Abstract
Polymer additive manufacturing (AM) is a powerful method for medical device prototyping, producing low-cost medical devices for resource limited settings and patient-specific customization. Plastic medical devices created with AM must undergo sterilization prior to in vivo experiments or use with patients. While prior studies have verified the feasibility and safety of sterilization of such devices, the impact on mechanical performance has not been studied. Temperatures during autoclave sterilization, a commonly used and widely available method, can match or exceed the melt temperature of Nylon-12 used for selective laser sintering. Here we tested the impact of single or multiple cycles of autoclave sterilization on the mechanical and functional performance of plastic components used with interventional radiology related medical devices (catheter hub, ablation probe handle, and implantable port). We found that up to 2 cycles of sterilization did not tangibly impact the manufacturability, function or surface finish of these devices. However, we found that more than one cycle of sterilization can compromise the mechanical strength of the devices, with geometric-linked variations in the level of change in stiffness (ranging from 12 – 23 %). In conclusion, autoclave sterilization is safe for single use AM medical devices, where repetition of the sterilization for device reuse can compromise mechanical performance.
Keywords: Interventional Radiology, 3D printing, Additive Manufacturing, Medical Devices, Rapid Prototyping
Introduction
Additive manufacturing (AM), commonly termed “3D printing”, has been widely adopted and used for producing customized patient-specific medical devices [1], proof of principle prototypes [2], and to bring low-cost devices in resource restricted environments [3]. 3D printing has been used to produce both metal and plastic components for medical devices, the former being extensively used with patients undergoing orthopedic, dental, and neural surgery [1], and the latter for image-guided interventional procedures [4,5].
As with all medical devices intended for surgical use, 3D-printed parts must be sterilized prior to use in preclinical experiments or with patients. While literature suggests that sterilization of 3D printed medical devices to feasible and safe [6], the specific impact of sterilization on the mechanical and functional performance has not been previously studied. Several sterilization techniques are used with medical devices, namely chemical techniques that use ethylene dioxide or hydrogen peroxide, γ-irradiation, and autoclaves. Not all techniques are suitable for sterilizing 3D-printed plastic parts; for example the use of γ-irradiation may degrade polymer components [6–8]. While chemical techniques are safer for use with plastic parts, there is risk of toxicity from residual chemicals, and recently the Food and Drug Administration has begun discouraging the use of ethylene dioxide for sterilization [9,10].
Autoclave sterilization is therefore the most ubiquitous and cost-effective technique but the temperatures within the autoclave can exceed the melting temperature of polymers commonly used for 3D printing [11]. The objective of this work was to examine whether dry heat autoclave would be safe for sterilization of 3D-printed medical device components for image-guided interventions. We hypothesized that 3D-printed plastic medical devices can be sterilized multiple times without compromising the parts’ mechanical or functional performance.
Materials and methods
Prototype modeling and manufacture
Three common interventional oncology devices were chosen for rapid prototyping, namely a vascular catheter (5Fr SoftVu, Angiodynamics, MA) with catheter hub (Qosina, Ronkonoma, NY), ablation probe handle (Nanoknife, Angiodynamics, MA) and an implantable port (Titanium, Bard Medical). Models of these devices were built using a commercially available computer aided design (CAD) software (Inventor, Autodesk Inc., San Rafael, California). The CAD models were exported to a stereolithography format (.STL) for additive manufacturing. All parts were fabricated with a selective laser sintering printer (SLS) (Formiga P110, Krailling, Germany) using Nylon-12 powder. The choice of printer and resin material was driven by manufacturer advertised biocompatibility and the theoretical ability to tolerate autoclave sterilization [11]. A minimum of 5 copies of each part was used for testing from a batch of approximately 8–10 prints. An example of each part is shown in Fig. 1.
Fig. 1. Sample 3D printed parts of medical devices using SLS printer.

A, D.) Body of a implantable port. B, E.) Hub of a endovascular catheter. C, F.) Handle of an ablation probe.
Experimental design
The overall workflow for autoclave sterilization and subsequent mechanical testing is described in Fig. 2. Sample parts were selected in a randomized fashion to undergo sterilization or be held as sham control. The sterilization process was repeated twice, with mechanical and visual testing performed immediately after each step.
Fig. 2.

Experimental workflow.
Sterilization procedure
Up to 2 cycles of sterilization was performed using a standard autoclave (44 Laboratory Autoclave, Tuttnauer, Breda, Noord-Brabant, Netherlands). Manufacturer protocols for the autoclave were used to perform the sterilization, where each cycle was carried out at 15 psi and 121 °C for a minimum of 15 min.
Mechanical fit check
A fit check was conducted for each test device by assembling the 3D-printed component to its corresponding mating feature within the device. Specifically, the back of the catheter hub was threaded onto the luer lock of a 5cc syringe, and the front was press fit with 5-French catheter tubing; a 22 Ga hypodermic needle was press fit into the ablation device handle, and the port device was press fit into a 5-French catheter tubing. The test was conducted by two independent operators in a semi-blinded fashion with qualitative verbal feedback on any perceived differences in the assembly of components between sham and treated parts.
Geometric measurements
Critical dimensions and external geometric shapes of each part were measured using a Coordinate Measuring Machine (CMM) (Nikon Altera 7.5.5 Coordinate Measuring Machine, Nikon, Tokyo, Japan). Specifically, the cross-sectional diameter of the ablation handle, the diameter of the catheter hub tip, circularity at different locations, flatness, and cylindrical measurements of the port device were measured. Custom fixtures were fabricated to secure the test parts onto the CMM base. The measurement locations for the three test devices are shown in Fig. 3. The measurements were repeated five times for each printed part. The variance of these five measurements for each trial and sham were compared.
Fig. 3. Critical dimensions measured using CMM (A, C, D) and CAD visualization of CMM fixture set-up (B, E, F).

Dotted line on devices depicts location of measurement. A-B.) Ablation Handle. C, E.) Catheter Hub. D, F.) Port device.
Optical inspection
Changes in the surface properties and appearance of the printed parts pre- and post- sterilization were qualitatively assessed via stereomicroscope imaging (KLA Tencor Alpha D-500, KLA Tencor, Milpitas, CA) monitoring changes at sharp edges and visual details of critical part features. These features were considered essential part attributes based on their involvement with the corresponding mating feature and the eventual user/operator of the part. The surfaces monitored were the ablation handle tab, the catheter luer lock, and the port device tip. The images were evaluated by two independent readers in a semi-blinded fashion to determine any visually perceivable difference between sham and treated parts.
Mechanical strength testing
The mechanical performance of the printed parts was evaluated by 3-Point bending and single-axis tensile testing using a Universal Test Machine (5500 Universal Test Machine, Instron, Norwood, MA). Machined aluminum fixtures were built for the 3-point bending test to suspend the center of each device for bending, as shown in Fig. 4. A ¼”–20 screw was used to mount the fixture to the base of the testing machine. The machine force load (0–5 kN) and part displacement (0–15 mm) were measured for each test. The final displacement and force applied to each part were collected for stiffness quantification.
Fig. 4. CAD representation of fixture setups for 3-point bending and compression mechanical tests using the universal test machine.

The red parts are the device stands and machine parts, and the gray parts are the medical devices. A.) Ablation handle. B.) Catheter hub. C.) Port Device.
Statistical analysis
The raw data collected from each print/device was recorded as mean and standard deviation by aggregating the data from the five trials. One-way Anova testing was performed to determine variation in outcomes, comparing the first and second round of sterilization with sham controls. A p-value of 0.05 or lower was designated to be statistically significant. The final displacements of the trials were compared amongst groups for the mechanical strength tests. All statistical analysis was performed using Graphpad Prism (Dotmatics, Boston, MA).
Results
Autoclave sterilization does not impact geometry, surface finish, or manufacturability of 3D printed plastic medical devices
We found that autoclave sterilization did not impact the geometry of printed parts based on our CMM data. The specific dimensions measured for each part are shown in Fig. 5. The dimensions of critical geometric features of all three parts were found to be no different from sham control measurements after one or two rounds of sterilization (p = 0.05). A weak trend appeared as a change in the dimension of the curved features of the port after the second sterilization (Fig. 5C, 5E), but this data was not found to be statistically significant when compared to sham control or one round of sterilization.
Fig. 5. Dimension measurements from CMM.

For all graphs, the circle datapoints represent control, square points represent the first sterilization trial, and trial points represent the second sterilization trial. A.) Ablation handle. B.) Catheter Hub. C-F.) dimensional measurements for the port device for the following dimensions labeled in Fig. 3. C.) Cone 2 circularity. D.) Cone 2 circularity. E.) cylindricity. F.) Plane flatness.
Consistent with CMM data, two independent operators were able to complete the assembly of test parts with the mating components, where they were unable to qualitatively distinguish between sham control or treated parts based on the goodness of fit and ease of assembly.
Visual inspection of surface finish of key geometric features did not reveal any appreciable changes in sterilized parts, where the images were rated as qualitatively indistinguishable based by two independent readers (Fig. 6).
Fig. 6. Stereomicroscopy inspection of critical surfaces.
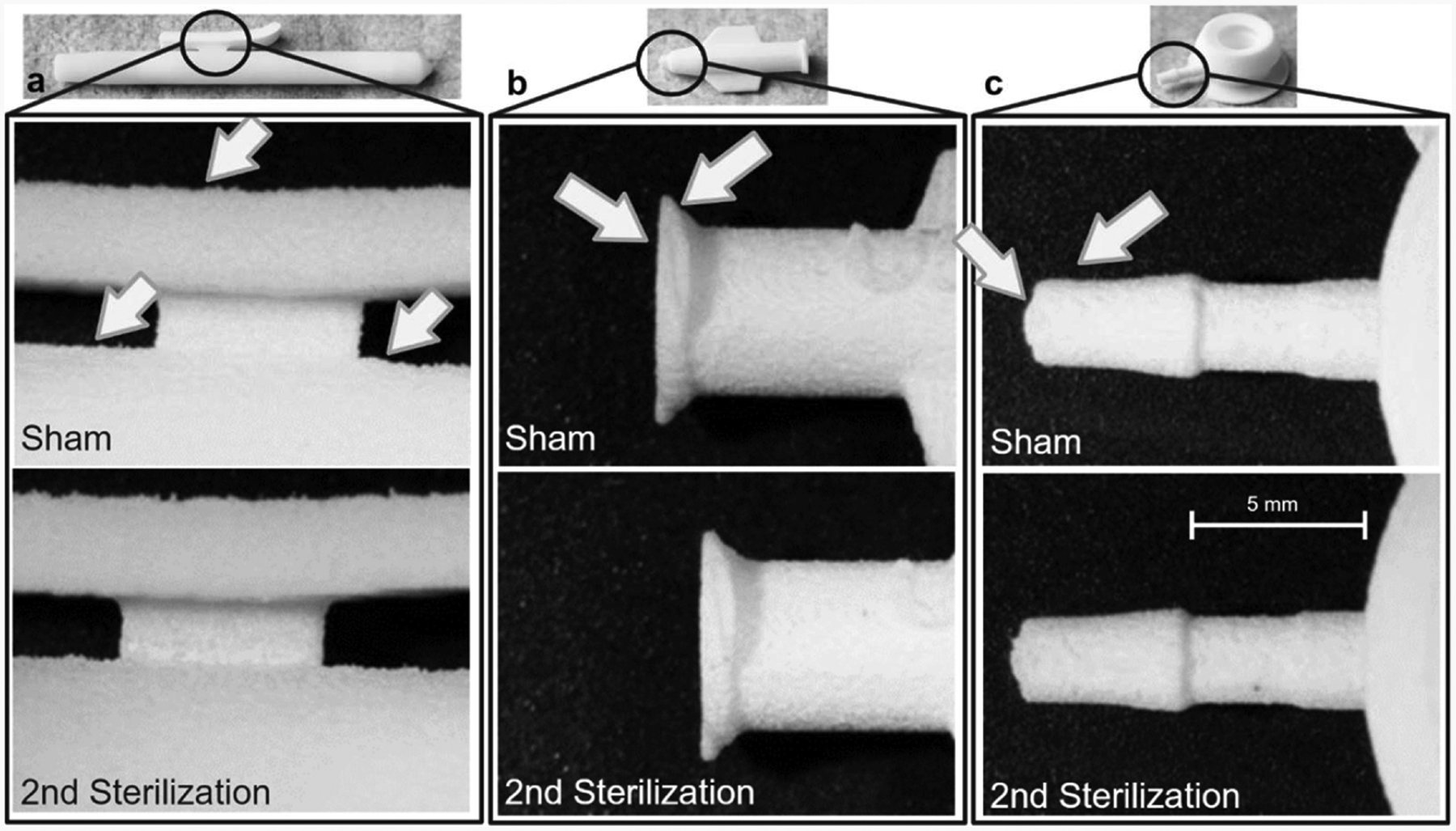
Pre- and post-second sterilization surface finishes were compared for each medical device. A.) Ablation handle. B.) Catheter hub. C.) Port device.
Repeated autoclave sterilization can compromise the mechanical strength of 3D printed plastic medical devices
Our results indicate that autoclave sterilization can impact the mechanical properties of 3D printed Nylon-12 parts, with variations in the level of change and the specific property affected based on device type and geometry (Fig. 7). In general, the mechanical strength of the parts was found to decrease roughly in proportion to the number of sterilization cycles. Both the flexural and compressive properties of the part were observed to change, where the form factor of the part geometry influenced the total change in strength. In the 3-point bend tests, the force-displacement curves of the ablation handle were similar across samples (Fig. 7A, 7D). However, a substantial difference in the treated and sham groups could be observed with the catheter hub (Fig. 7B, 7E) and port device prototypes (Fig. 7C, 7F). This was reflected in a 23 % and 18 % change in the parts’ mechanical stiffness, respectively. Similar outcomes were observed in the results of compression testing for the port device, showing a 12 % change in mechanical stiffness. In general, there were statistically significant differences in the displacement curves in parts after the first and second sterilization treatments.
Fig. 7. Elastic region of force versus displacement graphs for 3-point bending and compression mechanical tests.

A, D.) Ablation handle. B, E.) Port Device. C, F.) Catheter hub.
Discussion
Our results suggest that despite operating close to the material’s thermal limits, pre-use autoclave sterilization does not impact the mechanical performance of 3D printed medical device components. Deterioration in performance emerges upon additional rounds of sterilization that may limit the safe reusability of these devices. 3D printing has revolutionized the accessibility and availability of medical devices in resource-limited settings and provides a quick pathway to patient use. AM has been a significant contributor to the emerging innovation trend called frugal engineering, because of the low material costs, short manufacturing time, and use of commercially available printers. Frugal engineering with AM has led to an increase in patient-specific 3D printed devices, such as prosthetics and surgical guides, improving device specificity and device-to-patient compatibility while maintaining a low price point [12,13]. However, for true 3D printed product implementation, the entire lifecycle of the prototype must be amenable to low resource settings, implying that device material biocompatibility and sterilization via commercial autoclaves must be achieved. Our findings confirm that widely available autoclave sterilization may be used with these devices prior to patient use, clearing a crucial step in the product life cycle of medical devices.
Typical 3D printed medical devices, such as surgical guides used for knee replacements, mandible reconstruction, and spinal surgeries, are built using SLS printers and offer better accuracy and precision of surgical procedures by improving the alignment, simplifying techniques and handling, increasing surgeon’s safety, and reducing operation time when compared to non-patient specific surgical guides [13]. The biocompatibility of sterilized 3D printed surgical guides has been tested extensively, however the retention of mechanical performance post sterilization has been shown to be dependent on the material, device application, and sterilization technique [14–24]. Our findings add to this body of literature that autoclave sterilization is compatible with both sterility requirements without interfering with device performance for parts manufactured using Nylon-12. SLS-printed medical devices were found to tolerate dry heat sterilization via a commonly available autoclave, suggesting that 3D printed medical devices can be sterilized and are safe and effective for surgical use. There was slight mechanical performance deterioration, however for at least 2 cycles of sterilization the form, fit, and function of the tested medical devices were fully maintained. Mechanical property changes within the 3D printed medical devices are inversely proportional to the part wall thickness, where the thicker walled parts experienced less mechanical property change. The commercially available devices that were used as our test cases were all produced by injection molding, where our results suggest that specific design changes may be required to accommodate manufacture with 3D printing and post-processing prior to use. In addition, the true temperature range and ability to withstand environments beyond prescribed specifications have yet to be explored, despite Nylon-12′s inherent material properties of being heat-resistant, flame-retardant, and highly ductile [11]. During the sterilization process, the medical devices experienced at least 121 °C, approaching Nylon-12′s melting point of 175–189 °C, which begs the question: what are the true limits of Nylon-12? Can parts printed with Nylon-12 material withstand higher temperatures and steam sterilization procedures?
There are a few limitations to this study. First, experiments were conducted with one type of material and one type of printer; while nylon-12 is a highly common material used in SLS printing which has been widely used for 3D printing medical devices, additional biocompatible polymer plastics and other types of printers should be tested. Secondly, the dry heat sterilization procedure was assumed to be effective, and the sterility was not physically tested [25,26]. Third, despite the general mechanical properties being tested, potential changes to surface finish properties were not explored [22]. Lastly, while the mechanical assembly of the printed medical devices was tested in their respective assemblies, the devices were not deployed for in-vivo uses post-sterilization, meaning the actual function was not tested.
In conclusion, SLS printing is a promising manufacturing method for prototypes and patient-specific medical devices and can be utilized for the frugal engineering mindset to be more cost-effective for lower-income communities. Dry heat sterilization of SLS-printed medical devices with a common autoclave device can be used prior to surgical use and does not degrade part form, fit, and function.
Funding support
G.S. acknowledges grant and funding support from the National Institute of Diabetes, and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK129990, the National Science Foundation CBET under Award Number 2338949 and the Institute for Applied Life Sciences in the University of Massachusetts at Amherst. M.C.S acknowledges funding support from the National Science Foundation under the Graduate Research Fellowship Program (NSF GRFP). S.K. received support from the Massachusetts Space Grant Consortium.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Govindarajan Srimathveeravalli reports financial support was provided by National Institute of Diabetes, and Digestive and Kidney Diseases. Govindarajan Srimathveeravalli reports financial support was provided by National Science Foundation CBET. Mary Chase Sheehan reports financial support was provided by National Science Foundation under the Graduate Research Fellowship Program. Govindarajan Srimathveeravalli reports a relationship with Kunam Medical that includes: equity or stocks. Govindarajan Srimathveeravalli reports a relationship with Aperture Medical that includes: equity or stocks. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Disclosures
The authors report no relevant disclosures related to the work presented here. G.S. holds equity in Aperture Medical and Kunam Medical. M.C.S. holds equity in Kunam Medical.
References
- [1].Salmi M Additive manufacturing processes in medical applications. Materials 2021;14(1):191. 10.3390/ma14010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].da Silva LRR, Sales WF, dos AR. Campos, de Sousa JAG, Davis R, Singh A, Coelho RT, Borgohain B. A comprehensive review on additive manufacturing of medical devices. Progress in additive manufacturing, 6. Springer Science and Business Media Deutschland GmbH; 2021. p. 517–53. 10.1007/s40964-021-00188-0. Aug. 01. [DOI] [Google Scholar]
- [3].Fogarasi M, Snodderly KL, Di Prima MA. A survey of additive manufacturing trends for FDA-cleared medical devices. Nat Rev Bioeng 2023;1(10):687–9. 10.1038/s44222-023-00109-6. [DOI] [Google Scholar]
- [4].Sheth R, Balesh ER, Zhang YS, Hirsch JA, Khademhosseini A, Oklu R. Three-dimensional printing: an enabling technology for IR. J Vasc Interv Radiol, 2016;27 (6):859–65. 10.1016/j.jvir.2016.02.029. Elsevier Inc. Jun. 01. [DOI] [PubMed] [Google Scholar]
- [5].Green DE, McNeeley MF. Practice corner: a perfect fit. RadioGraphics 2012;32(7): 1975–6. 10.1148/rg.327125179. Nov. [DOI] [PubMed] [Google Scholar]
- [6].Perez M, Block M, Espalin D, Winker R, Hoppe T, Medina F, and Wicker R, “Sterilization of FDM-manufactured parts,” 2012. [Google Scholar]
- [7].Cunha JAM, Mellis K, Sethi R, Siauw T, Sudhyadhom A, Garg A, Goldberg K, Hsu IC, Pouliot J. Evaluation of PC-ISO for customized, 3D printed, gynecologic 192Ir HDR brachytherapy applicators. J Appl Clin Med Phys 2015;16(1):246–53. 10.1120/jacmp.v16i1.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harrell CR, Djonov V, Fellabaum C, Volarevic V. Risks of using sterilization by gamma radiation: the other side of the coin. Int J Med Sci 2018;15(3):274–9. 10.7150/ijms.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mendes GCC, Brandão TRS, Silva CLM. Ethylene oxide sterilization of medical devices: a review. American journal of infection control, 35. Mosby Inc.; 2007. p. 574–81. 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- [10].“Ethylene Oxide Sterilization Facility Updates,” U.S. Food and Drug Administration, Jun. 02, 2021. https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/ethylene-oxide-sterilization-facility-updates (accessed Feb. 15, 2024). [Google Scholar]
- [11].“PA 2200 EU Safety Data Sheet,” Jan. 2024.
- [12].Maric J, Rodhain F, Barlette Y. Frugal innovations and 3D printing: insights from the field. J Innov Econ Manag 2016;n 21(3):57–76. 10.3917/jie.021.0057. Sep. [DOI] [Google Scholar]
- [13].Yilmaz A, Badria AF, Huri PY, Huri G. 3D-printed surgical guides. Annals of joint, 4. AME Publishing Company;, 2019. 10.21037/aoj.2019.02.04. Feb. 01. [DOI] [Google Scholar]
- [14].Török G, Gombocz P, Bognár E, Nagy P, Dinya E, Kispélyi B, Hermann P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy - Pilot study. BMC Oral Health 2020;20(1). 10.1186/s12903-020-1005-0. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burkhardt F, Handermann L, Rothlauf S, Gintaute A, Vach K, Spies BC, Lüchtenborg J. Accuracy of additively manufactured and steam sterilized surgical guides by means of continuous liquid interface production, stereolithography, digital light processing, and fused filament fabrication. J Mech Behav Biomed Mater 2024;152. 10.1016/j.jmbbm.2024.106418. Apr. [DOI] [PubMed] [Google Scholar]
- [16].Toro M, Cardona A, Restrepo D, Buitrago L. Does vaporized hydrogen peroxide sterilization affect the geometrical properties of anatomic models and guides 3D printed from computed tomography images? 3D Print Med 2021;7(1). 10.1186/s41205-021-00120-w. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eveland R, Antloga K, Meyer A, Tuscano L. Low temperature vaporized hydrogen peroxide sterilization of 3D printed devices. 3D Print Med 2024;10(1). 10.1186/s41205-024-00206-1. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pop SI, Dudescu M, Mihali SG, Păcurar M, Bratu DC. Effects of disinfection and steam sterilization on the mechanical properties of 3D SLA-and DLP-printed surgical guides for orthodontic implant placement. Polym (Basel) 2022;14(10). 10.3390/polym14102107. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Keßler A, Dosch M, Reymus M, Folwaczny M. Influence of 3D-printing method, resin material, and sterilization on the accuracy of virtually designed surgical implant guides. J Prosthet Dent 2022;128(2):196–204. 10.1016/j.prosdent.2020.08.038. [DOI] [PubMed] [Google Scholar]
- [20].Ferràs-Tarragó J, Sabalza-Baztán O, Sahuquillo-Arce JM, Angulo-Sánchez MÁ, De-La-Calva Ceinos C, Amaya-Valero JV, Baixauli-García F. Autoclave sterilization of an in-house 3D-printed polylactic acid piece: biological safety and heat-induced deformation. Eur J Trauma Emerg Surg 2022;48(5):3901–10. 10.1007/s00068-021-01672-6. Oct. [DOI] [PubMed] [Google Scholar]
- [21].Rynio P, Galant K, Wójcik Ł, Grygorcewicz B, Kazimierczak A, Falkowski A, Gutowski P, Dołęgowska B, Kawa M. Effects of sterilization methods on different 3D printable materials for templates of physician-modified aortic stent grafts used in vascular surgery—a preliminary study. Int J Mol Sci 2022;23(7). 10.3390/ijms23073539. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].bin A Fadzil AF, Pramanik A, Basak AK, Prakash C, Shankar S. Role of surface quality on biocompatibility of implants - A review. Annals of 3D printed medicine, 8. Elsevier Inc.;, 2022. 10.1016/j.stlm.2022.100082. Oct. 01. [DOI] [Google Scholar]
- [23].Wiseman J, Rawther T, Langbart M, Kernohan M, Ngo Q. Sterilization of bedside 3D-printed devices for use in the operating room. Annals of 3D printed medicine, 5. Elsevier Inc.;, 2022. 10.1016/j.stlm.2022.100045. Mar. 01. [DOI] [Google Scholar]
- [24].Valls-Esteve A, Lustig-Gainza P, Adell-Gomez N, Tejo-Otero A, Englí-Rueda M, Julian-Alvarez E, Navarro-Sureda O, Fenollosa-Artés F, Rubio-Palau J, Krauel L, Munuera J. A state-of-the-art guide about the effects of sterilization processes on 3D-printed materials for surgical planning and medical applications: a comparative study. Int J Bioprint 2023;9(5). 10.18063/ijb.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aguado-Maestro I, De Frutos-Serna M, González-Nava A, Merino-De Santos AB, García-Alonso M. Are the common sterilization methods completely effective for our in-house 3D printed biomodels and surgical guides? Injury 2021;52(6):1341–5. 10.1016/J.INJURY.2020.09.014. Jun. [DOI] [PubMed] [Google Scholar]
- [26].Ramos CH, Wild PM, de C. Martins E. Effectiveness in sterilization of objects produced by 3D printing with polylactic acid material: comparison between autoclave and ethylene oxide methods. Rev Bras Ortop (Sao Paulo) 2021;58(2): 284–9. 10.1055/s-0042-1750751. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]


