Liquid Chromatograph) using a COSMOSIL C18-MS-II column (20 mm × 200 mm; Nacalai Tesque, Japan) on an Agilent 1200 system equipped with a diode array detector (DAD) (Agilent Technologies, USA). Flow cytometry analysis was performed using a CytoFLEX Flow Cytometer (Beckman Coulter, USA).
The synthesis of OSA-Cy7
Osimertinib (0.5 g, Mreda) and acrylic acid (3 ml, Sigma) were mixed in a reaction tube and heated at 40 °C for 4 h. The reaction was monitored by TLC plate and LC-MS. The yellow solid product was obtained by separation and purification via a reverse phase preparation HPLC. Compound 1 was obtained in about 60% yield (Fig. S1–S4, Table S1). Coupling reaction of compound 1 (0.03 g) and sulfo-Cy7 (0.05 g, Ruixibiotech) amine 2 with EDC (1 equivalent) and HOBt (1 equivalent) in the presence of 5 equivalents of DIPEA in DMF at 25 °C for 5 h resulted in probe OSA-Cy7. OSA-Cy7 (about 33% yield) was purified using semi-preparative HPLC under following condition: mobile phase A: water, B: acetonitrile, 0–2 min 15% B, 3–13 min 40% B, 14–40 min 70% B. The compound was monitored at 750 nm. Fractions corresponding to the OSA-Cy7 peak (retention time: 26.5 min) were collected and lyophilized. The purity and structure of OSA-Cy7 was confirmed by HPLC-HR MS/MS (Fig. S5 and S6).
Cell culture and Anti-proliferation assay
Cell lines (A549, H1975, and PC9) were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (Shanghai, China) and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (Gibco, ThermoFisher Scientific), as previously described [19]. All cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO₂. Cells were passaged upon reaching 80–90% confluence. Cytotoxicity was assessed using the Cell Counting Kit-8 (CCK-8) assay following the manufacturer’s instructions. Briefly, cells were seeded into 96-well plates, allowed to adhere overnight, and then treated with varying concentrations of OSA-Cy7 for 48 h, with osimertinib processed in parallel as a reference compound under identical conditions. After incubation, CCK-8 reagent was added and absorbance measured at 450 nm to determine cell viability. For the colony formation assay, 1,000 cells per well were plated in 6-well plates and incubated for 24 h before treatment. Cells were then exposed to different concentrations of OSA-Cy7 and cultured for 10 days. At the end of the treatment period, colonies were fixed with 4% paraformaldehyde, stained with crystal violet, and counted to evaluate long-term antiproliferative effects.
NIR fluorescence imaging analysis
Cells were placed in 96 well glass bottom plates for 24 h at 37 °C in a humidified atmosphere containing 5% CO₂. After washing three times with phosphate-buffered saline (PBS), 100 µL of 1 µM probe was added to the wells for 1 h in the dark at 37 °C. After washed three times with PBS, cell images were captured using ImageXpress Micro Confocal High-Content Imaging System (San Jose, USA) (Cy7 channel λex = 750 nm, λem = 780 nm). Mean fluorescence intensity (MFI) was quantified using ImageJ software.
Flow cytometry analysis
Cells were seeded at a density of 5 × 10⁵ per well in six-well plates. After 24 h, cells were left untreated or treated with OSA-Cy7 (1 µM) or sulfo-Cy7 (Cy7, 1 µM) for 1 h at 37 °C, then cells were digested using trypsin with EDTA and washed three times with PBS. Flow cytometric analysis was performed on a CytoFLEX flow cytometer (Beckman Coulter) using a 638 nm laser for excitation and a 763/43 BP emission filter (R763-APCA750 channel). Data analysis and graphical output were performed using FlowJo software.
Western blot analysis
Western blot analysis was performed to investigate the expression of EGFR and the downstream signaling pathway in various NSCLC cell lines. Cells were added at a density of 5 × 105 per well in six-well plates and allowed to adhere for 24 h. Cells were then treated with various concentrations of OSA-Cy7 in RPMI‑1640 containing 2% FBS for 24 h prior to EGF stimulation (10 ng/mL). After a PBS washing step, the cells were harvested and lysed in RIPA lysis buffer containing protease/phosphatase inhibitor cocktails (Roche). After sonication, cell lysates were centrifuged at 12,000×g at 4℃ for 10 min. The supernatant was collected and protein concentration was determined by a BCA protein quantitation kit (Thermo Fisher, USA). Subsequently, 30 µg of protein sample was loaded to each lane and resolved on a 10% SDS-PAGE gel. After that, proteins on the gel were transferred onto poly-vinylidene difluoride (PVDF) membranes. PVDF membranes were blocked with 5% BSA blocking solution for 1.5 h at room temperature, followed by incubation with different primary antibodies (4℃, 1:1000), including EGFR (CatNo. 4267, CST), phospho-EGFR (CatNo. 3777, CST), Akt (CatNo. 9272, CST), Phospho-Akt (CatNo. 4060, CST) and β-Actin (CatNo. 81115, proteintech). Next, the membranes were washed by TBST buffer (Tris buffer with 0.1% Tween-20), followed by incubation with HRP-conjugated rabbit secondary antibodies. Signal detection was performed using enhanced chemiluminescence (ECL) and visualized with a ChemiDoc imaging system (Bio-Rad, CA, USA).
Statistical
All statistical analyses were performed using GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA).
Results
Synthesis and characterization of OSA-Cy7
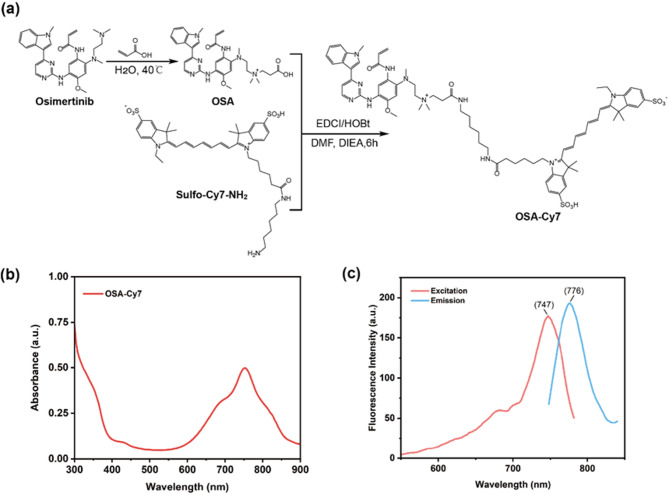
The synthetic route and structure of OSA-Cy7 is presented in Fig. 1a. The ultraviolet absorption and fluorescence spectrum of OSA-Cy7 (1 µM) were measured in PBS (20 mM, pH 7.4). It could be learned that the maximum ultraviolet absorption and maximum emission wavelength of OSA-Cy7 are around 750 nm, and 780 nm respectively (Fig. 1b, c), which is close to the original dye Cy7. Notably, we observed a subtle shoulder near 700 nm in both the absorption and excitation spectra of OSA-Cy7, which may be partially attributed to the aggregation. This is plausible given the amphiphilic nature of the molecule, which comprises a hydrophobic osimertinib core and a hydrophilic sulfo-Cy7 moiety, potentially promoting partial self-association. Nonetheless, the overall spectral profiles remain highly consistent with those of Cy7, indicating that the conjugation of osimertinib to Cy7-NH₂ does not significantly alter the intrinsic photophysical properties of Cy7.
Fig. 1.
Structure and optical property of OSA-Cy7. (a) Synthetic route and chemical structure of OSA-Cy7; (b): UV-Vis absorbance spectrum of OSA-Cy7; (c) Excitation and fluorescence spectrum of OSA-Cy7
Targeting selectivity of OSA-Cy7 to mutant EGFR cell lines
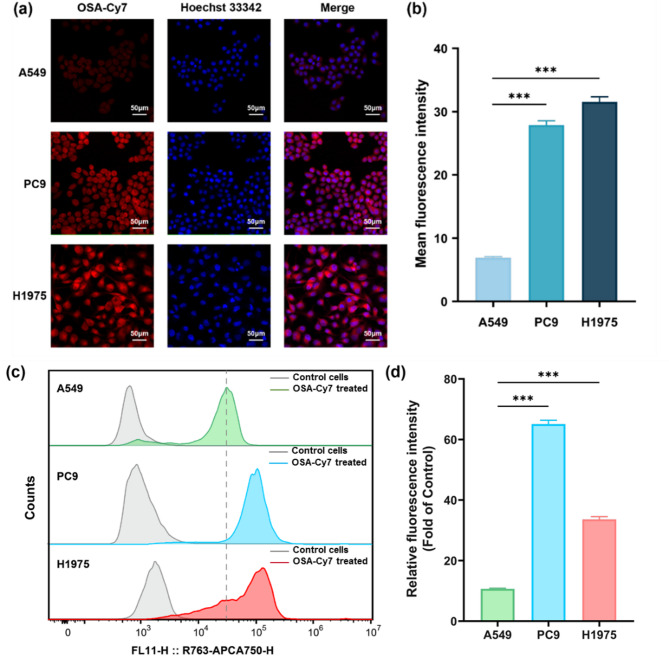
To investigate the selectivity of OSA-Cy7 in NSCLC cells, we first incubated various cell lines with OSA-Cy7 and analyzed the results using fluorescence microscopy. PC9 and H1975 cells, harboring the 19del and L858R/T790M mutations, respectively, are sensitive to third-generation EGFR TKIs, whereas A549 cells, which have wild-type EGFR, are not. All cell lines were incubated for 1 h with 1 µM OSA-Cy7. As shown in Fig. 2a and b, significant NIR fluorescence was observed exclusively in H1975 and PC9 cells, indicating strong binding of OSA-Cy7 to mutant EGFR. In contrast, A549 cells, which express wild-type EGFR, displayed very weak NIR fluorescence, suggesting minimal binding between OSA-Cy7 and wild-type EGFR. To confirm that the observed fluorescence in H1975 and PC9 cells was due to OSA-Cy7 binding and not the Cy7 dye alone, free Cy7 dye was incubated with all cell lines under the same conditions. As expected, no obvious binding of the free Cy7 dye was detected in H1975 or PC9 cells (supporting information Fig. S7), supporting the conclusion that the fluorescence observed in these cells was attributable to the Osimertinib core of OSA-Cy7, rather than the Cy7 dye tag.
Fig. 2.
Selective targeting of active mutant EGFR by OSA-Cy7 in various NSCLC cell lines. (a) Confocal fluorescence imaging (20× magnification) of NSCLC cells treated with OSA-Cy7: PC9 (EGFR 19del mutant), H1975 (L858R/T790M mutant), and A549 (wild-type EGFR). The red fluorescence represents the Cy7 signal, nuclei are counter stained with Hoechst 33,342 (blue channel). (b) Quantitative analysis of mean fluorescence intensity (MFI) from images in (a), demonstrating differential uptake of OSA-Cy7 among cell lines (n = 3). (c) Flow cytometry analysis assessing the selectivity of OSA-Cy7 binding in PC9, H1975, and A549 cell lines (the dotted line represents the MFI of A549 cells). (d) Quantification of MFI from (c) after normalization to the corresponding MFI of untreated control cells, demonstrating the preferential accumulation of OSA-Cy7 in EGFR-mutant cells. All statistical P values were calculated by the two-tailed student’s t test (n = 3, ***P < 0.001, data represent the mean ± SD)
Further, flow cytometry was used to validate the binding selectivity of OSA-Cy7 to EGFR protein at the cellular level (Fig. 2c and d). Consistent with the fluorescence microscopy results, stronger probe uptake was observed in EGFR mutant H1975 and PC9 cells compared to WT A549 cells. Additionally, uptake of free Cy7 was lower overall and showed no selectivity toward EGFR-mutant cells (supporting information Fig. S8). Collectively, demonstrate that OSA-Cy7 exhibits a selective binding affinity for EGFR mutations associated with third-generation EGFR TKI sensitivity, making it a promising tool for predicting patient response to third-generation EGFR TKI therapy.
Anti-proliferation and EGFR TK inhibitory activity of OSA-Cy7
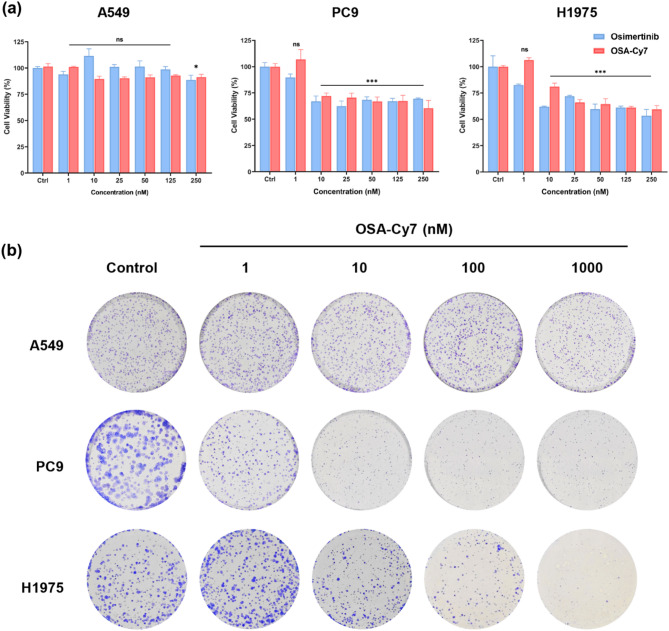
Subsequently, we assessed the cytotoxicity of OSA-Cy7 on NSCLC cell lines A549 (wild-type EGFR), PC9 (Ex 19del), and H1975 (L858R/T790M) using CCK8 assay. As shown in Fig. 3a, the drug-dye conjugate OSA-Cy7 exhibited potent antiproliferative activity comparable to that of its parent compound osimertinib (Fig. 3a). To further confirm the selective inhibitory effects of OSA-Cy7 on EGFR mutant cells, we performed a colony formation assay. As shown in Fig. 3b, OSA-Cy7 treatment significantly reduced the number of colonies in EGFR mutant PC9 and H1975 cell lines, while the proliferation of wild-type A549 cells was unaffected.
Fig. 3.
Effects of OSA-Cy7 on cell proliferation and colony formation in three NSCLC cell lines. (a) Cytotoxicity of OSA-Cy7 and osimertinib was evaluated in A549 (wild-type EGFR), H1975 (L858R/T790M mutant), and PC9 (19del mutant) cells after 48 h of treatment (n = 3, student’s t-test, *P < 0.05, ***P < 0.001, ns = not significant). (b) Representative image of colony formation, with colonies stained using crystal violet
Additionally, OSA-Cy7 also potently and dose-dependently inhibited phosphorylation of EGFR and its downstream protein, AKT, in EGFR-mutant cell lines (H1975 and PC9 cells), while having less activity against EGFR phosphorylation in the A549 wild-type EGFR cell line, consistent with the known selectivity of osimertinib [9]. These findings suggest that OSA-Cy7 can effectively suppress the cellular phosphorylation of mutant EGFR, leading to the inhibition of cell proliferation. Overall, it could be concluded that OSA-Cy7 not only retains the mutant EGFR-targeting ability of osimertinib, while also exhibiting potent antitumor activity.
Discussion
The therapeutic efficacy of TKIs in NSCLC is closely linked to the specific types of EGFR mutations present [11]. Thus, EGFR testing of NSCLC is required to predict the sensitivity to TKIs and to identify TKI applicable patients. Currently, gene mutation analysis is the gold standard for to identify patients who may benefit from targeted therapy [20]. However, this approach suffers various limitations, like invasiveness, high cost, inaccuracy caused by sample quality, and can not capture the holistic picture of tumor EGFR status.
In response, imaging-based assays for EGFR TKI response have obtained significant attention [14–16, 21]. In this study, we developed an Osimertinib-based near-infrared (NIR) probe for identifying tumor cells sensitive to third-generation EGFR-TKIs. Osimertinib, the most widely approved and utilized third-generation EGFR-TKI, was initially approved for the treatment of NSCLC patients harboring the EGFR T790M resistance mutation [22]. More recently, it has also been approved as a first-line therapy for patients with common EGFR mutations, such as exon 19del and the L858R mutation [10, 23]. Compared with the first and second generation TKI, osimertinib could irreversible binding to EGFR, form covalent bonds with the cysteine 797 residue, even when mutant EGFR exhibit resistance to first- and second-generation EGFR-TKIs [24]. The irreversible binding to EGFR make it a ideal target molecule for construction of sensitive imaging probe [22]. Moreover, NIR imaging offers several key advantages for diagnostic applications [25]. It provides high resolution and signal-to-background scaling, enabling effective real-time imaging with minimal interference from background fluorescence. Unlike other imaging modalities, NIR is non-radioactive and safe, eliminating the risks of radiation exposure. Additionally, it does not require expensive equipment, making it a cost-effective option for both clinical and research settings. Our results demonstrated that OSA-Cy7 could preferably accumulated in Osimertinib sensitive H1975 (L858R/T790M) and PC9 (19del) cells compared with A549 (WT) cells (Fig. 2). Stronger NIR intensity was observed in H1975 and PC-9 cells, indicating the NIR probe could selectively target the sensitive H1975 and PC9 cells which is consistent with its parent compound Osimertinib, and could be used to discriminating activating mutant and WT EGFR. Given its distinct fluorescence characteristics in EGFR-sensitive and non-sensitive cells, we propose that OSA-Cy7’s NIR fluorescence could serve as a valuable tool for screening NSCLC patients eligible for third-generation EGFR-TKI therapy. A direct application scenario would be the use of flow cytometry or fluorescence imaging analysis of OSA-Cy7 treated surgical samples to screen for eligibility for third-generation EGFR-TKI therapy [14]. Furthermore, based on the biological properties of NIR, OSA-Cy7 holds potential for in vivo applications [26]. The OSA-Cy7 probe could be potentially utilized as a tool to monitor acquired drug resistance and evaluate the efficacy of third-generation TKI therapy. This is particularly important as acquired resistance to third-generation EGFR-TKIs can arise through the EGFR C797S mutation, which impairs Osimertinib binding [27]. By detecting this resistance early, physicians could make informed decisions, such as switching to alternative treatments, thus preserving the therapeutic window and avoiding ineffective treatment.
Furthermore, OSA-Cy7 also possesses potent anti-cancer activity. Cytotoxicity, colony formation assay together with cellular phosphorylation studies have also collectively showed that OSA-Cy7 is highly potent against both activating and T790M resistant EGFR mutant cells, with a wide selectivity margin over wild-type EGFR activity (Fig. 3). Thus, it also retains the potent antitumor activity of Osimertinib. EGFR-TKIs exert their anticancer effects primarily by inhibiting the phosphorylation of EGFR [9]. Western blot analysis demonstrated that OSA-Cy7 selectively inhibits the phosphorylation of mutant EGFR in NSCLC cell lines. Specifically, treatment with OSA-Cy7 resulted in a concentration-dependent decrease in EGFR phosphorylation levels in H1975 and PC9cells. In contrast, A549 cells, which possess wild-type EGFR, exhibited no significant changes in phosphorylation levels upon OSA-Cy7 treatment (Fig. 4). These findings suggest that OSA-Cy7 preferentially targets mutant forms of EGFR, aligning with the known selectivity profile of osimertinib, the parent compound of OSA-Cy7 [9]. This inhibition prevents the activation of downstream signaling pathways that promote tumor cell proliferation and survival.
Fig. 4.
Effect of OSA-Cy7 on EGFR phosphorylation in representative mutant EGFR lines (PC-9 and H1975) and wild-type EGFR cell line (A549). EGFR phosphorylation (p) and typical downstream AKT signaling pathway were significantly inhibited by OSA-Cy7 in PC-9 and H1975 cells, while exhibiting minimal activity against EGFR phosphorylation in A549 cells. The specific blot for phosphorylated and total EGFR, AKT derives from parallel gels run with identical lysate aliquots, representative bands were selected based on band clarity
Despite its promising results, this study has certain limitations. While the ability to screen for TKI sensitivity in vitro suggests that OSA-Cy7 could be applied to patient-derived samples, such as fluorescence analysis of surgical specimens, its in vivo application requires further validation. To this end, NSCLC xenograft models will be employed to evaluate its real-time imaging capability, tumor-targeting efficiency, and therapeutic response monitoring. A foreseeable challenge is that the in vivo performance of OSA-Cy7 may be compromised by metabolic conversion to known osimertinib derivatives (e.g., AZ5104 and AZ7550) [28] or by nonspecific cleavage of the Cy7 linker, either of which could diminish tumor selectivity and fluorescence signal intensity. To address these potential limitations, future studies will focus on evaluating the metabolic stability, pharmacokinetics, and biodistribution of OSA-Cy7, as well as optimizing its molecular structure to maintain its theranostic integrity. These efforts will be essential to validate the probe’s performance in vivo and refine its design for potential clinical translation.
Conclusions
In this study, we have developed a novel NIR fluorescent probe, OSA-Cy7, by conjugating the third-generation EGFR-TKI osimertinib with the Cy7 fluorophore. OSA-Cy7 demonstrates high sensitivity and selectivity for detecting common activating EGFR mutations in NSCLC cells, such as exon 19 deletion and L858R/T790M mutations. Additionally, OSA-Cy7 could effectively inhibit EGFR phosphorylation, resulting in significant antiproliferative effects against EGFR-mutant tumor cells. These findings suggest that OSA-Cy7 holds promise as a valuable theranostic agent that combines selective molecular imaging with targeted therapy for EGFR-mutant NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Ms. Jane Q.Q Feng for her review of the manuscript and valuable suggestions.
Author contributions
D.Y., and L.J.H. methodology, investigation and formal analysis. W.J, H.L, and L.X.Q., data curation and validation, H.X.Z. and Z.P.W conceptualization, supervision and writing.
Funding
This work was supported by National Natural Science Foundation of China (NSFC) (No: 82202639), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab) (No: 2020B1212030006 and MY2022KF01); Traditional Chinese Medicine Bureau of Guangdong Province (No: 20241122 and 20254048), and the Guangdong Academy of Chinese Medicine (YN2023QN14, YN2024GZRPY002, and YN2024HK14).
Data availability
All data supporting the findings reported in this study are provided within the main manuscript and its Supplementary Information files.
Declarations
Ethics approval and consent to participate
This study did not involve human participants, animals, human tissue, human data, or clinical trials. All cell lines were obtained from commercial repositories and used under standard laboratory protocols. Ethical approval was not required for this research and compliance with the Declaration of Helsinki was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Dong and Jinhang Li contributed equally to this work.
Contributor Information
Xianzhang Huang, Email: huangxz020@gzucm.edu.cn.
Pengwei Zhang, Email: pwzhang2020@gzucm.edu.cn.
References
- 1.Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Zhao W, Li X, Zhang S, Zhou C, Zhou D, et al. Mutation spectrum of EGFR from 21,324 Chinese patients with Non-Small cell lung cancer (NSCLC) successfully tested by multiple methods in a CAP-Accredited laboratory. Pathol Oncol Res. 2021;27:602726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Yang H, Teo ASM, Amer LB, Sherbaf FG, Tan CQ, et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat Genet. 2020;52:177–86. [DOI] [PubMed] [Google Scholar]
- 4.Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in people’s Republic of China. J Thorac Oncol. 2020;15:1567–76. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto I, Mitsudomi T, Nakagawa K, Fukuoka M. The emerging role of epidermal growth factor receptor (EGFR) inhibitors in first-line treatment for patients with advanced non-small cell lung cancer positive for EGFR mutations. Ther Adv Med Oncol. 2010;2:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karachaliou N, Fernandez-Bruno M, Bracht JWP, Rosell R. EGFR first- and second-generation TKIs—there is still place for them in EGFR-mutant NSCLC patients. Translational Cancer Res. 2019;8(Suppl 1):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferro A, Marinato GM, Mulargiu C, Marino M, Pasello G, Guarneri V, et al. The study of primary and acquired resistance to first-line osimertinib to improve the outcome of EGFR-mutated advanced Non-small cell lung cancer patients: the challenge is open for new therapeutic strategies. Crit Rev Oncol/Hematol. 2024;196:104295. [DOI] [PubMed] [Google Scholar]
- 8.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross DAE, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-Mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-Mutated advanced Non-Small-Cell lung cancer. N Engl J Med. 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 11.Medical Advisory Secretariat. Epidermal growth factor receptor mutation (EGFR) testing for prediction of response to EGFR-Targeting tyrosine kinase inhibitor (TKI) drugs in patients with advanced Non-Small-Cell lung cancer: an Evidence-Based analysis. Ont Health Technol Assess Ser. 2010;10:1–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K. Primary resistance to EGFR tyrosine kinase inhibitors (TKIs): contexts and comparisons in EGFR-Mutated lung cancer. J Respiration. 2023;3:223–36. [Google Scholar]
- 13.Shepherd FA. Should EGFR tyrosine kinase inhibitors be used in non-small cell lung cancer in the absence of EGFR mutations? Yes, there is a role for EGFR TKIs in these patients. Clin Adv Hematol Oncol. 2016;14:41–3. [PubMed] [Google Scholar]
- 14.Deng H, Lei Q, Wang C, Wang Z, Chen H, Wang G, et al. A fluorogenic probe for predicting treatment response in non-small cell lung cancer with EGFR-activating mutations. Nat Commun. 2022;13:6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu X, Wu M, Luo Q. Development of a NIR fluorescent probe for fluorescence-assisted EGFR-TKI applicable patients screening and drug resistance monitoring. Eur J Med Chem. 2023;261:115818. [DOI] [PubMed] [Google Scholar]
- 16.Högnäsbacka A, Poot AJ, Kooijman E, Schuit RC, Schreurs M, Verlaan M et al. Synthesis and preclinical evaluation of two osimertinib isotopologues labeled with carbon-11 as PET tracers targeting the tyrosine kinase domain of the epidermal growth factor receptor. Nucl Med Biol. 2023;120–1:108349. [DOI] [PubMed]
- 17.Kim MH, Kim S-G, Kim D-W. A novel Dual-labeled peptide for multimodal imaging of EGFR with L858R mutation. Curr Radiopharm. 2024;17:174–83. [DOI] [PubMed] [Google Scholar]
- 18.Lei Q, Zhou X, Li Y, Zhao S, Yang N, Xiao Z, et al. Image-Based phenotypic profiling enables rapid and accurate assessment of EGFR-Activating mutations in tissues from lung cancer patients. J Am Chem Soc. 2025;147:4552–70. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Zhang P, Xu F, Hu S, Liu J, Tan Y, et al. Ynamide electrophile for the profiling of ligandable carboxyl residues in live cells and the development of new covalent inhibitors. J Med Chem. 2022;65:10408–18. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian J, Gregg J, Berktas M, Li J, Leighl NB. EGFR testing practices, treatment choice and clinical outcomes in advanced NSCLC in a real-world setting: A retrospective analysis of a US-based electronic health records database. Lung Cancer. 2025;201:108412. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhang L, Lin X, Ke L, Li B, Xu L, et al. Dual-responsive nanosystem for precise molecular subtyping and resistant reversal of EGFR targeted therapy. Chem Eng J. 2019;372:483–95. [Google Scholar]
- 22.Jänne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99. [DOI] [PubMed] [Google Scholar]
- 23.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-Mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 24.Finlay MRV, Anderton M, Ashton S, Ballard P, Bethel PA, Box MR, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57:8249–67. [DOI] [PubMed] [Google Scholar]
- 25.Bernhard W, El-Sayed A, Barreto K, Gonzalez C, Fonge H, Geyer CR. Near infrared imaging of epidermal growth factor receptor positive xenografts in mice with domain I/II specific antibody fragments. Theranostics. 2019;9:974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Refaat A, Yap ML, Pietersz G, Walsh APG, Zeller J, del Rosal B, et al. In vivo fluorescence imaging: success in preclinical imaging paves the way for clinical applications. J Nanobiotechnol. 2022;20:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansour A, AboulMagd MM, Abbas AH, Abdel-Rahman SM, Abdel-Aziz H. Insights into fourth generation selective inhibitors of (C797S) EGFR mutation combating non-small cell lung cancer resistance: a critical review. RSC Adv. 2023;13:18825–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickinson PA, Cantarini MV, Collier J, Frewer P, Martin S, Pickup K, et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos. 2016;44:1201–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings reported in this study are provided within the main manuscript and its Supplementary Information files.