Abstract
The liver is a crucial organ in the human body and is responsible for various functions, including digestion, detoxification, metabolism, and immune response. Proper hepatic function is vital for maintaining systemic homeostasis, and dysregulation of liver signaling pathways contributes to various diseases. Recepteur d’Origine Nantais (RON) is a transmembrane receptor tyrosine kinase that is activated by macrophage-stimulating protein (MSP) and coordinates cell fate decisions through the activation of downstream signaling cascades. As the predominant source of MSP in humans, the liver establishes a liver-specific MSP‒RON autocrine‒paracrine signaling axis that contributes to hepatic regeneration, metabolism, and immune functions. Extensive research has demonstrated that MSP-RON signaling is involved in steatotic liver diseases, hepatitis, cirrhosis, cholestatic liver disease, and liver cancer, highlighting the importance of RON in the development of liver diseases. This review demonstrates the role of the MSP-RON pathway both in maintaining liver homeostasis and in driving disease onset and progression while exploring its signaling mechanisms and therapeutic potential for liver disorders.
Keywords: MSP-RON signaling axis, Liver pathobiology, Liver metabolism, Liver immunology, Liver homeostasis, Targeted therapies
Background
Macrophage-stimulating protein (MSP) was first reported in 1976—E. J. Leonard and A. Skeel discovered a protein in human and fetal bovine serum that promotes the chemotaxis, movement, and spread of mouse peritoneal macrophages [1]. Because of its structural similarities to hepatocyte growth factor (HGF), it was designated hepatocyte growth factor-like protein (HGFL) [2, 3]. Compared with mRNAs from other organs, MSP cDNA was noted to hybridize strongly with mRNAs from the liver, indicating that hepatocytes are the primary source [4]. As the exclusive receptor for MSP, Recepteur d’Origine Nantais (RON) is a receptor tyrosine kinase that regulates downstream signaling to control cell fate decisions, metabolism, immune responses, and cancer progression [5, 6]. Research has shown that RON is expressed in both hepatocytes and Kupffer cells of the liver, thereby establishing a specific autocrine‒paracrine loop in the liver [7, 8]. Loss of RON receptor signaling exacerbates high-fat, high-cholesterol diet-induced nonalcoholic steatohepatitis in apolipoprotein E-deficient mice [9]. Transfection of the murine MSP gene into human SCLC cell lines significantly increased liver metastasis, whereas the bone and lung metastatic burdens were not significantly different [10]. Recent evidence has also demonstrated that MSP-RON signaling modulates hepatic fibrogenesis by activating transforming growth factor-beta (TGF-β)-dependent epithelial‒mesenchymal transition (EMT) mechanisms [11, 12]. Collectively, these findings establish the MSP-RON pathway as an important regulator of hepatic homeostasis and liver disease. This review focuses on the roles of MSP-RON signaling in various liver disorders and the pivotal role of this pathway in maintaining liver homeostasis. Given the established evidence that RON is a promising therapeutic target in oncology [13–15], we discuss its potential for pharmacological intervention in hepatic disorders.
Overview of the MSP-RON signaling pathway
Components of the MSP-RON signaling pathway
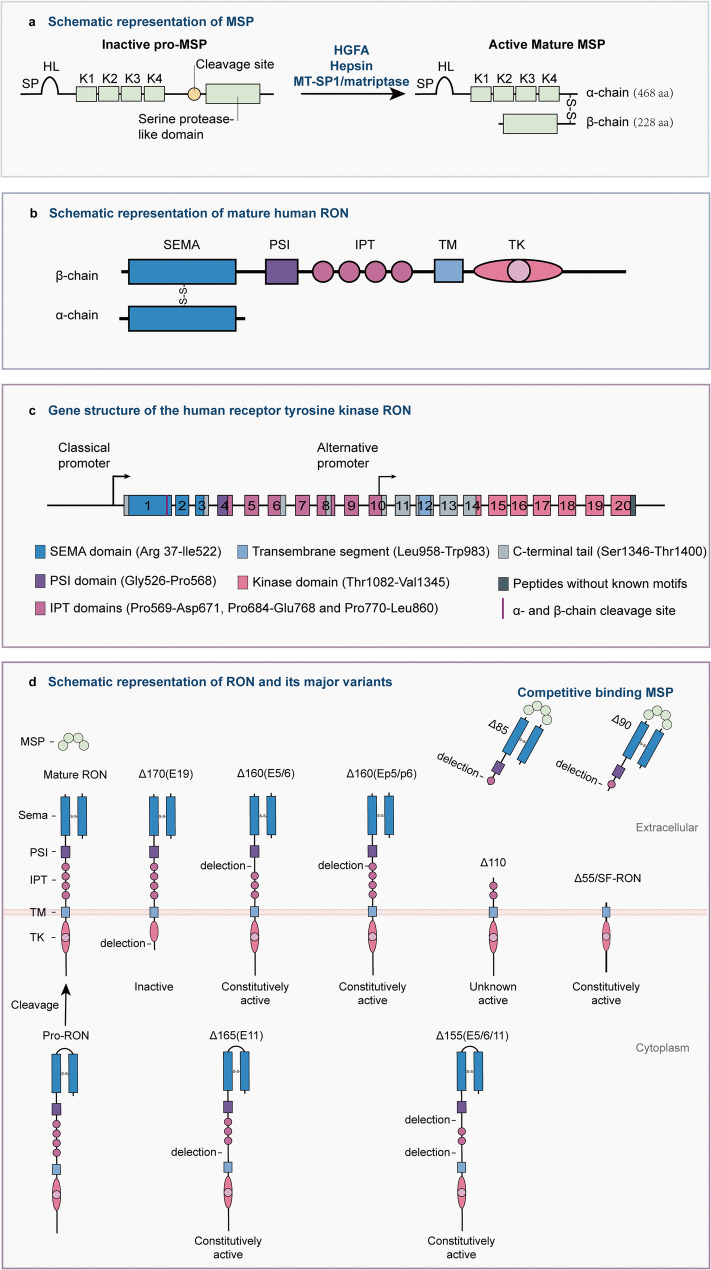
The MSP-RON signaling pathway is primarily composed of the ligand MSP, the receptor RON, and downstream effectors triggered by receptor activation. The genes encoding human MSP and RON are both located in the chromosome 3p21 region [16]. MSP expression in the liver is primarily controlled by transcription factor-coactivator complexes. Hepatocyte nuclear factor 4 (HNF4) is essential for the liver-specific expression of MSP, while nuclear transcription factor Y (NF-Y) cooperates with HNF4 to activate its transcription [5, 17]. It is initially synthesized by the liver as an inactive precursor (pro-MSP) and is secreted into systemic circulation [2, 4]. Multiple members of the trypsin family of serine proteases capable of promoting MSP maturation have been identified across diverse tissues, such as hepatocyte growth factor activator (HGFA) [18, 19], membrane-type serine protease 1 (MT-SP1/matriptase) [20], and hepsin (Fig. 1a) [21]. Proteolytic cleavage generates mature MSP as a disulfide-bonded α/β heterodimer, a process fundamentally required for its biological functionality [22, 23].
Fig. 1.
Schematic of MSP, RON, and RON variants. a MSP and its maturation process. b Mature RON. c All 20 exons encoding RON and their corresponding structure. d RON and its major variants. Exon 11 deletion prevents RON from maturing from RONΔ165 (E11) and RONΔ155 (E5/6/11) into the α/β two-chain form, leading to protein accumulation in the cytoplasm. HL hairpin loop, K1-4 kringle domains 1–4, HGFA hepatocyte growth factor activator, MT-SP1 membrane-type serine protease 1, SEMA semaphoring, PSI plexin-semaphorin-integrin, IPT immunoglobulin-like plexin and transcription, TM transmembrane, TK tyrosine kinase
RON is widely expressed on various epithelial cells [24], tissue macrophages [7, 8, 25, 26], and multiple types of cancer cells [5, 27–30]. Human RON contains 20 exons that encode pro-RON, an approximately 180-kDa glycosylated single-chain protein consisting of 1,400 amino acids (Fig. 1b) [31]. Similar to MET, pro-RON needs to undergo proteolytic cleavage to become a biologically active mature RON [31]. The structure of it closely resembles that of MET, sharing key domains such as the SEMA domain; however, their roles in cellular signaling differ significantly [32]. Mature RON consists of a 35-kDa α-chain and 145-kDa β-chain; furthermore, the α-chain of RON includes the N-terminus of the SEMA domain, whereas its β-chain contains the C-terminus of the SEMA domain, a PSI motif, four IPT domains, a transmembrane region, an intracellular kinase domain, and a carboxy-terminal tail (Fig. 1c) [31]. MSP and RON are highly conserved across species [4, 33–35]. In mice, a primary model for investigating MSP-RON signaling biology, both genes are located on chromosome 9qF1 [16]. Transgenic mice overexpressing human wild-type RON developed multiple lung adenomas. This suggests functional similarity between human and rodent RON, given its role in human cancers including lung cancer [36]. However, human and murine RON proteins exhibit 75.42% sequence similarity [37], suggesting potential structural variations between these orthologs. Thus, certain therapeutic antibodies targeting human RON show limited binding to murine RON [38], which impedes the development of RON-directed antibody therapeutics.
Canonical MSP-RON signaling pathway
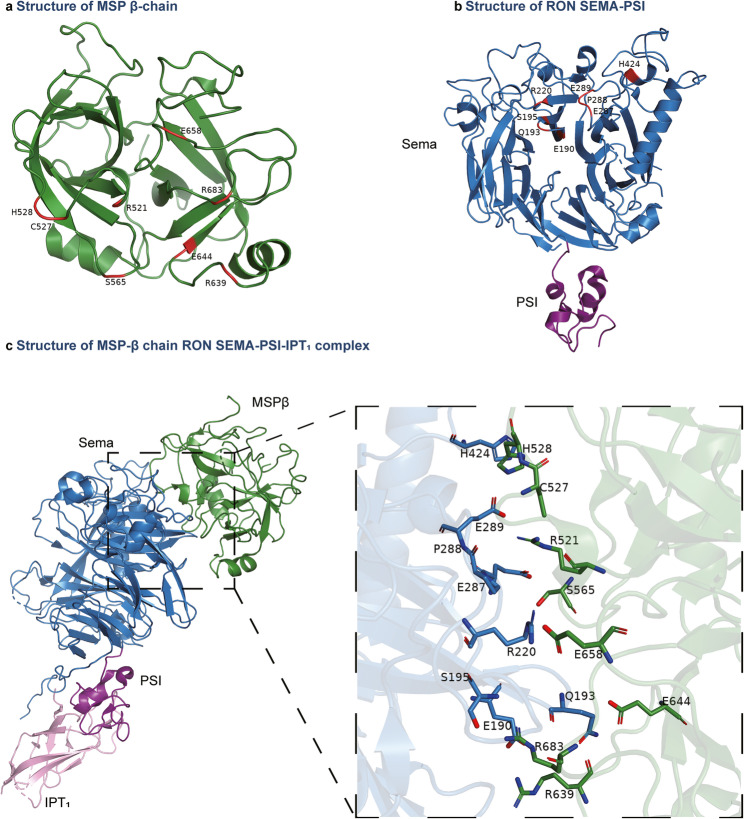
The canonical MSP-RON signaling cascade is initiated through three sequential steps: ligand-induced receptor dimerization upon MSP binding to the RON extracellular domain, trans-autophosphorylation of specific tyrosine residues within the intracellular kinase domain, and recruitment of adaptor proteins to activate downstream effectors [5]. MSP contains two structurally distinct receptor-binding domains. The β-chain of MSP contains a high-affinity binding site that specifically interacts with the RON SEMA domains. Figure 2 illustrates the structure of the MSP β-chain (Fig. 2a), RON SEMA domains (Fig. 2b), and partial structures of the MSP-RON complex, with a focused annotation of critical residues involved in the binding interface (Fig. 2c) [31, 39]. The low-affinity site in the α-chain of MSP subsequently binds to RON [23, 31]. This sequential engagement triggers conformational rearrangements in the RON β-chain, leading to the autophosphorylation of Tyr1238 and Tyr1239 within the intracellular kinase domain [40] and then promoting the phosphorylation of Tyr1353 and Tyr1360 located on the C-terminal docking site on the intracellular tail [41]. The docking site recruits downstream signaling effectors, thereby activating diverse intracellular cascades, including the Src [42, 43], Ras/Erk [44, 45], phosphoinositide 3-kinase (PI3K)/AKT [46, 47], β-catenin [48, 49], NF-κB [7, 50, 51], JAK/STAT [52, 53], and transforming growth factor (TGF) β/Smad [5, 54] pathways, which coordinate critical cellular responses (Fig. 3).
Fig. 2.
Crystal structure of the MSP–RON complex. The image includes color-coded domains for the MSP β-chain (forest green) and RON domains, including SEMA (sky blue), PSI (deep purple), and IPT1 (pink). a Crystal structure of the MSP β-chain (PBD ID: 2ASU). Residues involved in the MSP–RON interaction are marked in red. b Crystal structure of RON-SEMA-PSI (PBD ID: 4FWW). Residues involved in the MSP–RON interaction are marked in red. c Crystal structure of the MSP-RON complex (PBD ID: 4QT8). The zoomed-in view shows the spatial arrangement of residues at the interaction interface
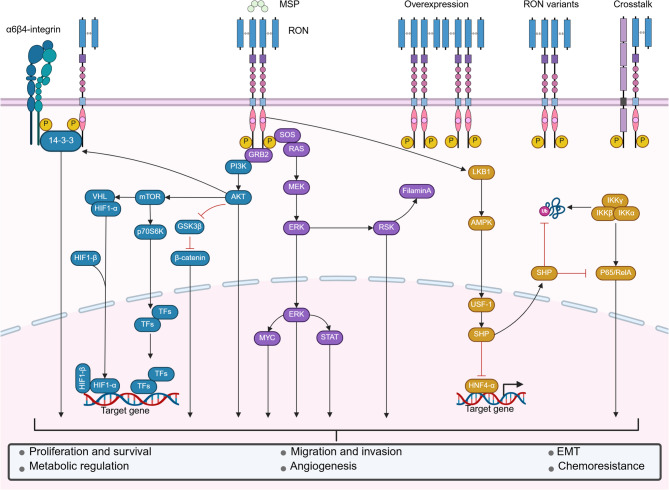
Fig. 3.
Overview of MSP-RON signaling in epithelial and cancer cells. RON exhibits various forms of activation, including MSP-induced RON dimerization, spontaneous dimerization due to RON overexpression, spontaneous activation caused by RON variants and point mutations, and crosstalk with other RTKs. RON activation induces the activation of downstream signaling cascades and various biological processes. GRB2 growth factor receptor-bound protein 2, SOS son of sevenless, STAT signal transducer and activator of transcription, GS3Kβ glycogen synthase kinase 3β, mTOR mammalian target of rapamycin, p70S6K p70S6 kinase, VHL von Hippel–Lindau, HIF1 hypoxia-inducible factor 1, TFs transcription factors, RSK2 p90 ribosomal S6 kinase 2, LKB1 liver kinase B1, AMPK AMP-activated protein kinase, USF1 upstream transcription factor 1, SHP small heterodimer partner, HNF4-α hepatocyte nuclear factor 4α, IKK inhibitor of κB kinase, EMT epithelial–mesenchymal transition. Figure created with BioRender.com
Noncanonical MSP-RON signaling pathway
In some instances, RON can be aberrantly activated in a ligand-independent manner, typically manifested as its overexpression [55, 56], the production of variants [57, 58], and crosstalk with other RTKs. These forms of the noncanonical MSP-RON signaling pathway are key in tumorigenesis, malignant progression, angiogenesis, and chemoresistance in many cancers [5, 27–30].
RON overexpression on certain cancer cell surfaces results in spontaneous dimerization, leading to continuous phosphorylation [5]. Notably, increased RON expression is associated with the production of its variants [59]. The three main mechanisms underlying the production of RON variants are alternative mRNA splicing, protein truncation, and alternative transcription [5, 27, 60]. The identified variants have been named on the basis of the predicted molecular weight of the protein and the exon regions affected by alternative mRNA splicing. Thus far, the following major variants have been characterized (Table 1): RONΔ170(E19) [61, 62], RONΔ165(E11) [57, 63–66], RONΔ165(E2) [67], RONΔ160(E5/6) [30, 49, 57, 62, 64, 65, 68], RONΔ160(E2/3) [69], RONΔ155(E5/6/11) [57, 64, 65], RONΔ110 [27, 70], RONΔ90 [71], RONΔ85 [72], and RONΔ55/SF-RON [58, 73–78].
Table 1.
Summary of structural bases and functional characteristics of human RON variants
| RON Variant | Exons Involved | Structural Characteristics | Key Functional Characteristics | References |
|---|---|---|---|---|
| RON170 | Deletion in exon 19 | Frameshift mutation generating a premature termination codon, resulting in deletion of the kinase domain and multifunctional docking site. | Lacks kinase activity. | [61, 62] |
| RON165E11 | Deletion in exon 11 | Deletion of β-chain IPT domain 4 impairs proteolytic processing, causing intracellular accumulation of single-chain RON165. | Exhibits constitutive phosphorylation. | [57, 63–66, 217] |
| RON165E2 | Deletion in exon 2 | Partial deletion of the SEMA domain. | Lacks tyrosine kinase activity but constitutively activates PI3K/AKT via PTEN hyperphosphorylation. | [67] |
| RON160E5E6 | Deletion in exon 5,6 | Deletion of β-chain IPT domain 1. | Exhibits constitutive phosphorylation. | [30, 49, 57, 62, 64, 65, 68, 218] |
| RON160EP5P6 | Partial splicing of exons 5 and 6 | Partial deletion of β-chain IPT domain 1. | Exhibits constitutive phosphorylation. | [73, 219] |
| RON160E2E3 | Deletion of exon 2, 3 | Partial deletion of the SEMA domain. | Lacks tyrosine kinase activity but constitutively activates PI3K/AKT. | [69] |
| RON155 | Deletion in exon 5,6,11 | Deletion of β-chain IPT domain 1 and 4, causing intracellular accumulation of single-chain RON155. | Exhibits constitutive phosphorylation. | [57, 64, 65] |
| RON110 | Insertion between exons 5 and 6 | Trypsin-like protease cleavage at residues Arg631–Lys632 in the extracellular domain of the β-chain. | Exhibits constitutive phosphorylation. | [27, 70] |
| RON90 | Deletion in exon6 | Frameshift and premature termination codon in exon 7 generate a truncated soluble protein with an intact ligand-binding domain. | Competitively inhibiting MSP-mediated RON activation. | [71] |
| RON85 | Insertion between exons 5 and 6 | Frameshift and premature termination codon generate a truncated soluble protein with an intact ligand-binding domain. | Competitively inhibiting MSP-mediated RON activation and blocking dimerization/signaling of full-length RON and variants. | [72] |
| RON55/sf-RON | Alternative transcription in exon 11 | Lacking most extracellular domains. | Exhibits constitutive phosphorylation. | [74, 83, 220] |
Most RON variants are generated via alternative mRNA splicing and have constitutive kinase activity (Fig. 1d). For example, the deletion of exon 11 results in the absence of 49 amino acids located in the fourth IPT domain in the extracellular sequence of the its β-chain, allowing RONΔ165(E11) to undergo constitutive phosphorylation and maintain continuous activation of downstream signaling cascades [57, 63]. However, the variant RONΔ170(E19), with deletion of exon 19, which encodes 46 amino acids leading to elimination of a multifunctional docking site and truncation of the C-terminus, lacks kinase activity [62]. Although the mechanism underlying alternative splicing of RON mRNA remains unclear, the heterogeneous nuclear ribonucleoprotein (hnRNP) family may be widely involved in this process [79–82]. The splicing of exon 11 is controlled by silencers and enhancers located in exons 11 and 12, and hnRNP H promotes the skipping of exon 11 by binding to the exon splicing silencer (ESS) within exon 11 [81]. Moreover, SRSF1 (SF2/ASF) in exon 12 facilitates exon 11 skipping by binding to the adjacent exon splicing enhancer (ESE) [79]. In contrast, hnRNP A1 binds to ESS, inhibiting the interaction between SRSF1 and ESE and promoting exon 11 inclusion [66]. In addition, hnRNP A2/B1 can increase exon 11 skipping; however, the underlying mechanisms must be characterized [80]. A recent study revealed that hnRNP A1 is also critical for promoting exon 5/6 inclusion, reducing RONΔ160(E5/6) production [82].
RONΔ110 is a typical truncated RON variant produced by digestion with the RONE5/6in protease [70]. RONE5/6in is derived from a transcript featuring a 20-amino acid insertion between exons 5 and 6. This modification renders the protein highly susceptible to protease digestion, resulting in the formation of the truncated variant RONΔ110. Notably, two other soluble truncated RON variants exist [71, 72]. The insertion and deletion at exon 6 lead to a frameshift mutation, followed by the generation of a premature termination codon, resulting in RONΔ85 and RONΔ90, respectively [71, 72]. The absence of part of the extracellular domains and the entire transmembrane and intracellular domains renders these proteins unable to anchor to the surface of the cell membrane and then become soluble splice variants of RON. However, the retention of the SEMA and PSI domains and part of the first IPT domain of the RON extracellular domain allows them to bind competitively to MSP, inhibiting MSP-RON signaling [71, 72].
SF-RON/RONΔ55, one of the most widely studied RON variants, is produced through alternative transcription, which initiates at an intragenic promoter located in exon 10, resulting in a truncated RON lacking most extracellular amino acids [74, 83]. The generation of two distinct transcripts may be associated with the methylation status of two CpG islands in the proximal classic RON promoter [83]. Hypomethylation of CpG island 1 and methylation of CpG island 2 are associated with the promotion of FL-RON transcription. In contrast, hypermethylation of CpG island 1 and strong methylation of CpG island 2 correlate with the absence of FL-RON and the presence of SF-RON [83]. Moreover, SF-RON has constitutive kinase activity mediated by spontaneous dimerization [83, 84]. The expression distribution, formation mechanism, and biological characteristics of RON variants warrant further research.
RON can interact with other RTKs in many tumor cells, including MET [85–87], epidermal growth factor receptor (EGFR) [88], platelet-derived growth factor receptor (PDGFR) [89], and insulin-like growth factor (IGF) 1 receptor (IGF1R) [90, 91]. For example, RON can form heterodimers with MET on the cell surface, producing synergistic effects through mutual phosphorylation [85]. Therefore, HGF (the ligand of MET) can induce the phosphorylation of RON and vice versa [85]. In addition, RON variants can crosstalk with full-length RON [62, 83], other variants [62, 72], and even other tyrosine kinase receptors [86, 92]. This mechanism expands the cellular functions and regulatory manners of RON signaling (Fig. 3). Notably, MET [85, 87], EGFR [93], PDGFR [94], and IGF1R [95] also play crucial roles in various physiological functions and diseases of the liver. Thus, the crosstalk between these receptors may be a potential mechanism underlying RON signaling–associated liver homeostasis regulation. However, research on RON crosstalk with other RTKs has thus far been conducted primarily in oncology [5, 96]. The interactions among these receptors in the liver warrant further investigation. Understanding the expression and biological properties of RON and its variants in different cells, as well as RON’s interactions with other receptors, is essential for the development of rational therapeutic strategies [5].
MSP-RON signaling in liver repair and regeneration
MSP-RON signaling is strongly involved in tissue repair. RON is expressed in wound healing response components, including keratinocytes, macrophages, and capillaries [3, 97]. In wound exudates, more pro-MSP is cleaved into mature MSP, promoting RON activation [97]. This activation enhances fibroblast migration and significantly upregulates the mRNA expression of type I and III collagen, as well as matrix metalloproteinase 1, all of which are major markers of wound healing [3, 98]. In addition, MSP can also regulate α6β4 integrin-mediated keratinocyte adhesion and migration during wound healing by activating the PI3K-AKT pathway [99].
The liver has a strong regenerative capacity and can restore its function by repairing and regenerating damaged tissues after injury [100, 101]. The MSP-RON pathway is also involved in liver damage repair. Enzo et al. reported that RON activation can promote the division, migration, invasion, scattering growth, and polarization of hepatic progenitor cells [102]. RON activation can also induce complex morphological changes in hepatic progenitor cells, including the formation of cord-like branching structures similar to ducts [102]. Some studies have demonstrated the dynamic modulation of MSP expression levels during liver injury. Compared with those in sham-operated controls, MSP mRNA levels in rats after 70% partial hepatectomy were transiently increased 93% within 1 h and returned to baseline within 2 h [103]. However, the early upregulation of mRNA expression was not directly associated with DNA synthesis, suggesting that MSP is involved in the initial stage of liver regeneration and promotes the entry of liver cells into the cell cycle. In addition, in fulminant liver failure, massive liver cell necrosis leads to a decrease in MSP synthesis, which may be detrimental to early liver repair by impairing the phagocytosis of macrophages [104]. This may be because the liver cells remaining after liver failure exhibit extensive necrosis and functional impairment, in contrast to the liver cells remaining after partial hepatectomy, which retain normal function [103, 104]. An increase in MSP expression is a compensatory mechanism that may be impeded in severely damaged livers. In general, the activation of the MSP-RON signaling pathway contributes to the early stage of liver regeneration, and early administration of MSP may be a feasible strategy for alleviating liver injury. Although MSP-RON signaling engages in extensive crosstalk with key hepatic developmental regulators like the HGF-MET signaling [85, 105], direct functional evidence for its role in liver development and growth remains lacking, warranting further investigation.
MSP-RON signaling in modulating liver immune responses
Modulation of immune cell function by MSP-RON signaling
MSP-RON signaling is a basic component of liver innate immunity (Fig. 4a) [106]. MSP was originally described as a serum protein that stimulates the movement, chemotaxis, and spread of peritoneal resident macrophages. It enhances the ability of these macrophages to bind via αMβ2 integrin to intercellular adhesion molecule 1 (ICAM1) and to phagocytose C3bi-coated red blood cells [107]. Notably, Atsushi et al. analyzed the phenotype of exudative macrophages on consecutive days after a stimulus was elicited and reported a continuous increase in RON expression. Thus, RON may be a marker for the terminal differentiation of macrophages [108]. In addition to being expressed in peritoneal resident macrophages, RON is expressed in certain tissue macrophages, such as Kupffer cells [7, 8], osteoclasts [25], and microglia [26]. Kupffer cells play a critical role in liver tissue homeostasis and disease [109]. RON signaling activation can result in macrophage polarization from the M1 to the M2 phenotype [2, 110]. On the basis of their activation state and function, macrophages can be divided into M1 (classically activated) and M2 (alternatively activated) types [111]. M1 macrophages, which are characterized by high inducible nitric oxide synthase (iNOS) expression, enhance the inflammatory response and pathogen-killing ability. In contrast, M2 macrophages, characterized by high ARG1 expression, promote inflammation resolution, tissue repair, and tumor growth [112]. In macrophages, RON inhibits iNOS expression through the PI3K-AKT pathway, thereby reducing nitric oxide synthesis [113]. Moreover, MSP activates the MAPK pathway, upregulates FOS expression, and enhances the binding of FOS to the AP-1 site in the ARG1 promoter, ultimately leading to increased ARG1 expression [110]. MSP-RON signaling can also inhibit type I interferon (IFN) expression through the regulation of macrophage polarization and the toll-like receptor (TLR) 4 signaling pathway, affecting innate immunity in humans (Fig. 4b) [114, 115].
Fig. 4.
MSP-RON signaling in the modulation of liver immunity. a Overall landscape of MSP-RON signaling in regulating interactions among liver immune cells. Most MSP, produced by the liver, circulates throughout the body via the bloodstream to regulate systemic RON signaling. MSP secreted by hepatocytes can also act on RON receptors expressed by hepatocytes themselves, forming an autocrine loop. Moreover, because tissue-resident macrophages express FL-RON, MSP can modulate Kupffer cell function, thereby influencing the activity of other immune cells and cytokine release. In T cells, RON is expressed as SF-RON, which is critical for T-cell function regulation. b MSP-RON signaling in Kupffer cells. MSP-mediated activation of full-length RON is the main form of RON signaling in macrophages. The MSP-RON pathway plays crucial regulatory roles in macrophage functions, including chemotaxis, phagocytosis, polarization, and cytokine release. PDL1 programmed death-ligand 1, MCP1 monocyte chemoattractant protein-1, MIP2 macrophage inflammatory protein-2, iC3b inactivated C3b, ICAM1 intercellular adhesion molecule 1, CR3 complement receptor 3, PKCζ protein kinase c zeta, iNOS inducible nitric oxide synthase, ARG1 arginase 1, CIITA class II major histocompatibility complex transactivator, MHCII major histocompatibility complex class II, LPS lipopolysaccharide, TLR4 toll-like receptor 4, MYD88 myeloid differentiation primary response 88, IRAK1 interleukin-1 receptor-associated kinase 1, TRAF6 tumor necrosis factor receptor associated factor 6. Figure created with BioRender.com
MSP-RON signaling can influence the function of immune cells (other than macrophages) through its effects on cytokine and chemokine secretion in macrophages [116]. For example, RON may facilitate neutrophil mobilization and activation. Targeted deletion of the RON tyrosine kinase domain (RON TK−/−) in murine sepsis models significantly reduces the secretion of mediators critical for neutrophil recruitment, including monocyte chemoattractant protein 1 (MCP1/CCL2), macrophage inflammatory protein 2 (MIP2/CCL3), and interleukin-6 (IL-6) [117]. Furthermore, neutrophils from TK−/− mice demonstrated a significant decrease in spontaneous oxidative burst activity in vitro [117]. Similarly, reduced neutrophil migration to and translocation within the liver are accompanied by considerable increases in liver tissue injury and systemic organ colonization [117]. In the context of endotoxin-induced innate immune responses, RON activation reduces the production of IL-12 by macrophages, which is crucial for IFN-γ production in natural killer cells [52]. Atakan Ekiz et al. reported that after 7 h of MSP stimulation, the mRNA levels of programmed death ligand 1 (PDL1), PDL2, B7H3, and CD80 increased significantly in macrophages [118]. Furthermore, MSP reduces IFN-γ-induced STAT1 phosphorylation and CIITA expression, reducing the levels of MHC class II molecules on the cell surface and potentially diminishing the T-cell activation capacity [52].
RON expression in the immune system was initially believed to be restricted to certain types of tissue macrophages. However, recent studies have shown that SF-RON mRNA is also expressed in CD4+ and CD8+ T cells [119]. Using embryonic stem cell-based homologous recombination gene targeting, researchers generated SF-RON isoform-deficient mice [119, 120]. These animals presented a marked increase in CD4+ and CD8+ T cells accompanied by a considerable decrease in regulatory T cells (Tregs) in the spleen [119]. SF-RON, a truncated variant of RON, has strong intrinsic tyrosine kinase activity [74] with a protective role in T-cell-mediated acute liver injury [120]. T-cell activation with either concanavalin A (an APC-dependent activator) or an anti-CD3/T-cell receptor (TCR) antibody (an APC-independent activator) leads to SF-RON-dependent inhibition of IFN-γ production, suggesting that SF-RON might suppress T-cell effector function directly or indirectly [120]. Furthermore, the inhibitory effects of SF-RON on IFN-γ production indicate RON’s potential to regulate the balance between type I and II immune responses [120, 121].
MSP-RON signaling in liver inflammation
MSP-RON signaling activation negatively regulates the production of inflammatory factors and plays a major role in anti-inflammatory processes [24, 52, 122, 123]. RON signaling forms the molecular basis of MSP-mediated anti-inflammatory effects, primarily by blocking the transcription of inflammatory factor genes [124] and inhibiting the function of inflammatory signaling molecules, such as nuclear factor κB (NF-κB) [125, 126]. MSP-RON signaling activates the LKB1-AMP-activated protein kinase (AMPK) signaling pathway, further inducing the transcription of orphan nuclear receptor small heterodimer partner (SHP). By blocking the entry of p65 into the nucleus and preventing the ubiquitination and degradation of TRAF6, SHP effectively inhibits NF-κB signaling and reduces the TLR-mediated inflammatory response [127].
Given the anti-inflammatory role of RON, TK−/− mice typically display exaggerated inflammatory responses across various inflammation models [24, 52, 122, 123]. Paradoxically, the inhibition of RON signaling may contribute to liver protection in endotoxin-induced acute liver failure. Leonis et al. reported that the loss of RON kinase activity led to a protective effect in a lipopolysaccharide (LPS)/GalN-induced acute liver failure model [128]. Compared with control TK+/+ mice, TK−/− mice presented lower serum levels of aminotransferase (a liver injury marker) after LPS/GalN injection. Histological analysis further revealed reduced hepatocyte apoptosis in these mice [128]. In contrast, the levels of tumor necrosis factor-α (TNF-α), the key inflammatory mediator of this model [129, 130], were significantly elevated in TK−/− mice after LPS/GalN injection [128]. This result is supported by the observation that, compared with control media, conditioned media from LPS-treated TK−/− Kupffer cells exhibited greater toxicity to hepatocytes [8]. Notably, TK−/− hepatocytes demonstrated increased resistance to death compared with TK+/+ hepatocytes, indicating that RON plays a role in both epithelial and inflammatory cell compartments in acute liver injury regulation [8]. Further study revealed that the lack of RON relieves the inhibition of certain survival signals, including NF-κB, B-cell lymphoma 6, and the suppressor of cytokine signaling, reducing hepatocyte sensitivity to Kupffer cell products and increasing hepatocyte resistance to death [8, 53, 128]. Researchers verified this by using the Alb-Cre and Lys-Cre systems to construct hepatocyte-specific and myeloid cell-specific RON knockout mouse models and conducted LPS stimulation experiments [8]. The results demonstrated that the loss of RON signaling in hepatocytes alleviated liver injury and significantly prolonged survival in mice, whereas the absence of it in myeloid cells led to the opposite effects [8].
MSP-RON signaling and infections
Considering its complex regulatory roles in the immune system and inflammation, the MSP-RON signaling pathway may be involved in the infection processes of different pathogens, such as HIV-1 [131, 132] and Listeria monocytogenes [133]. In murine models of polymicrobial peritonitis induced by cecal ligation and puncture (CLP) and systemic Listeria monocytogenes infection, compared with TK−/− mice, TK+/+ mice presented a significantly reduced hepatic bacterial burden and increased survival rates [117, 133].
Viral hepatitis, which leads to liver inflammation, is a major issue in health care worldwide. While the interplay between hepatitis viruses and RON signaling remains unexplored, some evidence suggests that RON may modulate virus‒host immune interactions and contribute to chronic viral hepatitis progression through its immunoregulatory functions, including but not necessarily limited to regulating cytokine balance and macrophage polarization, as previously described [24, 52, 122]. Given the prevalence of hepatitis B virus (HBV)-related chronic hepatitis, we exemplify this mechanism through HBV pathogenesis. In HBV infection, effective antiviral defense by the immune system depends on the coordination of antiviral mediators produced by hepatocytes and Kupffer cells, including IFN-α/β, IFN-γ, IL-18, and TNF-α [134–139]. Through complex interactions with the host, HBV disrupts the liver’s natural immune response by interfering with antiviral mediator production [134–139], leading to persistent viral infection. Several studies have indicated that HBV promotes M2 macrophage activation [140, 141]. Moreover, increased M2 macrophage infiltration has been associated with the progression of HBV-associated liver diseases, such as liver fibrosis and hepatocellular carcinoma (HCC) [142, 143]. As previously discussed, MSP-RON signaling critically modulates hepatic cytokine profiles and macrophage polarization. Notably, RON’s involvement in oncogenic pathways of other viruses also suggests potential mechanisms in viral hepatitis-associated carcinogenesis [78, 144]. In conclusion, elucidating the role of RON signaling in viral hepatitis pathogenesis and progressive liver disease is particularly interesting and promising.
MSP-RON signaling in liver fibrosis
RON mediates liver fibrosis progression by promoting epithelial‒mesenchymal transition (EMT) [145], the process through which polarized epithelial cells with intercellular connections transform into motile, invasive mesenchymal cells. EMT plays a crucial role in the fibrosis of various organs [146–148], contributes to liver fibrosis and serves as an important source of liver myofibroblasts [149, 150].
In a previous study, the human renal proximal tubular epithelial cell line HK-2 and the interstitial fibroblast line NRK49F transiently transfected with RON demonstrated significant EMT, as evidenced by the upregulation of the fibrosis marker proteins N-cadherin, vimentin, TGF-β, α smooth muscle actin (SMA), and fibronectin [43]. Similar results were obtained by Weng et al. [11] In the normal human liver cell line, RON, ERK1/2, and Smad2/3 phosphorylation increased (in the indicated order of magnitude) within 1 h of stimulation with exogenous MSP. After 48 h, N-cadherin, vimentin, α-SMA, and type I collagen levels increased, whereas E-cadherin levels decreased. Notably, galunisertib, a TGF-β pathway inhibitor, inhibited this effect [11]. In summary, MSP-RON signaling can promote the transformation of hepatocytes into mesenchymal cells through the TGF-β-related EMT pathway, promoting liver fibrosis.
Weng et al. reported that cirrhosis patients with higher MSP expression had poorer clinical outcomes [11]. These patients presented decreased platelet counts and estimated glomerular filtration rates, increased blood creatinine, urea nitrogen, and uric acid levels, increased international normalized ratios, and prolonged prothrombin times. Notably, serum MSP levels demonstrated comparable accuracy [area under the curve (AUC) = 0.769] to Model for End-Stage Liver Disease (AUC = 0.825) and Child‒Pugh classification (AUC = 0.799) scores in predicting poor prognosis in patients with cirrhosis [11]. A murine model of CCl4-induced liver fibrosis demonstrated elevated liver RON expression. Compared with control mice, mice treated with antibodies against MSP and small-molecule inhibitors of RON exhibited significant reductions in liver fibrosis [11]. Thus, MSP is a potential diagnostic and prognostic marker for cirrhosis. Liver fibrosis is a critical stage in the progression from chronic liver disease to cirrhosis, making it a key determinant of disease prognosis. Targeting the MSP-RON signaling pathway is a promising strategy for the reversal of liver fibrosis and cirrhosis.
Metabolic functions of MSP-RON signaling in the liver
MSP-RON signaling-mediated regulation of glucose and lipid metabolism
The AMPK pathway is not only a key metabolism regulator but also an important participant in anti-inflammatory processes [151]. It also plays a significant role in steatotic liver diseases pathogenesis [152]. One study evaluated the potential role of MSP in liver gluconeogenesis in primary human hepatocytes (PHHs) exposed to cAMP/Dex treatment [153]. The results showed that MSP negatively regulates the expression levels of two key gluconeogenesis enzymes, namely, PEPCK and Glc-6-Pase, and significantly inhibits gluconeogenesis in PHHs. This mechanism depends on the AMPK-SHP pathway [153]. MSP treatment significantly increased SHP mRNA expression within 3 h by increasing gene promoter activity in a time- and dose-dependent manner [153]. MSP increased the phosphorylation of AMPK and acetyl-CoA carboxylase (a downstream AMPK target) within 15 min of treatment. However, pretreatment with compound C (an AMPK inhibitor) or Ad-siSHP reversed MSP-mediated reductions in cAMP/Dex-mediated glucose production [153]. These results reconfirm that the MSP-RON-AMPK-SHP pathway is crucial for the regulation of liver gluconeogenesis in hepatocytes [153]. Liver gluconeogenesis and hepatic glucose production are closely related regulatory processes. Glucagon and glucocorticoids exert their effects through the cAMP-dependent pathway, whereas insulin exerts its effects through the PI3K pathway. Thus, the crosstalk between RON and IGFR and the crucial effects of classical MSP-RON signaling on PI3K may represent potential forms of RON signaling involved in liver glucose metabolism [90].
In 1998, Jorge et al. developed MSP−/− mice to assess the role of it in growth [154]. Compared with wild-type mice, MSP−/− mice demonstrated lipid accumulation in the cytoplasmic vacuoles of hepatocytes, even when both groups of mice were fed a normal diet [154]. Thus, MSP may play a significant role in liver lipid metabolism. Because palmitic acid (PA) is the major fatty acid in circulation, researchers have exposed HepG2 (a human liver cancer cell line) to PA with or without MSP to assess the effects of it on PA-induced lipid accumulation [155]. MSP stimulation was noted to significantly reduce lipid accumulation; this effect was potentially dependent on the AMPK pathway [155]. Treatment with MSP or AICAR (a well-established AMPK activator) resulted in considerable reductions in the expression of key lipogenic proteins, sterol regulatory element-binding protein 1, fatty acid synthase, and peroxisome proliferator-activated receptor α [155]. However, the inhibition of AMPK by Comp. C significantly attenuated this antilipogenic effect of MSP (or AICAR). In contrast, MSP or AICAR treatment considerably reversed PA- and LPS-induced inhibition of key fatty acid oxidation genes, such as aconitase, carnitine palmitoyl transferase 1, and peroxisome proliferator-activated receptor γ coactivator 1α [155]. Therefore, MSP can improve liver lipid metabolism by activating the AMPK pathway, enhancing fatty acid oxidation, and inhibiting adipogenesis and the inflammatory response [155].
In summary, MSP-RON signaling positively regulates liver glucose and lipid metabolism (Fig. 5). Furthermore, in breast cancer, RON promotes MAPK-dependent MYC expression and β-catenin-dependent SREBP2 expression, both of which are critical for tumor metastasis and recurrence [156]. SREBP2 is a key cholesterol biosynthesis regulator [157]. MYC can also regulate liver metabolic homeostasis through mechanisms such as promoting glycolysis and inducing endoplasmic reticulum (ER) stress [158, 159]. Therefore, SREBP2 and MYC may represent potential underlying mechanisms in RON-mediated hepatic metabolic regulation.
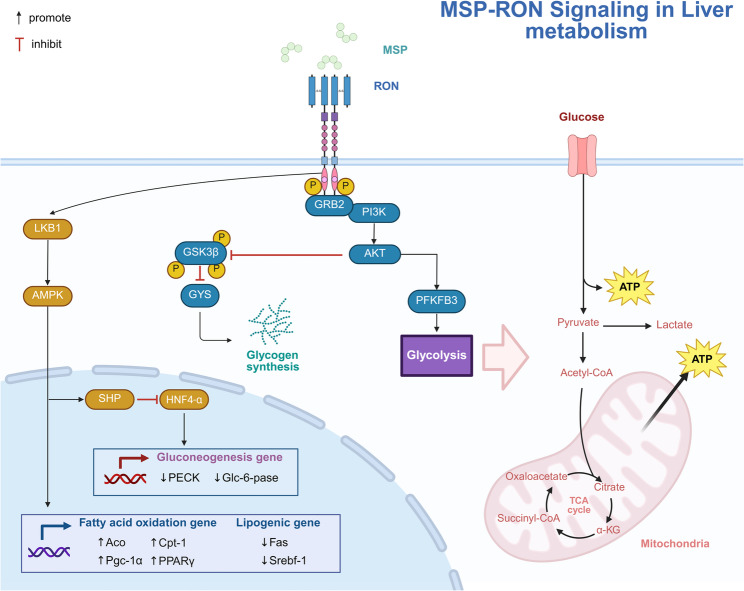
Fig. 5.
MSP-RON signaling in liver metabolism regulation. RON primarily modulates metabolism by regulating the transcription and activity of key enzymes involved in carbohydrate and lipid metabolism, predominantly facilitating glycemic and lipid reduction. PFKFB3 phosphofructokinase-2/fructose-2,6-bisphosphatase 3, GYS glycogen synthase, Fas fatty acid synthase, SREBF1 sterol regulatory element-binding protein 1, ACO aconitase, CPT1 carnitine palmitoyltransferase 1, PGC-1α peroxisome proliferator-activated receptor γ coactivator 1α, PECK phosphoenolpyruvate carboxykinase, Glc-6-pase glucose-6-phosphatase. Figure created with BioRender.com
MSP-RON signaling in steatotic liver diseases
In response to an updated understanding of the disease and the need to reduce stigma, international liver societies have recently revised the definition of NAFLD and renamed it metabolic dysfunction-associated steatotic liver disease (MASLD) [160]. Although this change is controversial, the research outcomes related to NAFLD obtained before the aforementioned revision remain valid [161]. NAFLD is a complex disease influenced by various mechanisms involving metabolic, genetic, environmental, and gut microbial factors [162]. The disease can be divided into two histological categories according to the presence of features of hepatocellular injury: nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) [163, 164]. Further progression of these histological categories leads to the development of fatty liver fibrosis, cirrhosis, and even liver cancer [165]. Notably, RON is involved in almost all steps of this process.
NASH, which is generally considered the liver manifestation of metabolic syndrome, is characterized by lipid accumulation within hepatocytes, which ultimately induces lipotoxicity and inflammatory injury [166, 167]. However, research on the involvement of the MSP-RON signaling pathway in NASH has yielded conflicting results. Apolipoprotein E (APOE)-knockout (KO) mice are frequently used as metabolic syndrome models in NASH research [168]. Some researchers have employed double KO (DKO) mice lacking both APOE and RON (in which the ligand binding domain of RON is deleted) to investigate the role of RON in NASH. These results demonstrated that MSP-RON signaling plays a protective role in NASH [9, 169, 170]. High-fat diet (HFD) consumption has been noted to lead to paler-appearing livers, along with greater lipid deposition and liver damage (as indicated by hematoxylin‒eosin and Oil Red O staining), in DKO mice than in APOE-KO mice [169, 170]. Moreover, a significant increase in the expression of proinflammatory cytokines and ARG1 in DKO mice livers indicates that the absence of RON causes Kupffer cell transformation from the M2 phenotype to the M1 phenotype, exacerbating liver inflammation [169, 170].
In contrast to prior findings, a recent investigation utilizing RON TK−/− mouse models demonstrated diametrically opposed phenotypic outcomes [171]. A HFD was noted to lead to 50% less weight gain in TK−/− mice than in controls; moreover, TK−/− mice’s livers were considerably less steatotic and weighed significantly less than the livers of TK+/+ mice. Compared with control mice, TK−/− mice also presented significantly higher serum levels of proinflammatory cytokines, such as IL-6 and IFN-γ [171]. These conflicting results may be attributed to the differences between experimental models. The deletion of Ron tyrosine kinase signaling domain and the ligand binding domain of Ron will lead to different effects on the MSP-RON signaling pathway [5]. In RON TK−/− mouse models, RON may retain intact transmembrane and extracellular domains despite the deletion of the tyrosine kinase domain. In mice with targeted deletion of the RON ligand-binding domain, RON may retain partial extracellular segments, an intact transmembrane domain, and a functional intracellular tyrosine kinase domain, even including the variants that naturally lack this domain [27]. Notably, distinct RON-deficient models may implicate differential outcomes of targeting specific components within the MSP-RON axis. The mechanisms underlying these discrepant outcomes and their implications require further investigation.
To elucidate the effects of MSP in the early stages of NASH, a study fed mice lacking low-density lipoprotein receptor expression a high-fat, high-cholesterol diet for 7 days and administered MSP via minipumps in the final 4 days [172]. Compared with saline-treated control mice, MSP-treated mice presented increased gene expression of proinflammatory and proapoptotic mediators in the liver; however, the underlying mechanisms remain unclear. Some scholars believe that the aforementioned studies did not report the dose of MSP incorporated into the liver, which may have affected the experimental results [6]. Future studies should explore the long-term effects of MSP on the development of NASH, as well as the effects of MSP in an advanced-stage NASH model.
The presence of liver fibrosis is the most important determinant of NAFLD prognosis [173]. Some studies in which RON’s ligand-binding domain was deleted in mice demonstrated that RON signaling has an antifibrotic effect on a NASH model [169, 170]. This finding contrasts with the aforementioned finding that RON activation promotes liver fibrosis development [11]. This might be due to the complex and diverse biological effects of RON activation in NAFLD. The protective effect of RON on liver fibrosis may have originated from the positive effects of RON on liver lipid metabolism and inflammation inhibition [169, 170].
Taken together, these findings highlight the importance of considering the stages and systemic effects of NAFLD when designing treatment strategies [165]. Future studies investigating the potential for regulating MSP-RON signaling at different stages of NAFLD development are warranted.
MSP-RON signaling in cholestatic liver disease
Cholestatic liver disease, caused by bile stasis, results in liver damage and fibrosis [174]. Currently, the evidence regarding MSP-RON in cholestatic liver disease primarily focuses on primary sclerosing cholangitis (PSC). PSC is characterized by idiopathic, progressive inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, and occurs in association with inflammatory bowel disease (IBD) in 70–80% of patients [175]. In 2011, Espen Melum et al. identified SNP rs3197999 in MST1 as a susceptibility locus for PSC through genome-wide association analysis (P = 1.1 × 10− 16). The A→G nucleotide substitution at this locus results in a p.Arg689Cys missense variant, replacing arginine with cysteine at position 689 of the MSP protein [176]. This missense variant is situated within a critical region of the high-affinity receptor-binding interface, suggesting its potential to functionally impair protein activity [39, 177]。In 2012, Häuser et al. demonstrated that the p.Arg689Cys variant substantially enhances MSP’s stimulatory effects on macrophage chemotaxis and proliferation [177]. Notably, this non-synonymous coding variant has been robustly replicated as a risk variant for IBD in multiple independent studies [177–181]. This association underscores the pathogenic significance of the rs3197999 variant in PSC patients with IBD, although the precise mechanistic role of the MSP-RON signaling axis in PSC pathogenesis remains incompletely defined. Future research should prioritize elucidating disease-relevant biological processes mediated by dysregulated MSP signaling and developing targeted therapeutic strategies to modulate this pathway.
MSP-RON signaling in liver cancer
MSP-RON signaling in HCC progression
Aberrant RON activation is key in the tumorigenesis, malignant progression, angiogenesis, and chemoresistance of many cancers [5, 27–29]. RON overexpression is observed in only a minority of HCC cases (5.7%), in stark contrast to its higher prevalence in breast cancer (55.6%) and colorectal cancer (51.1%) cohorts [55, 182]. However, RON plays a critical role in HCC. A study employing siRNA to investigate its function in HCC cell lines revealed that RON is associated with the invasive and carcinogenic phenotypes of cancer cells, including migration, invasion, antiapoptotic behavior, and cell cycle arrest, through the regulation of AKT, RAF, and ERK signaling molecules [183]. To date, few studies have systematically evaluated both the expression profiles of RON variants in HCC and the fundamental differences in RON signaling activation between malignant and normal hepatocytes. These knowledge gaps underscore the need for comprehensive investigations into the molecular heterogeneity of RON during hepatic carcinogenesis.
MSP expression is markedly elevated in HCC. Zhu et al. demonstrated that MSP mRNA expression is higher in clinical samples of HCC than in normal liver tissue cells [184, 185]. Research using the antibody microarray technique indicates that MSPα is a serum biomarker for predicting HBV-related HCC [186]. Thus, the MSP-RON signaling pathway may play a role in HBV-related HCC progression. Recently, Xi et al. reported that MSP activates γδT cells within the peripheral blood mononuclear cells (PBMCs) of patients with HCC [187]. These activated γδT cells exhibit cytotoxic activity against HepG2 cells [187]. Further characterization of these γδT cells revealed that T cells primarily recognize MSP through their TCRs [185]. Compared with those from healthy controls, γδT cells from the PBMCs of patients with HCC significantly increased the expression of antigen-presenting molecules, including MHC-I, MHC-II, CD86, and CD11a, after MSP stimulation [185]. Although the underlying mechanisms remain unclear, MSP has potential as a therapeutic target for HCC and in γδT-cell–based treatment for HCC [188–190].
Emerging evidence implicates RON signaling in the pathogenesis of cholangiocarcinoma in addition to HCC. Preclinical studies have demonstrated that the small-molecule inhibitor BMS-777607 suppresses cholangiocarcinoma cell proliferation [191]. However, as BMS-777607 exhibits multikinase inhibitory activity targeting both RON and MET receptors, the specific mechanistic contribution of RON requires further validation.
RON and MET in the prognosis of patients with hepatobiliary carcinoma
RON and MET expression are strongly correlated with the prognosis of patients with hepatobiliary cancer. Compared with MET-negative HCC patients, MET-positive HCC patients presented higher 5-year overall recurrence rates (65.3% vs. 54.3%, P = 0.041) [192]. In another study, advanced perihilar cholangiocarcinoma (CCA) patients with both MET and RON positivity presented worse 5-year survival rates than those with other expression patterns (16.7% vs. 39.8%, P = 0.021) [193]. Compared with the MET/RON intermediate group (48.5%), the MET/RON negativity and MET/RON positivity groups of patients with extrahepatic CCA had significantly lower overall 5-year survival rates (28.3% and 32.4%, respectively; P = 0.01) [194]. Notably, MET or RON negativity was associated with nodal metastasis in extrahepatic CCA [193]. Krawczyk et al. conducted genotyping on the rs3197999 variant of MSP in 223 patients with CCA and discovered a tendency for overexpression of the MSP variant in these patients [195]. Thus, MSP may play a role in modulating the genetic risk of CCA [195].
Taken together, these results reveal that RON and MET may be prognostic biomarkers for clinical hepatobiliary cancer. The examination of RON and MET expression may provide a novel method for patient classification than traditional pathological classification systems.
RON as a potential target in liver metastasis therapy
Accumulating evidence highlights the crucial role of MSP-RON signaling in promoting cancer metastasis [5, 96]. RON activation can promote the invasive growth of cancer cells through various signaling pathways [48, 58, 145]. MSP-RON signaling also promotes cancer metastasis by modulating the tumor microenvironment (TME) [5]. RON is expressed in various cellular components of the TME and regulates their functions, including tumor-associated macrophages [110], myeloid-derived suppressor cells [116], and cancer-associated fibroblasts [196]. Current evidence indicates that MSP-RON activation generates a complex immunoregulatory gene signature, ultimately suppressing CD8 + T-cell activity to promote tumor progression and metastasis [114, 116, 118, 197]. Notably, RON signaling in T cells is mediated by SF-RON. Lai et al. reported that the absence of SF-RON is sufficient to completely abolish breast cancer metastases in mice. In mice with SF-RON deletion, spleen cells show a decrease in Treg and CD11b + Ly6G + myeloid cells, along with an increase in stem-like CD4 + T cells and tumor-specific CD8 + T cells within the TME. RON activation also promotes angiogenesis in prostate cancer through the upregulation of VEGFR, CXCL1, CXCL2, CXCL5, and CXCL8 expression [50, 51].
In tumor cells, MSP alone is sufficient to induce liver metastasis of lung cancer. Sato et al. transfected SBC-5 cells with MSP to explore its effects on tumor metastasis behavior and the tumor immune microenvironment [10]. SBC-5 is a small-cell lung cancer cell line that does not express RON; therefore, MSP does not affect these cells directly [10]. The authors reported that SBC-5 cells expressing MSP predominantly metastasized to the liver rather than to bones or the lungs [10]. Immunohistochemical staining indicated that macrophage infiltration in liver metastases of SBC-5 expressing MSP was significantly greater than that in controls, with a greater density of tumor-associated microvessels—which may facilitate liver metastasis [10]. However, the mechanisms underlying MSP-dependent hepatic tropism in tumor metastasis remain unclear. One possible explanation is that MSP, RON, and HGFA expression in the liver is widely believed to be higher than that in other organs [10, 198]. HGFA is a trypsin-like serine protease that can activate the single-chain precursor pro-MSP into mature form [199]. MSP and HGFA are synthesized mainly by the liver, and the constitutive expression of RON in hepatocytes and Kupffer cells generates the most advanced environment for receiving MSP signals [10, 18]. Certain cancer cells with high RON expression have been observed to escape paracrine regulation by establishing an autocrine circuit through autonomous MSP expression, thereby enabling self-sustaining oncogenic signaling [196, 200, 201]. In addition, analysis of The Cancer Genome Atlas PanCancer data revealed that MSP expression is significantly and positively correlated with RON expression in almost all available cancer datasets [201]. Therefore, the liver may be susceptible to cancer cells with high RON and MSP expression. In other words, cancer cells with strong RON and MSP expression may have an increased risk of liver metastasis. Overall, targeting MSP-RON signaling may represent a potential strategy to simultaneously suppress primary tumors and their liver metastases.
Therapeutic strategies targeting MSP-RON signaling
To date, therapeutic strategies targeting RON in oncology have focused primarily on three modalities: small-molecule inhibitors, monoclonal antibodies, and antibody‒drug conjugates (ADCs). Table 2 summarizes the current therapeutic strategies targeting the MSP-RON signaling pathway by the end of 2024.
Table 2.
Summary of drugs targeting the RON receptor tyrosine kinase
| Agents | Type | Mechanism and Target Receptor | Phase | Characteristics | References |
|---|---|---|---|---|---|
| Crizotinib (PF-2341066) | Small Molecule Inhibitor | ATP-competitive inhibitor of RON, Met, ALK and ROS | FDA-approved (NSCLC) | Inhibits ATP binding, effectively suppressing cancer cell growth and metastasis. | [221, 222] |
| Foretinib (GSK1363089) | Small Molecule Inhibitor | Multi-target tyrosine kinase inhibitor of RON, Met, VEGFRs, AXL, ROS, and TIE-2 | Phase I/II Clinical Trial | Shows antitumor activity in various cancers, including HCC. | [223, 224] |
| BMS-777607 | Small Molecule Inhibitor | ATP-competitive inhibitor of RON, Met, and other RTKs | Phase I/II Clinical Trial | Demonstrates potential in inhibiting tumor growth and metastasis across different cancers. | [203, 225] |
| Golvatinib (E7050) | Small Molecule Inhibitor | Multi-target tyrosine kinase inhibitor of RON, Met, and c-Kit | Phase I/II Clinical Trial | Inhibits tumor growth and metastasis in various cancer models; effectively blocks HAV infection. | [226–228] |
|
Glesatinib (MGCD265) |
Small Molecule Inhibitor | Multi-target tyrosine kinase inhibitor including MET, RON, VEGFR1, VEGFR2, VEGFR3, and TIE2 | Phase I/II Clinical Trial | Showing antitumor activity in various cancers. | [229, 230] |
| Compound I | Small Molecule Inhibitor | Specific inhibitor of MET and RON | Preclinical Studies | Effective in xenograft models, showing potential in various cancer cell lines. | [231] |
| MK-2461 | Small Molecule Inhibitor | ATP-competitive inhibitor of RON and Met | Phase I/II Clinical Trial | Effective in xenograft models, showing potential in various cancer cell lines. | [232] |
| MK-8033 | Small Molecule Inhibitor | ATP-competitive inhibitor of RON and Met | Phase I Clinical Trial | Effective in xenograft models, showing potential in various cancer cell lines. | [233, 234] |
| Lcrf-0004 | Small Molecule Inhibitor | Selective inhibitor of RON | Preclinical Studies | Showing potential in various cancer cell lines. | [235] |
| WM-S1-030 | Small Molecule Inhibitor | Selective inhibitor of RON and RON variants | Phase I Clinical Trial | More potent against the RON variants, including RON, RON Δ155, Δ160, and Δ165. | [205] |
| IMC-41A10 | Monoclonal antibody |
Binds to the β-chain extracellular domain of RON, prevents MSP binding, and blocks MSP-RON signaling |
Preclinical Studies | Effective in xenograft models, showing potential in various cancer cell lines. | [236] |
| Narnatumab (IMC-RON8) | Monoclonal Antibody |
Binds to RON, prevents MSP binding, and blocks MSP-RON signaling |
Phase I Clinical Trial | Well-tolerated with limited antitumor activity. | [208] |
| 29B06/07F01 (humanized) | Monoclonal Antibody | Humanized monoclonal antibodies targeting RON | Preclinical Studies | Effective in xenograft models, potential in various cancer cell lines. |
EORTC- NCI-AACR International Symposium‡ |
| Ig4/Ig7/Ig10 (human) | Monoclonal Antibody | Binds to the SEMA domain of RON, and blocks MSP-RON signaling | Preclinical Studies | Effective in various cancer cell lines, but not in xenograft models. | [237] |
| Zt/f2 (mouse) | Monoclonal Antibody | Binds to the 49 amino acid sequence coded by exon 11 in the RON β-chain extracellular sequences. Does not compete with MSP for binding to RON. Induces RON internalization, reducing RON expression and impairing downstream signaling. | Preclinical Studies | Effective as a single agent or in combination with 5-FU in xenograft tumors, showing potential in various cancer cell lines. | [206, 238] |
| Zt/g4 (mouse) | Monoclonal Antibody | Binds to the SEMA domain of RON. Induces RON internalization, reducing RON expression and impairing downstream signaling. | Preclinical Studies | Effectively induces RON internalization, diminishes RON expression, and impairs downstream signaling. | [62, 206, 207] |
| Zt/g4-MMAE | Antibody-Drug Conjugate | Zt/g4 antibody conjugated with MMAE, targeting RON receptor | Preclinical Studies | Shows antitumor activity in PDAC, and TNBC models. | [38, 209, 210] |
| Zt/g4-DM1 | Antibody-Drug Conjugate | Zt/g4 antibody conjugated with DM1, targeting RON receptor | Preclinical Studies | Shows antitumor activity in PDAC, CRC, and NSCLC models. | [14, 239, 240] |
| H5B14-MMAE | Antibody-Drug Conjugate | H5B14 antibody conjugated with MMAE, targeting RON PSI domain | Preclinical Studies | Effective against various cancer cell lines and xenograft models. | [241] |
| H5B14-DCM | Antibody-Drug Conjugate | H5B14 antibody conjugated with MMAE, targeting RON PSI domain | Preclinical Studies | Effective against various cancer cell lines and xenograft models. Stronger tumor elimination ability than H5B14-MMAE. | [241] |
NSCLC non-small cell lung cancer, HCC hepatocellular carcinoma, HAV hepatitis A virus, PDAC pancreatic ductal adenocarcinoma, TNBC triple-negative breast cancer, CRC colorectal cancer
‡Data presented at the EORTC-NCI-AACR International Symposium, 16–19 Nov 2010, Berlin, Germany
Owing to the resemblance of ATP pocket structures across various kinases, many inhibitors that target the ATP binding sites of tyrosine kinases, such as the RON inhibitor BMS-777607, impact multiple tyrosine kinases and exhibit off-target effects [202, 203]. Moreover, variants constitute a major oncogenicity component of RON signaling [96]. Given the structural heterogeneity of RON variants, which exhibit varying divergence from FL-RON, constitutes a major obstacle for targeted therapies. Many small-molecule inhibitors frequently demonstrate selective efficacy against FL-RON while showing minimal activity against variants [73, 204]. Comfortingly, the recent discovery of WM-S1-030 has afforded a paradigm for the development of small-molecule inhibitors that target RON variants [205]. By potently suppressing phosphorylation in RONΔ155, Δ160, and Δ165 variants, this compound exhibits enhanced antitumor efficacy over conventional RON inhibitor BMS-777607 [205].
RON monoclonal antibodies specifically bind to the extracellular domain, inhibiting MSP-RON signaling through antibody-mediated receptor internalization and lysosomal degradation [62, 206, 207]. Despite demonstrating favorable tolerability in clinical trials, the therapeutic efficacy of narnatinib appears to be limited [208]. A potential explanation is that monoclonal antibodies fail to effectively target RON variants. The structural alterations between FL-RON and its variants result in a lack of shared antibody recognition epitopes, which is the primary reason for the failure of monoclonal antibodies to target RON during cancer therapy [5]. Recent advances in RON-targeted antibody delivery platforms have demonstrated significant therapeutic potential. A prime example is the RON-directed antibody‒drug conjugate Ztg4-MMAE, which achieves complete tumor eradication in RON-overexpressing xenograft models, thereby expanding the therapeutic potential of RON-targeted strategies through precision payload delivery [209, 210]. Notably, ADCs directly eliminate RON-overexpressing cells, largely overcoming the limitation of conventional targeted therapies in suppressing oncogenic signaling from RON variants. In summary, how to effectively target variants remains one of the greatest difficulties faced by RON-targeted therapies. In the future, screening and characterizing more small-molecule drugs targeting pathogenic RON variants and developing more antibody-based delivery therapies might be the key to overcoming this difficulty.
Furthermore, emerging strategies targeting the MSP-RON signaling axis warrant attention. For example, identifying natural inhibitors from traditional Chinese medicine may be a promising research strategy [211–213]. Targeting enzymes such as MT-SP1/matriptase and HGFA, which are involved in the MSP maturation process, could be novel strategies for modulating the MSP-RON signaling pathway [18–21, 198, 214]. Notably, given the crosstalk between RON and multiple RTKs, the exclusive use of RON inhibitors may lead to compensatory activation of other signaling pathways. The combination of other kinase inhibitors may lead to improved therapeutic effects for cancer [92]. RON is also likely involved in resistance to treatments targeting receptors engaging in crosstalk with it [92]. Table 3 summarizes liver treatment strategies targeting these receptors.
Table 3.
Summary of mainly small molecule inhibitors and monoclonal antibodies of key receptors involved in RON crosstalk in the field of liver diseases
| Agents | Type | Target Receptor | Phase | Characteristics | References |
|---|---|---|---|---|---|
| MET | |||||
| Cabozantinib (INC280) | Small Molecule Inhibitor | MET, VEGFR1/2/3, RET, KIT, FLT3, AXL, NTRK, ROS1 | FDA-approved (NSCLC) | Effective in HCC. Demonstrates preventive effects on NASH. Inhibits HBV RNA transcription. | [242–246] |
| Tepotinib (Tepmetko) | Small Molecule Inhibitor | MET | FDA-approved (NSCLC) | Effective in HCC and ICC with high-level MET amplification. | [246, 247] |
| Tivantinib (ARQ 197) | Small Molecule Inhibitor | MET | Phase III clinical trial | Almost no effect on RON. Effective in cirrhotic patients with HCC. | [248] |
| Capmatinib | Small Molecule Inhibitor | MET | FDA-approved (NSCLC) | Effective in HCC with high-level MET amplification. | [249, 250] |
| Savolitinib (Volitinib) | Small Molecule Inhibitor | MET | Phase III clinical trial | Effective in HCC with high-level MET amplification. | [251, 252] |
| EGFR | |||||
| Erlotinib (Tarceva) | Small Molecule Inhibitor | EGFR | FDA-approved (NSCLC) | Well tolerated with modest disease-control benefits in HCC. Demonstrated a comprehensive anti-HBV potential. Reduction in fibrogenesis. | [253–258] |
| Gefitinib (Iressa) | Small Molecule Inhibitor | EGFR | FDA-approved (NSCLC) | Induces growth inhibition, apoptosis, and cell cycle arrest in human HCC cells, attributed to blunted HSC activation. | [259–261] |
| Osimertinib (AZD9291) | Small Molecule Inhibitor | EGFR | FDA-approved (NSCLC) | Significantly induces apoptosis in a panel of HCC cell lines, irrespective of EGFR expression levels. Targets both tumor cells and angiogenesis. | [262, 263] |
| Afatinib (Gilotrif) | Small Molecule Inhibitor | EGFR, Her2 | FDA-approved (NSCLC) | Inhibits the viability, migration, and invasion of HCC cells. | [264–266] |
| Pelitinib (EKB-569) | Small Molecule Inhibitor | EGFR, Her2 | Phase II clinical trial | Inhibition of HCC cell migration and invasion occurs through suppression of EMT. Combination with sorafenib may overcome HCC resistance to EGFR-TK inhibitors. | [267, 268] |
| Cetuximab (Erbitux) | Monoclonal Antibody | EGFR | FDA-approved (mCRC) | Inhibits the growth of HCC cell lines and has the potential for combination therapy with other drugs for HCC. | [269–275] |
| Panitumumab | Monoclonal Antibody | EGFR | FDA-approved (mCRC) | Induces significant pre-G1 and G2/M cell cycle arrest in HepG2 cells when combined with sorafenib. | [276] |
| Nimotuzumab | Monoclonal Antibody | EGFR | Approved (III/IV NPC) | Effective in certain HCC cell lines. Showing potential in HCC. | [277] |
| PDGFR | |||||
| Nintedanib | Small Molecule Inhibitor | PDGFRα, PDGFRβ, VEGFR1, VEGFR2, VEGFR3 | FDA-approved (IPF) | Reduces steatohepatitis and hepatic fibrosis pathology. Inhibits ICC aggressiveness by suppressing cytokines derived from activated cancer-associated fibroblasts. | [278–280] |
| Regorafenib (Stivarga) | Small Molecule Inhibitor | PDGFRβ, VEGFR1/2/3, Kit, RET, Raf-1, BRAF, TIE2 | FDA-approved (HCC) | Prevented the formation of liver metastases in colon cancer models. Demonstrated acceptable tolerability and evidence of antitumor activity in patients with HCC. | [281–286] |
| Sorafenib (Nexavar) | Small Molecule Inhibitor | PDGFRβ, VEGFR2, VEGFR3, FLT3, c-Kit | FDA-approved (HCC) | Demonstrates antitumor activity in HCC and potential for combination with other anticancer agents. Efficiently blocks HCV replication in vitro | [287–290] |
| Sunitinib (Sutent) | Small Molecule Inhibitor | PDGFRβ, VEGFR2, FLT3 | FDA-approved (RCC, GIST, PNET) | Exhibits modest antitumor activity in advanced HCC with manageable adverse effects. Reduces inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Weakly reactivates HBV. | [291–296] |
| Pazopanib (Votrient) | Small Molecule Inhibitor | PDGFRβ, VEGFR1, VEGFR2, VEGFR3, c-Kit, FGFR1, c-Fms | FDA-approved (RCC) | Demonstrates a manageable safety profile. Antitumor activity in patients with advanced HCC is attributed to antiangiogenic effects. In vitro, the proliferation of various HCC cell lines was not inhibited. | [297, 298] |
| Lenvatinib | Small Molecule Inhibitor | PDGFRα/β, VEGFR1/2/3, FGFR1 | FDA-approved (HCC) | Demonstrates an antitumor activity in advanced HCC with manageable adverse effects. Exhibits preventive effects on experimental liver fibrosis and sinusoidal capillarization, as well as on the in vitro phenotypes of HSC. | [299–302] |
| Olaratumab | Monoclonal Antibody | PDGFRα | FDA-approved (STS) | Contributes to human HSC proliferation and migration. Showing potential in HEHE. | [303, 304] |
| IGFR | |||||
| Linsitinib (OSI-906) | Small Molecule Inhibitor | IGF-1R, IR, INSR | Phase III clinical trial | Effective against certain HCC cell lines. Strongly enhances the inhibitory effect of Sorafenib and/or vitamin K1 on HCC cell migration. | [305–307] |
| BMS-754,807 | Small Molecule Inhibitor | IGF-1R, INSR, Met, Aurora A/B, TrkA/B, Ron | Phase II clinical trial | Effective in certain HCC cell lines. | [308, 309] |
| NVP-AEW541 (AEW541) | Small Molecule Inhibitor | IGF-1R, INSR | Preclinical Studies | Induces growth inhibition, apoptosis, and cell cycle arrest in human HCC cell lines without accompanying cytotoxicity. | [310, 311] |
NSCLC non-small cell lung cancer, HCC hepatocellular carcinoma, NASH non-alcoholic steatohepatitis, HBV hepatitis B virus, HSC hepatic stellate cell, mCRC metastatic colorectal cancer, IPF idiopathic pulmonary fibrosis, ICC intrahepatic cholangiocarcinoma, HCV hepatitis C virus, RCC renal cell carcinoma, GIST gastrointestinal stromal tumor, PNET pancreatic neuroendocrine tumor, STS soft tissue sarcoma, HEHE hepatic epithelioid hemangioendothelioma
Conclusions
In conclusion, this comprehensive review highlights the multiple involvement of MSP-RON signaling in liver pathobiology and its therapeutic potential in liver disease. In bacterial peritonitis, MSP-RON signaling activation significantly reduces bacterial loads in the liver [117]. It may also be associated with HBV infection [110, 140, 141], highlighting the need for further relevant research. By regulating lipid metabolism and suppressing inflammation, the MSP-RON signaling pathway might provide potential benefits to patients with early NAFLD [114, 115, 153]. Moreover, RON inhibitors can reverse liver fibrosis progression, and serum MSP levels may be effective prognostic markers in patients with liver cirrhosis [11]. Given the strong association of the MSP rs3197999 variant with PSC and CCA, further research is warranted to define its pathogenic mechanisms. However, given the intricate complexity of hepatic MSP-RON signaling, therapeutic strategies must be contextually tailored to specific disease etiologies and their associated molecular landscapes. Future research must elucidate its cell type-selective roles and spatiotemporal dynamics, but developing cell type-selective targeting approaches remains a critical challenge.
Targeting MSP-RON signaling may be a novel, promising strategy for the treatment of primary tumors and their liver metastases [5, 10]. However, from the therapeutic perspective of targeting primary cancers with RON overexpression, RON-targeted therapies face persistent challenges, including off-target effects and limited efficacy against RON variants [215, 216]. Recent advancements in the structural biology of the MSP-RON signaling pathway have provided a theoretical foundation, enhancing the current understanding of its complex signaling mechanisms. Moreover, AlphaFold and RosettaFold are anticipated to further accelerate the development of drugs that target MSP-RON signaling and targeted degradation technologies, such as molecular glues and PROTACs. These advancements are likely to accelerate the translation of basic research into clinical application.
Acknowledgements
Not applicable.
Abbreviations
- ACO
Aconitase
- ADCs
Antibody‒drug conjugates
- AMPK
AMP-activated protein kinase
- APOE
Apolipoprotein E
- ARG1
Arginase 1
- AUC
Area under the curve
- CCA
Cholangiocarcinoma
- CIITA
Class II major histocompatibility complex transactivator
- CLP
Ligation and puncture
- CPT1
Carnitine palmitoyltransferase 1
- CR3
Complement receptor 3
- DKO
Double knockout
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial–mesenchymal transition
- ER
Endoplasmic reticulum
- ESE
Exon splicing enhancer
- ESS
Exon splicing silencer
- Fas
Fatty acid synthase
- Glc-6-pase
Glucose-6-phosphatase
- GRB2
Growth factor receptor-bound protein 2
- GS3Kβ
Glycogen synthase kinase 3β
- GYS
Glycogen synthase
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HFD
High-fat diet
- HGF
Hepatocyte growth factor
- HGFA
Hepatocyte growth factor activator
- HGFL
Hepatocyte growth factor-like protein
- HIF1
Hypoxia-inducible factor 1
- HL
Hairpin loop
- HNF4
Hepatocyte nuclear factor 4
- HNF4-α
Hepatocyte nuclear factor 4α
- iC3b
Inactivated C3b
- ICAM1
Intercellular adhesion molecule 1
- IFN
Interferon
- IGF1R
Insulin-like growth factor 1 receptor
- IKK
Inhibitor of κB kinase
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- IPT
Immunoglobulin-like plexin and transcription
- IRAK1
Interleukin-1 receptor-associated kinase 1
- K1-4
Kringle domains 1–4
- KO
Knockout
- LKB1
Liver kinase B1
- LPS
Lipopolysaccharide
- MASLD
Metabolic dysfunction associated steatotic liver disease
- MCP
Monocyte chemoattractant protein
- MHCII
Major histocompatibility complex class II
- MIP2
Macrophage inflammatory protein 2
- MSP
Macrophage-stimulating protein
- mTOR
Mammalian target of rapamycin
- MT-SP1/matriptase
Membrane-type serine protease 1
- MYD88
Myeloid differentiation primary response 88
- NAFL
Nonalcoholic fatty liver
- NASH
Nonalcoholic steatohepatitis
- NF
Nuclear factor
- NF-Y
Nuclear factor Y
- p70S6K
p70S6 kinase
- PBMCs
Peripheral blood mononuclear cells
- PDGFR
Platelet-derived growth factor receptor
- PDL1
Programmed death ligand 1
- PECK
Phosphoenolpyruvate carboxykinase
- PFKFB3
Phosphofructokinase-2/fructose-2,6-bisphosphatase isoform 3
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator 1α
- PHHs
Primary human hepatocytes
- PI3K
Phosphoinositide 3-kinase
- PKCζ
Protein kinase C zeta
- PSI
Plexin-semaphorin-integrin
- RON
Recepteur d’Origine Nantais
- RSK2
p90 ribosomal S6 kinase 2
- SEMA
Semaphorin
- SF2/ASF
also known as serine/arginine-rich splicing factor 1 (SRSF1)
- SHP
Small heterodimer partner
- SMA
Smooth muscle actin
- SOS
Son of sevenless
- SREBF1
Sterol regulatory element transcription factor 1
- STAT
Signal transducer and activator of transcription
- TCR
T-cell receptor
- TFs
Transcription factors
- TGF-β
Transforming growth factor-beta
- TK
Tyrosine kinase
- TLR
Toll-like receptor
- TLR4
Toll-like receptor 4
- TM
Transmembrane
- TME
Tumor microenvironment
- TNF
Tumor necrosis factor
- TRAF6
Tumor necrosis factor receptor-associated factor 6
- Tregs
Regulatory T cells
- USF1
Upstream transcription factor 1
- VHL
Von Hippel–Lindau
Author contributions
HPY and MHW conceived the central concept of this review and delineated its overall structure. KW and HPY wrote the original version of the manuscript. JJ and KW created the figures. JYP, MJZ, JLZ, TS, DL, and MDW participated in the reference collection and table organizing. HPY and MHW provided guidance and supervision during the writing process and critically reviewed the manuscript. All the authors have read and approved the article.
Funding
This work was supported by grants from the National Natural Sciences Foundation of China [grant number 82473937], the Zhejiang Plan for the Special Support for Top-notch Talents in China [grant number 2022R52029], and Fundamental Research Funds for the Central Universities [grant number 2022ZFJH003].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Minghai Wang, Email: minghai.wang@ttuhsc.edu.
Hangping Yao, Email: yaohangping@zju.edu.cn.
References
- 1.Leonard EJ, Skeel A. A serum protein that stimulates macrophage movement, chemotaxis and spreading. Exp Cell Res. 1976;102:434–8. [DOI] [PubMed] [Google Scholar]
- 2.Skeel A, Yoshimura T, Showalter SD, Tanaka S, Appella E, Leonard EJ. Macrophage stimulating protein: purification, partial amino acid sequence, and cellular activity. J Exp Med. 1991;173:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasbey JC, Sanders AJ, Bosanquet DC, Ruge F, Harding KG, Jiang WG. Expression of hepatocyte growth Factor-Like protein in human wound tissue and its biological functionality in human keratinocytes. Biomedicines. 2015;3:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura T, Yuhki N, Wang MH, Skeel A, Leonard EJ. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993;268:15461–8. [PubMed] [Google Scholar]
- 5.Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013;13:466–81. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Fang X, Shi D, Yao S, Wu W, Fang Q, Yao H. MSP-RON pathway: potential regulator of inflammation and innate immunity. Front Immunol. 2020;11:569082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaidis NM, Gray JK, Gurusamy D, Fox W, Stuart WD, Huber N, Waltz SE. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock. 2010;33:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology. 2011;53:1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen JN, Dey A, Cai J, Zhang J, Tian Y, Kennett M, Ma Y, Liang TJ, Patterson AD, Hankey-Giblin PA. Metabolic profiling reveals aggravated Non-Alcoholic steatohepatitis in High-Fat High-Cholesterol Diet-Fed Apolipoprotein E-Deficient mice lacking Ron receptor signaling. METABOLITES. 2020;10:1–24. [DOI] [PMC free article] [PubMed]
- 10.Sato S, Hanibuchi M, Kuramoto T, Yamamori N, Goto H, Ogawa H, Mitsuhashi A, Van TT, Kakiuchi S, Akiyama S, et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis. 2013;30:333–44. [DOI] [PubMed] [Google Scholar]
- 11.Weng T, Yan D, Shi D, Zhu M, Liu Y, Wu Z, Tang T, Zhu L, Zhang H, Yao H, Li L. The MSP-RON pathway regulates liver fibrosis through transforming growth factor beta-dependent epithelial-mesenchymal transition. Liver Int. 2021;41:1956–68. [DOI] [PubMed] [Google Scholar]
- 12.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baird AM, Easty D, Jarzabek M, Shiels L, Soltermann A, Klebe S, Raeppel S, MacDonagh L, Wu C, Griggs K, et al. When RON MET TAM in mesothelioma: all druggable for one, and one drug for all? Front Endocrinol (Lausanne). 2019;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng L, Yao HP, Zhou YQ, Zhou J, Zhang R, Wang MH. Biological evaluation of antibody-maytansinoid conjugates as a strategy of RON targeted drug delivery for treatment of non-small cell lung cancer. J Exp Clin Cancer Res. 2016;35:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomuleasa C, Tigu AB, Munteanu R, Moldovan CS, Kegyes D, Onaciu A, Gulei D, Ghiaur G, Einsele H, Croce CM. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct Target Ther. 2024;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt BG, Fox LH, Davis JC, Jones A, Lu Z, Waltz SE. An introduction and overview of RON receptor tyrosine kinase signaling. Genes (Basel). 2023;14:517. [DOI] [PMC free article] [PubMed]
- 17.Waltz SE, Gould FK, Air EL, McDowell SA, Degen SJF. Hepatocyte nuclear Factor-4 is responsible for the Liver-specific expression of the gene coding for hepatocyte growth Factor-like protein. J Biol Chem. 1996;271:9024–32. [DOI] [PubMed] [Google Scholar]
- 18.Iida I, Johkura K, Teng R, Kubota S, Cui L, Zhao X, Ogiwara N, Okouchi Y, Asanuma K, Nakayama J, Sasaki K. Immunohistochemical localization of hepatocyte growth factor activator (HGFA) in developing mouse liver tissues. Heterogeneous distribution of HGFA protein. J Histochem Cytochem. 2003;51:1139–49. [DOI] [PubMed] [Google Scholar]
- 19.Han Z, Harris PK, Jones DE, Chugani R, Kim T, Agarwal M, Shen W, Wildman SA, Janetka JW. Inhibitors of HGFA, matriptase, and hepsin Serine proteases: A nonkinase strategy to block cell signaling in cancer. ACS Med Chem Lett. 2014;5:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt AS, Welm A, Farady CJ, Vásquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci U S A. 2007;104:5771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan R, Kolumam GA, Lin SJ, Xie MH, Santell L, Wu TD, Lazarus RA, Chaudhuri A, Kirchhofer D. Proteolytic activation of pro-macrophage-stimulating protein by hepsin. Mol Cancer Res. 2011;9:1175–86. [DOI] [PubMed] [Google Scholar]
- 22.Waltz SE, McDowell SA, Muraoka RS, Air EL, Flick LM, Chen YQ, Wang MH, Degen SJ. Functional characterization of domains contained in hepatocyte growth factor-like protein. J Biol Chem. 1997;272:30526–37. [DOI] [PubMed] [Google Scholar]
- 23.Danilkovitch A, Miller M, Leonard EJ. Interaction of macrophage-stimulating protein with its receptor. Residues critical for beta chain binding and evidence for independent alpha chain binding. J Biol Chem. 1999;274:29937–43. [DOI] [PubMed] [Google Scholar]
- 24.Muraoka RS, Sun WY, Colbert MC, Waltz SE, Witte DP, Degen JL, Friezner Degen SJ. The ron/stk receptor tyrosine kinase is essential for peri-implantation development in the mouse. J Clin Invest. 1999;103:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade K, Fornetti J, Zhao L, Miller SC, Randall RL, Anderson N, Waltz SE, McHale M, Welm AL. RON kinase: A target for treatment of cancer-induced bone destruction and osteoporosis. Sci Transl Med. 2017;9:eaai9338. [DOI] [PMC free article] [PubMed]
- 26.Dey A, Allen JN, Fraser JW, Snyder LM, Tian Y, Zhang L, Paulson RF, Patterson A, Cantorna MT, Hankey-Giblin PA. Neuroprotective role of the Ron receptor tyrosine kinase underlying central nervous system inflammation in health and disease. Front Immunol. 2018;9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007;257:157–64. [DOI] [PubMed] [Google Scholar]
- 28.Wang MH, Yao HP, Zhou YQ. Oncogenesis of RON receptor tyrosine kinase: a molecular target for malignant epithelial cancers. Acta Pharmacol Sin. 2006;27:641–50. [DOI] [PubMed] [Google Scholar]
- 29.Tong X, Zhang X, Fan J, Tong Y, Li S, Jin J, Yao H. The RON receptor tyrosine kinase is a potential therapeutic target in Burkitt lymphoma. Cancer Biol Ther. 2013;14:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res. 2000;261:229–38. [DOI] [PubMed] [Google Scholar]
- 31.Chao KL, Tsai IW, Chen C, Herzberg O. Crystal structure of the Sema-PSI extracellular domain of human RON receptor tyrosine kinase. PLoS ONE. 2012;7:e41912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. [DOI] [PubMed] [Google Scholar]
- 33.Han S, Stuart LA, Degen SJ. Characterization of the DNF15S2 locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry. 1991;30:9768–80. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Aoki S, Takahashi T, Matsumoto K, Kiyohara T, Nakamura T, COMMUNICATIONS. Cloning and expression of xenopus HGF-like protein (HLP) and ron/hlp receptor implicate their involvement in early neural development. Volume 224. BIOCHEMICAL AND BIOPHYSICAL RESEARCH; 1996. pp. 564–73. [DOI] [PubMed]
- 35.Bassett DI. Identification and developmental expression of a macrophage stimulating 1/ hepatocyte growth factor-like 1 orthologue in the zebrafish. Dev Genes Evol. 2003;213:360–2. [DOI] [PubMed] [Google Scholar]
- 36.Chen YQ, Zhou YQ, Fu LH, Wang D, Wang MH. Multiple pulmonary adenomas in the lung of Transgenic mice overexpressing the RON receptor tyrosine kinase. Recepteur d’origine Nantais. Carcinogenesis. 2002;23:1811–9. [DOI] [PubMed] [Google Scholar]
- 37.UniProt. The universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao HP, Feng L, Suthe SR, Chen LH, Weng TH, Hu CY, Jun ES, Wu ZG, Wang WL, Kim SC, et al. Therapeutic efficacy, Pharmacokinetic profiles, and toxicological activities of humanized antibody-drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy. J Immunother Cancer. 2019;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao KL, Gorlatova NV, Eisenstein E, Herzberg O. Structural basis for the binding specificity of human recepteur d’origine Nantais (RON) receptor tyrosine kinase to macrophage-stimulating protein. J Biol Chem. 2014;289:29948–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Yennawar N, Hankey PA. Autoinhibition of the Ron receptor tyrosine kinase by the juxtamembrane domain. Cell Commun Signal. 2014;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao ZQ, Chen YQ, Wang MH. Requirement of both tyrosine residues 1330 and 1337 in the C-terminal tail of the RON receptor tyrosine kinase for epithelial cell scattering and migration. Biochem Biophys Res Commun. 2000;267:669–75. [DOI] [PubMed] [Google Scholar]
- 42.Danilkovitch-Miagkova A, Angeloni D, Skeel A, Donley S, Lerman M, Leonard EJ. Integrin-mediated RON growth factor receptor phosphorylation requires tyrosine kinase activity of both the receptor and c-Src. J Biol Chem. 2000;275:14783–6. [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Choi HI, Kim DH, Kim CS, Bae EH, Ma SK, Kim SW. RON receptor tyrosine kinase regulates epithelial mesenchymal transition and the expression of Pro-Fibrotic markers via src/smad signaling in HK-2 and NRK49F cells. Int J Mol Sci. 2019;20:5489. [DOI] [PMC free article] [PubMed]
- 44.Chaudhuri A, Xie MH, Yang B, Mahapatra K, Liu J, Marsters S, Bodepudi S, Ashkenazi A. Distinct involvement of the Gab1 and Grb2 adaptor proteins in signal transduction by the related receptor tyrosine kinases RON and MET. J Biol Chem. 2011;286:32762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Q, Guin S, Padhye SS, Zhou YQ, Zhang RW, Wang MH. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol Cancer. 2011;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Rajput A, Kan JL, Rose R, Liu XQ, Kuropatwinski K, Hauser J, Beko A, Dominquez I, Sharratt EA, et al. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J Biol Chem. 2009;284:10912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang MH, MonteroJulian FA, Dauny I, Leonard EJ. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene. 1996;13:2167–75. [PubMed] [Google Scholar]
- 48.Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE. Β-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene. 2011;30:3694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu XM, Zhou YQ, Wang MH. Mechanisms of cytoplasmic β-catenin accumulation and its involvement in tumorigenic activities mediated by oncogenic splicing variant of the receptor originated from Nantes tyrosine kinase. J Biol Chem. 2005;280:25087–94. [DOI] [PubMed] [Google Scholar]
- 50.Thobe MN, Gray JK, Gurusamy D, Paluch AM, Wagh PK, Pathrose P, Lentsch AB, Waltz SE. The Ron receptor promotes prostate tumor growth in the TRAMP mouse model. Oncogene. 2011;30:4990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thobe MN, Gurusamy D, Pathrose P, Waltz SE. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene. 2010;29:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson CB, Ray M, Lutz M, Sharda D, Xu J, Hankey PA. The RON receptor tyrosine kinase regulates IFN-gamma production and responses in innate immunity. J Immunol. 2008;181:2303–10. [DOI] [PubMed] [Google Scholar]
- 53.Kulkarni RM, Kutcher LW, Stuart WD, Carson DJ, Leonis MA, Waltz SE. Ron receptor-dependent gene regulation in a mouse model of endotoxin-induced acute liver failure. Hepatobiliary Pancreat Dis Int. 2012;11:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao S, Ammanamanchi S, Brattain M, Cao L, Thangasamy A, Wang J, Freeman JW. Smad4-dependent TGF-beta signaling suppresses RON receptor tyrosine kinase-dependent motility and invasion of pancreatic cancer cells. J Biol Chem. 2008;283:11293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213:402–11. [DOI] [PubMed] [Google Scholar]
- 56.Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE, Lowy AM. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–82. [DOI] [PubMed] [Google Scholar]
- 57.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–97. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Zhao L, Derose YS, Lin YC, Bieniasz M, Eyob H, Buys SS, Neumayer L, Welm AL. Short-Form Ron promotes spontaneous breast cancer metastasis through interaction with phosphoinositide 3-Kinase. Genes Cancer. 2011;2:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao HP, Hudson R, Wang MH. RON receptor tyrosine kinase in pancreatic ductal adenocarcinoma: pathogenic mechanism in malignancy and pharmaceutical target for therapy. Biochim Biophys Acta Rev Cancer. 2020;1873:188360. [DOI] [PubMed] [Google Scholar]
- 60.Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage-stimulating protein receptor, the RON receptor tyrosine kinase. Carcinogenesis. 2003;24:1291–300. [DOI] [PubMed] [Google Scholar]
- 61.Angeloni D, Duh FM, Moody M, Dean M, Zabarovsky ER, Sentchenko V, Braga E, Lerman MI. C to A single nucleotide polymorphism in intron 18 of the human MST1R (RON) gene that maps at 3p21.3. Mol Cell Probes. 2003;17:55–7. [DOI] [PubMed] [Google Scholar]
- 62.Wang MH, Lao WF, Wang D, Luo YL, Yao HP. Blocking tumorigenic activities of colorectal cancer cells by a splicing RON receptor variant defective in the tyrosine kinase domain. Cancer Biol Ther. 2007;6:1121–9. [DOI] [PubMed] [Google Scholar]
- 63.Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer S, Hirschfeld M, Jaeger M, Pies S, Iborra S, Erbes T, Stickeler E. RON alternative splicing regulation in primary ovarian cancer. Oncol Rep. 2015;34:423–30. [DOI] [PubMed] [Google Scholar]
- 65.Greenbaum A, Rajput A, Wan G. RON kinase isoforms demonstrate variable cell motility in normal cells. Heliyon. 2016;2:e00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonomi S, di Matteo A, Buratti E, Cabianca DS, Baralle FE, Ghigna C, Biamonti G. HnRNP A1 controls a splicing regulatory circuit promoting mesenchymal-to-epithelial transition. Nucleic Acids Res. 2013;41:8665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ling Y, Kuang Y, Chen LL, Lao WF, Zhu YR, Wang LQ, Wang D. A novel RON splice variant lacking exon 2 activates the PI3K/AKT pathway via PTEN phosphorylation in colorectal carcinoma cells. Oncotarget. 2017;8:39101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis. 2000;21:1507–12. [PubMed] [Google Scholar]
- 69.Wang D, Lao WF, Kuang YY, Geng SM, Mo LJ, He C. A novel variant of the RON receptor tyrosine kinase derived from colorectal carcinoma cells which lacks tyrosine phosphorylation but induces cell migration. Exp Cell Res. 2012;318:2548–58. [DOI] [PubMed] [Google Scholar]
- 70.Ma Q, Zhang K, Guin S, Zhou Y-Q, Wang M-H. Deletion or insertion in the first immunoglobulin-plexin-transcription (IPT) domain differentially regulates expression and tumorigenic activities of RON receptor tyrosine kinase. Mol Cancer. 2010;9:307. [DOI] [PMC free article] [PubMed]
- 71.Eckerich C, Schulte A, Martens T, Zapf S, Westphal M, Lamszus K. RON receptor tyrosine kinase in human gliomas: expression, function, and identification of a novel soluble splice variant. J Neurochem. 2009;109:969–80. [DOI] [PubMed] [Google Scholar]
- 72.Ma Q, Zhang K, Yao H-P, Zhou Y-Q, Padhye S, Wang M-H. Inhibition of MSP-RON signaling pathway in cancer cells by a novel soluble form of RON comprising the entire Sema sequence. Int J Oncol. 2010;36:1551–61. [DOI] [PubMed] [Google Scholar]
- 73.Chakedis J, French R, Babicky M, Jaquish D, Mose E, Cheng P, Holman P, Howard H, Miyamoto J, Porras P, et al. Characterization of RON protein isoforms in pancreatic cancer: implications for biology and therapeutics. Oncotarget. 2016;7:45959–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bardella C, Costa B, Maggiora P, Patane S, Olivero M, Ranzani GN, De Bortoli M, Comoglio PM, Di Renzo MF. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64:5154–61. [DOI] [PubMed] [Google Scholar]
- 75.Maniscalco L, Guil-Luna S, Iussich S, Gattino F, Trupia C, Millan Y, Martin de Las Mulas J, Sanchez Cespedez R, Saeki K, Accornero P, De Maria R. Expression of the short form of RON/STK in feline mammary carcinoma. Vet Pathol. 2019;56:220–29. [DOI] [PubMed] [Google Scholar]
- 76.Gurska L. RON kinase as a novel therapeutic target in myeloproliferative neoplasms. 2020;13:162.
- 77.Moxley KM, Wang L, Welm AL, Bieniasz M. Short-form Ron is a novel determinant of ovarian cancer initiation and progression. Genes Cancer. 2016;7:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He S, Ni S, Hegde S, Wang X, Sharda DR, August A, Paulson RF, Hankey PA. Activation of the N-terminally truncated form of the Stk receptor tyrosine kinase Sf-Stk by friend virus-encoded gp55 is mediated by cysteine residues in the ecotropic domain of gp55 and the extracellular domain of Sf-Stk. J Virol. 2010;84:2223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghigna C, Giordano S, Shen HH, Benvenuto F, Castiglioni F, Comoglio PM, Green MR, Riva S, Biamonti G. Cell motility is controlled by SF2/ASF through alternative splicing of the < i > ron protooncogene. Mol Cell. 2005;20:881–90. [DOI] [PubMed] [Google Scholar]
- 80.Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakàcs A, Coppola L, Karni R. Splicing factor HnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71:4464–72. [DOI] [PubMed] [Google Scholar]
- 81.Lefave CV, Squatrito M, Vorlova S, Rocco GL, Brennan CW, Holland EC, Pan YX, Cartegni L. Splicing factor HnRNPH drives an oncogenic splicing switch in gliomas. Embo J. 2011;30:4084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu S, Chen C, Chen M, Liang J, Jiang K, Lou B, Lu J, Zhu X, Zhou D. MAGOH promotes gastric cancer progression via hnRNPA1 expression inhibition-mediated RON∆160/PI3K/AKT signaling pathway activation. J Experimental Clin Cancer Res. 2024;43:32. [DOI] [PMC free article] [PubMed]
- 83.Angeloni D, Danilkovitch-Miagkova A, Ivanova T, Braga E, Zabarovsky E, Lerman MI. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene. 2007;26:4499–512. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Yang Y, Hu S, He J, Wu Z, Qi Z, Huang M, Liu R, Lin Y, Tan C, et al. Short-form RON (sf-RON) enhances glucose metabolism to promote cell proliferation via activating β-catenin/SIX1 signaling pathway in gastric cancer. Cell Biol Toxicol. 2021;37:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–49. [DOI] [PubMed] [Google Scholar]
- 86.Wu Z, Zhang Z, Ge X, Lin Y, Dai C, Chang J, Liu X, Geng R, Wang C, Chen H, et al. Identification of short-form RON as a novel intrinsic resistance mechanism for anti-MET therapy in MET-positive gastric cancer. Oncotarget. 2015;6:40519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu HS, Hsu PY, Lai MD, Chang HY, Ho CL, Cheng HL, Chen HT, Lin YJ, Wu TJ, Tzai TS, Chow NH. An unusual function of RON receptor tyrosine kinase as a transcriptional regulator in Cooperation with EGFR in human cancer cells. Carcinogenesis. 2010;31:1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi T, Furukawa Y, Kikuchi J, Ito C, Miyata Y, Muto S, Tanaka A, Kusano E. Transactivation of RON receptor tyrosine kinase by interaction with PDGF receptor beta during steady-state growth of human mesangial cells. Kidney Int. 2009;75:1173–83. [DOI] [PubMed] [Google Scholar]
- 90.Jaquish DV, Yu PT, Shields DJ, French RP, Maruyama KP, Niessen S, Hoover H, Cravatt DAC, Lowy B. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32:1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Potratz JC, Saunders DN, Wai DH, Ng TL, McKinney SE, Carboni JM, Gottardis MM, Triche TJ, Jürgens H, Pollak MN, et al. Synthetic lethality screens reveal RPS6 and MST1R as modifiers of insulin-like growth factor-1 receptor inhibitor activity in childhood sarcomas. Cancer Res. 2010;70:8770–81. [DOI] [PubMed] [Google Scholar]
- 92.Wang Q, Quan H, Zhao J, Xie C, Wang L, Lou L. RON confers lapatinib resistance in HER2-positive breast cancer cells. Cancer Lett. 2013;340:43–50. [DOI] [PubMed] [Google Scholar]
- 93.Komposch K, Sibilia M. EGFR signaling in liver diseases. Int J Mol Sci. 2015;17:30. [DOI] [PMC free article] [PubMed]
- 94.Borkham-Kamphorst E, Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2016;28:53–61. [DOI] [PubMed] [Google Scholar]
- 95.Gui R, Li W, Li Z, Wang H, Wu Y, Jiao W, Zhao G, Shen Y, Wang L, Zhang J, et al. Effects and potential mechanisms of IGF1/IGF1R in the liver fibrosis: A review. Int J Biol Macromol. 2023;251:126263. [DOI] [PubMed] [Google Scholar]
- 96.Cazes A, Childers BG, Esparza E, Lowy AM. The MST1R/RON tyrosine kinase in cancer: oncogenic functions and therapeutic strategies. Cancers (Basel). 2022;14:2037. [DOI] [PMC free article] [PubMed]
- 97.Nanney LB, Skeel A, Luan J, Polis S, Richmond A, Wang MH, Leonard EJ. Proteolytic cleavage and activation of pro-macrophage-stimulating protein and upregulation of its receptor in tissue injury. J Invest Dermatol. 1998;111:573–81. [DOI] [PubMed] [Google Scholar]
- 98.Zhao J, Hu L, Gong N, Tang Q, Du L, Chen L. The effects of macrophage-stimulating protein on the migration, proliferation, and collagen synthesis of skin fibroblasts in vitro and in vivo. Tissue Eng Part A. 2015;21:982–91. [DOI] [PubMed] [Google Scholar]
- 99.Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates α6β4 and α3β1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell. 2003;5:257–71. [DOI] [PubMed] [Google Scholar]
- 100.Sugimoto A, Saito Y, Wang G, Sun Q, Yin C, Lee KH, Geng Y, Rajbhandari P, Hernandez C, Steffani M et al. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature. 2025;640:752–61. [DOI] [PMC free article] [PubMed]
- 101.Saviano A, Henderson NC, Baumert TF. Single-cell genomics and Spatial transcriptomics: discovery of novel cell States and cellular interactions in liver physiology and disease biology. J Hepatol. 2020;73:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medico E, Mongiovi AM, Huff J, Jelinek MA, Follenzi A, Gaudino G, Parsons JT, Comoglio PM. The tyrosine kinase receptors Ron and sea control scattering and morphogenesis of liver progenitor cells in vitro. Mol Biol Cell. 1996;7:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bezerra JA, Laney DW Jr., Degen SJ. Increased expression of mRNA for hepatocyte growth factor-like protein during liver regeneration and inflammation. Biochem Biophys Res Commun. 1994;203:666–73. [DOI] [PubMed] [Google Scholar]
- 104.Harrison P, Degen SJ, Williams R, Farzaneh F. Hepatic expression of hepatocyte-growth-factor-like/macrophage-stimulating protein mRNA in fulminant hepatic failure. Lancet. 1994;344:27–9. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. [DOI] [PubMed] [Google Scholar]
- 106.Wang MH, Zhou YQ, Chen YQ. Macrophage-stimulating protein and RON receptor tyrosine kinase: potential regulators of macrophage inflammatory activities. Scand J Immunol. 2002;56:545–53. [DOI] [PubMed] [Google Scholar]
- 107.Lutz MA, Correll PH. Activation of CR3-mediated phagocytosis by MSP requires the RON receptor, tyrosine kinase activity, phosphatidylinositol 3-kinase, and protein kinase C zeta. J Leukoc Biol. 2003;73:802–14. [DOI] [PubMed] [Google Scholar]
- 108.Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard EJ, Suda T. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–403. [PubMed] [Google Scholar]
- 109.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21. [DOI] [PubMed] [Google Scholar]
- 110.Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, Morris SM Jr., Hankey PA. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J Immunol. 2011;187:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 113.Chen YQ, Fisher JH, Wang MH. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phosphatidylinositol-3 kinase is required for RON-mediated Inhibition of iNOS expression. J Immunol. 1998;161:4950–9. [PubMed] [Google Scholar]
- 114.Chaudhuri A, Wilson NS, Yang B, Paler Martinez A, Liu J, Zhu C, Bricker N, Couto S, Modrusan Z, French D, et al. Host genetic background impacts modulation of the TLR4 pathway by RON in tissue-associated macrophages. Immunol Cell Biol. 2013;91:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaudhuri A. Regulation of macrophage polarization by RON receptor tyrosine kinase signaling. Front Immunol. 2014;5:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurusamy D, Gray JK, Pathrose P, Kulkarni RM, Finkleman FD, Waltz SE. Myeloid-specific expression of Ron receptor kinase promotes prostate tumor growth. Cancer Res. 2013;73:1752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caldwell CC, Martignoni A, Leonis MA, Ondiveeran HK, Fox-Robichaud AE, Waltz SE. Ron receptor tyrosine kinase-dependent hepatic neutrophil recruitment and survival benefit in a murine model of bacterial peritonitis. Crit Care Med. 2008;36:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ekiz HA, Lai SA, Gundlapalli H, Haroun F, Williams MA, Welm AL. Inhibition of RON kinase potentiates anti-CTLA-4 immunotherapy to shrink breast tumors and prevent metastatic outgrowth. Oncoimmunology. 2018;7:e1480286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lai SA, Gundlapalli H, Ekiz HA, Jiang A, Fernandez E, Welm AL. Blocking Short-Form Ron eliminates breast cancer metastases through accumulation of Stem-Like CD4 + T cells that subvert immunosuppression. Cancer Discov. 2021;11:3178–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wetzel CC, Leonis MA, Dent A, Olson MA, Longmeier AM, Ney PA, Boivin GP, Kader SA, Caldwell CC, Degen SJ, Waltz SE. Short-form Ron receptor is required for normal IFN-gamma production in Concanavalin A-induced acute liver injury. Am J Physiol Gastrointest Liver Physiol. 2007;292:G253–61. [DOI] [PubMed] [Google Scholar]
- 121.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–35. [DOI] [PubMed] [Google Scholar]
- 122.Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, Wang MH, Kaestner KH, Degen SJ. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest. 2001;108:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Correll PH, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct. 1997;1:69–83. [DOI] [PubMed] [Google Scholar]
- 124.Wang MH, Cox GW, Yoshimura T, Sheffler LA, Skeel A, Leonard EJ. Macrophage-stimulating protein inhibits induction of nitric oxide production by endotoxin- or cytokine-stimulated mouse macrophages. J Biol Chem. 1994;269:14027–31. [PubMed] [Google Scholar]
- 125.Ray M, Yu S, Sharda DR, Wilson CB, Liu Q, Kaushal N, Prabhu KS, Hankey PA. Inhibition of TLR4-induced IκB kinase activity by the RON receptor tyrosine kinase and its ligand, macrophage-stimulating protein. J Immunol. 2010;185:7309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benight NM, Waltz SE. Ron receptor tyrosine kinase signaling as a therapeutic target. Expert Opin Ther Targets. 2012;16:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuk JM, Shin DM, Lee HM, Kim JJ, Kim SW, Jin HS, Yang CS, Park KA, Chanda D, Kim DK, et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat Immunol. 2011;12:742–51. [DOI] [PubMed] [Google Scholar]
- 128.Leonis MA, Toney-Earley K, Degen SJ, Waltz SE. Deletion of the Ron receptor tyrosine kinase domain in mice provides protection from endotoxin-induced acute liver failure. Hepatology. 2002;36:1053–60. [DOI] [PubMed] [Google Scholar]
- 129.Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaplowitz N. Mechanisms of liver cell injury. J Hepatol. 2000;32:39–47. [DOI] [PubMed] [Google Scholar]
- 131.Feng T, Gan J, Qin A, Huang X, Wu N, Hu H, Yao H. HIV–1 downregulates the expression and phosphorylation of receptor tyrosine kinase by targeting the NF–κB pathway. Mol Med Rep. 2016;14:1947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cary DC, Clements JE, Henderson AJ. RON receptor tyrosine kinase, a negative regulator of inflammation, is decreased during Simian immunodeficiency Virus–Associated central nervous system disease. J Immunol. 2013;191:4280–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lutz MA, Gervais F, Bernstein A, Hattel AL, Correll PH. STK receptor tyrosine kinase regulates susceptibility to infection with Listeria monocytogenes. Infect Immun. 2002;70:416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris). 2010;58:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. [DOI] [PubMed] [Google Scholar]
- 136.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut. 2012;61(Suppl 1):i6–17. [DOI] [PubMed] [Google Scholar]
- 137.Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16–23. [DOI] [PubMed] [Google Scholar]
- 138.Ait-Goughoulte M, Lucifora J, Zoulim F, Durantel D. Innate antiviral immune responses to hepatitis B virus. Viruses. 2010;2:1394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–49. [DOI] [PubMed] [Google Scholar]
- 140.Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142–51. [DOI] [PubMed] [Google Scholar]
- 141.Shi B, Ren G, Hu Y, Wang S, Zhang Z, Yuan Z. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLoS ONE. 2012;7:e44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019; 179:829 – 45.e20. [DOI] [PubMed]
- 143.Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y, et al. Hepatitis B virus infection and Immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chou YC, Lin SJ, Lu J, Yeh TH, Chen CL, Weng PL, Lin JH, Yao M, Tsai CH. Requirement for LMP1-induced RON receptor tyrosine kinase in Epstein-Barr virus-mediated B-cell proliferation. Blood. 2011;118:1340–9. [DOI] [PubMed] [Google Scholar]
- 145.Côté M, Miller AD, Liu SL. Human RON receptor tyrosine kinase induces complete epithelial-to-mesenchymal transition but causes cellular senescence. Biochem Biophys Res Commun. 2007;360:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang J, Chen Y, Gu D, Zhang G, Chen J, Zhao J, Wu P. Ketamine-induced bladder fibrosis involves epithelial-to-mesenchymal transition mediated by transforming growth factor-β1. Am J Physiol Ren Physiol. 2017;313:F961–72. [DOI] [PubMed] [Google Scholar]
- 148.Zhang HX, Li YN, Wang XL, Ye CL, Zhu XY, Li HP, Yang T, Liu YJ. Probucol ameliorates EMT and lung fibrosis through restoration of SIRT3 expression. Pulm Pharmacol Ther. 2019;57:101803. [DOI] [PubMed] [Google Scholar]
- 149.Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol. 2013;305:G881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang W, He H, Wang T, Su N, Zhang F, Jiang K, Zhu J, Zhang C, Niu K, Wang L, et al. Single-Cell transcriptomic analysis reveals a hepatic stellate Cell-Activation roadmap and myofibroblast origin during liver fibrosis in mice. Hepatology. 2021;74:2774–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023;24:255–72. [DOI] [PubMed] [Google Scholar]
- 152.Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J, Han D. The AMPK pathway in fatty liver disease. Front Physiol. 2022;13:970292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chanda D, Li T, Song K-H, Kim Y-H, Sim J, Lee CH, Chiang JYL, Choi H-S. Hepatocyte growth factor family negatively regulates hepatic gluconeogenesis via induction of orphan nuclear receptor small heterodimer partner in primary hepatocytes. J Biol Chem. 2009;284:28510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bezerra JA, Carrick TL, Degen JL, Witte D, Degen SJ. Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J Clin Invest. 1998;101:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chanda D, Li J, Oligschlaeger Y, Jeurissen ML, Houben T, Walenbergh SM, Shiri-Sverdlov R, Neumann D. MSP is a negative regulator of inflammation and lipogenesis in ex vivo models of non-alcoholic steatohepatitis. Exp Mol Med. 2016;48:e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hunt BG, Davis JC, Fox LH, Vicente-Muñoz S, Lester C, Wells SI, Waltz SE. RON-augmented cholesterol biosynthesis in breast cancer metastatic progression and recurrence. Oncogene. 2023;42:1716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–30. [DOI] [PubMed] [Google Scholar]
- 158.Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K, et al. SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep. 2013;5:654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nevzorova YA, Cubero FJ, Hu W, Hao F, Haas U, Ramadori P, Gassler N, Hoss M, Strnad P, Zimmermann HW, et al. Enhanced expression of c-myc in hepatocytes promotes initiation and progression of alcoholic liver disease. J Hepatol. 2016;64:628–40. [DOI] [PubMed] [Google Scholar]
- 160.Hoofnagle JH, Doo E. Letter to the editor: A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2024;79:E91–2. [DOI] [PubMed] [Google Scholar]
- 161.Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80:e54–6. [DOI] [PubMed] [Google Scholar]
- 162.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24. [DOI] [PubMed] [Google Scholar]
- 163.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- 164.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. [DOI] [PubMed] [Google Scholar]
- 165.Israelsen M, Francque S, Tsochatzis EA, Krag A. Steatotic liver disease. Lancet. 2024;404:1761–78. [DOI] [PubMed] [Google Scholar]
- 166.Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver. 2012;6:149–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fujii H, Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 2012;47:215–25. [DOI] [PubMed] [Google Scholar]
- 168.Schierwagen R, Maybüchen L, Zimmer S, Hittatiya K, Bäck C, Klein S, Uschner FE, Reul W, Boor P, Nickenig G, et al. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci Rep. 2015;5:12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yu S, Allen JN, Dey A, Zhang L, Balandaram G, Kennett MJ, Xia M, Xiong N, Peters JM, Patterson A, Hankey-Giblin PA. The Ron receptor tyrosine kinase regulates macrophage heterogeneity and plays a protective role in Diet-Induced obesity, atherosclerosis, and hepatosteatosis. J Immunol. 2016;197:256–65. [DOI] [PubMed] [Google Scholar]
- 170.Allen J, Zhang J, Quickel MD, Kennett M, Patterson AD, Hankey-Giblin PA. Ron receptor signaling ameliorates hepatic fibrosis in a Diet-Induced nonalcoholic steatohepatitis mouse model. J Proteome Res. 2018;17:3268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stuart WD, Brown NE, Paluch AM, Waltz SE. Loss of Ron receptor signaling leads to reduced obesity, diabetic phenotypes and hepatic steatosis in response to high-fat diet in mice. Volume 308. AMERICAN JOURNAL OF PHYSIOLOGY-ENDOCRINOLOGY AND METABOLISM; 2015. pp. E562–72. [DOI] [PMC free article] [PubMed]
- 172.Li J, Chanda D, van Gorp PJ, Jeurissen ML, Houben T, Walenbergh SM, Debets J, Oligschlaeger Y, Gijbels MJ, Neumann D, Shiri-Sverdlov R. Macrophage stimulating protein enhances hepatic inflammation in a NASH model. PLoS ONE. 2016;11:e0163843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hasegawa S, Yoneda M, Kurita Y, Nogami A, Honda Y, Hosono K, Nakajima A. Cholestatic liver disease: current treatment strategies and new therapeutic agents. Drugs. 2021;81:1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Melum E, Franke A, Schramm C, Weismüller TJ, Gotthardt DN, Offner FA, Juran BD, Laerdahl JK, Labi V, Björnsson E, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Häuser F, Deyle C, Berard D, Neukirch C, Glowacki C, Bickmann JK, Wenzel JJ, Lackner KJ, Rossmann H. Macrophage-stimulating protein polymorphism rs3197999 is associated with a gain of function: implications for inflammatory bowel disease. Genes Immun. 2012;13:321–7. [DOI] [PubMed] [Google Scholar]
- 178.Goyette P, Lefebvre C, Ng A, Brant SR, Cho JH, Duerr RH, Silverberg MS, Taylor KD, Latiano A, Aumais G, et al. Gene-centric association mapping of chromosome 3p implicates MST1 in IBD pathogenesis. Mucosal Immunol. 2008;1:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Häuser F, Rossmann H, Laubert-Reh D, Wild PS, Zeller T, Müller C, Neuwirth S, Blankenberg S, Lackner KJ. Inflammatory bowel disease (IBD) locus 12: is glutathione peroxidase-1 (GPX1) the relevant gene? Genes Immun. 2015;16:571–5. [DOI] [PubMed] [Google Scholar]
- 180.Di Narzo AF, Telesco SE, Brodmerkel C, Argmann C, Peters LA, Li K, Kidd B, Dudley J, Cho J, Schadt EE, et al. High-Throughput characterization of blood serum proteomics of IBD patients with respect to aging and genetic factors. PLoS Genet. 2017;13:e1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brylak J, Nowak JK, Dybska E, Glapa-Nowak A, Kierkuś J, Osiecki M, Banaszkiewicz A, Radzikowski A, Szaflarska-Popławska A, Kwiecień J et al. Macrophage-Stimulating 1 polymorphism rs3197999 in pediatric patients with inflammatory bowel disease. Med (Kaunas). 2024;60:1243. [DOI] [PMC free article] [PubMed]
- 182.Chen Q, Seol DW, Carr B, Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997;26:59–66. [DOI] [PubMed] [Google Scholar]
- 183.Cho SB, Park YL, Song YA, Kim KY, Lee GH, Cho DH, Myung DS, Park KJ, Lee WS, Chung IJ, et al. Small interfering RNA-directed targeting of RON alters invasive and oncogenic phenotypes of human hepatocellular carcinoma cells. Oncol Rep. 2011;26:1581–6. [DOI] [PubMed] [Google Scholar]
- 184.Zhu M, Paddock GV. Expression of the hepatocyte growth factor-like protein gene in human hepatocellular carcinoma and interleukin-6-induced increased expression in hepatoma cells. Biochim Biophys Acta. 1999;1449:63–72. [DOI] [PubMed] [Google Scholar]
- 185.Du B, Yu R, Geng X, Li Y, Liu Y, Liu S, Li F, Yu Q, Guo Y, Xi X. The function of MSP-activated γδT cells in hepatocellular carcinoma. Int Immunopharmacol. 2023;124:110893. [DOI] [PubMed] [Google Scholar]
- 186.Liu T, Xue R, Dong L, Wu H, Zhang D, Shen X. Rapid determination of serological cytokine biomarkers for hepatitis B virus-related hepatocellular carcinoma using antibody microarrays. Acta Biochim Biophys Sin (Shanghai). 2011;43:45–51. [DOI] [PubMed] [Google Scholar]
- 187.Xi X, Guo Y, Zhu M, Qiu F, Lei F, Li G, Du B. Identification of new potential antigen recognized by γδT cells in hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:1917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–7. [DOI] [PubMed] [Google Scholar]
- 189.Ramutton T, Buccheri S, Dieli F, Todaro M, Stassi G, Meraviglia S. γδ T cells as a potential tool in colon cancer immunotherapy. Immunotherapy. 2014;6:989–99. [DOI] [PubMed] [Google Scholar]
- 190.van Diest E, Hernández López P, Meringa AD, Vyborova A, Karaiskaki F, Heijhuurs S, Gumathi Bormin J, van Dooremalen S, Nicolasen MJT, Gatti L et al. Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J Immunother Cancer. 2021;9:e003850. [DOI] [PMC free article] [PubMed]
- 191.Cheng CT, Chen YY, Wu RC, Tsai CY, Chiang KC, Yeh TS, Chen MH, Yeh CN. MET–RON dual inhibitor, BMS–777607, suppresses cholangiocarcinoma cell growth, and MET–RON upregulation indicates worse prognosis for intra–hepatic cholangiocarcinoma patients. Oncol Rep. 2018;40:1411–21. [DOI] [PubMed] [Google Scholar]
- 192.Koh YW, Park YS, Kang HJ, Shim JH, Yu E. MET is a predictive factor for late recurrence but not for overall survival of early stage hepatocellular carcinoma. Tumour Biol. 2015;36:4993–5000. [DOI] [PubMed] [Google Scholar]
- 193.Watanabe H, Yokoyama Y, Kokuryo T, Ebata T, Igami T, Sugawara G, Mizuno T, Shimoyama Y, Nagino M. Prognostic value of hepatocyte growth factor receptor expression in patients with Perihilar cholangiocarcinoma. Ann Surg Oncol. 2015;22:2235–42. [DOI] [PubMed] [Google Scholar]
- 194.Hayashi Y, Yamaguchi J, Kokuryo T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. The complete loss of tyrosine kinase receptors MET and RON is a poor prognostic factor in patients with extrahepatic cholangiocarcinoma. Anticancer Res. 2016;36:6585–92. [DOI] [PubMed] [Google Scholar]
- 195.Krawczyk M, Höblinger A, Mihalache F, Grünhage F, Acalovschi M, Lammert F, Zimmer V. Macrophage stimulating protein variation enhances the risk of sporadic extrahepatic cholangiocarcinoma. Dig Liver Dis. 2013;45:612–5. [DOI] [PubMed] [Google Scholar]
- 196.Li C, Morvaridi S, Lam G, Chheda C, Kamata Y, Katsumata M, Edderkaoui M, Yuan X, Nissen N, Pandol SJ, Wang Q. MSP-RON signaling is activated in the transition from pancreatic intraepithelial neoplasia (PanIN) to pancreatic ductal adenocarcinoma (PDAC). Front Physiol. 2019;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Benight NM, Wagh PK, Zinser GM, Peace BE, Stuart WD, Vasiliauskas J, Pathrose P, Starnes SL, Waltz SE. HGFL supports mammary tumorigenesis by enhancing tumor cell intrinsic survival and influencing macrophage and T-cell responses. Oncotarget. 2015;6:17445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kataoka H, Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. Febs J. 2010;277:2230–7. [DOI] [PubMed] [Google Scholar]
- 199.Kawaguchi M, Orikawa H, Baba T, Fukushima T, Kataoka H. Hepatocyte growth factor activator is a serum activator of single-chain precursor macrophage-stimulating protein. Febs J. 2009;276:3481–90. [DOI] [PubMed] [Google Scholar]
- 200.Ren X, Daa T, Yada N, Kashima K, Fujitomi Y, Yokoyama S. Expression and mutational status of RON in neoplastic lesions of the breast: analysis of MSP/RON signaling in ductal carcinoma in situ and invasive ductal carcinoma. Apmis. 2012;120:358–67. [DOI] [PubMed] [Google Scholar]
- 201.Hunt BG, Jones A, Lester C, Davis JC, Benight NM, Waltz SE. RON (MST1R) and HGFL (MST1) Co-Overexpression supports breast tumorigenesis through autocrine and paracrine cellular crosstalk. Cancers (Basel). 2022;14:2493. [DOI] [PMC free article] [PubMed]
- 202.Uckun FM, Qazi S, Cely I, Sahin K, Shahidzadeh A, Ozercan I, Yin Q, Gaynon P, Termuhlen A, Cheng J, Yiv S. Nanoscale liposomal formulation of a SYK P-site inhibitor against B-precursor leukemia. Blood. 2013;121:4348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schroeder GM, An Y, Cai ZW, Chen XT, Clark C, Cornelius LA, Dai J, Gullo-Brown J, Gupta A, Henley B, et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem. 2009;52:1251–4. [DOI] [PubMed] [Google Scholar]
- 204.Fialin C, Larrue C, Vergez F, Sarry JE, Bertoli S, Mansat-De Mas V, Demur C, Delabesse E, Payrastre B, Manenti S, et al. The short form of RON is expressed in acute myeloid leukemia and sensitizes leukemic cells to cMET inhibitors. Leukemia. 2013;27:325–35. [DOI] [PubMed] [Google Scholar]
- 205.Kim J, Koh D-I, Lee M, Park YS, Hong S-W, Shin J-S, Lee MS, Kim M-H, Lee JH, Jeong J, et al. Targeting isoforms of RON kinase (MST1R) drives antitumor efficacy. Cell Death Differ. 2023;30:2491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Z, Yao H, Guin S, Padhye SS, Zhou YQ, Wang MH. Monoclonal antibody (mAb)-induced down-regulation of RON receptor tyrosine kinase diminishes tumorigenic activities of colon cancer cells. Int J Oncol. 2010;37:473–82. [DOI] [PubMed] [Google Scholar]
- 207.Guin S, Yao HP, Wang MH. RON receptor tyrosine kinase as a target for delivery of chemodrugs by antibody directed pathway for cancer cell cytotoxicity. Mol Pharm. 2010;7:386–97. [DOI] [PubMed] [Google Scholar]
- 208.LoRusso PM, Gounder M, Jalal SI, André V, Kambhampati SRP, Loizos N, Hall J, Holzer TR, Nasir A, Cosaert J, et al. Phase 1 study of narnatumab, an anti-RON receptor monoclonal antibody, in patients with advanced solid tumors. Invest New Drugs. 2017;35:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Suthe SR, Yao HP, Weng TH, Hu CY, Feng L, Wu ZG, Wang MH. RON receptor tyrosine kinase as a therapeutic target for eradication of Triple-Negative breast cancer: efficacy of Anti-RON ADC Zt/g4-MMAE. Mol Cancer Ther. 2018;17:2654–64. [DOI] [PubMed] [Google Scholar]
- 210.Yao HP, Feng L, Weng TH, Hu CY, Suthe SR, Mostofa AGM, Chen LH, Wu ZG, Wang WL, Wang MH. Preclinical efficacy of Anti-RON Antibody-Drug conjugate Zt/g4-MMAE for targeted therapy of pancreatic cancer overexpressing RON receptor tyrosine kinase. Mol Pharm. 2018;15:3260–71. [DOI] [PubMed] [Google Scholar]
- 211.Chen Y, Zheng J, Mo L, Chen F, Li R, Wang Y, Liang Q, Chen Z, Dai W, Chen L, et al. Oroxylin A suppresses breast cancer-induced osteoclastogenesis and osteolysis as a natural RON inhibitor. Phytomedicine. 2024;129:155688. [DOI] [PubMed] [Google Scholar]
- 212.Sun Y, Weng J, Chen X, Ma S, Zhang Y, Zhang F, Zhang Z, Wang F, Shao J, Zheng S. Oroxylin A activates ferritinophagy to induce hepatic stellate cell senescence against hepatic fibrosis by regulating cGAS-STING pathway. Biomed Pharmacother. 2023;162:114653. [DOI] [PubMed] [Google Scholar]
- 213.Zhao D, Gao Y, Su Y, Zhou Y, Yang T, Li Y, Wang Y, Sun Y, Chen L, Zhang F, et al. Oroxylin A regulates cGAS DNA hypermethylation induced by methionine metabolism to promote HSC senescence. Pharmacol Res. 2023;187:106590. [DOI] [PubMed] [Google Scholar]
- 214.Fukushima T, Uchiyama S, Tanaka H, Kataoka H. Hepatocyte growth factor activator: A proteinase linking tissue injury with repair. Int J Mol Sci. 2018;19:3435. [DOI] [PMC free article] [PubMed]
- 215.Yao HP, Tong XM, Hudson R, Wang MH. MET and RON receptor tyrosine kinases in colorectal adenocarcinoma: molecular features as drug targets and antibody-drug conjugates for therapy. J Exp Clin Cancer Res. 2020;39:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen SL, Wang GP, Shi DR, Yao SH, Chen KD, Yao HP. RON in hepatobiliary and pancreatic cancers: pathogenesis and potential therapeutic targets. World J Gastroenterol. 2021;27:2507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhou D, Pan G, Zheng C, Zheng J, Yian L, Teng X. Expression of the RON receptor tyrosine kinase and its association with gastric carcinoma versus normal gastric tissues. BMC Cancer. 2008;8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yao H-P, Luo Y-L, Feng L, Cheng L-F, Lu Y, Li W, Wang M-H. Agonistic monoclonal antibodies potentiate tumorigenic and invasive activities of splicing variant of the RON receptor tyrosine kinase. Cancer Biol Ther. 2006;5:1179–86. [DOI] [PubMed] [Google Scholar]
- 219.Chakedis J, French R, Babicky M, Jaquish D, Howard H, Mose E, Lam R, Holman P, Miyamoto J, Walterscheid Z, Lowy AM. A novel protein isoform of the RON tyrosine kinase receptor transforms human pancreatic duct epithelial cells. Oncogene. 2016;35:3249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Persons DA, Paulson RF, Loyd MR, Herley MT, Bodner SM, Bernstein A, Correll PH, Ney PA. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–65. [DOI] [PubMed] [Google Scholar]
- 221.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–17. [DOI] [PubMed] [Google Scholar]
- 222.Giroux-Leprieur E, Fallet V, Cadranel J, Wislez M. Spotlight on Crizotinib in the first-line treatment of ALK-positive advanced non-small-cell lung cancer: patients selection and perspectives. Lung Cancer (Auckl). 2016;7:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM, Rini BI, Srinivas S, Stein MN, Adams LM, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yau TCC, Lencioni R, Sukeepaisarnjaroen W, Chao Y, Yen CJ, Lausoontornsiri W, Chen PJ, Sanpajit T, Camp A, Cox DS, et al. A phase I/II multicenter study of Single-Agent foretinib as First-Line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2017;23:2405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Weng TH, Yao MY, Xu XM, Hu CY, Yao SH, Liu YZ, Wu ZG, Tang TM, Fu PF, Wang MH, Yao HP. RON and MET Co-overexpression are significant pathological characteristics of poor survival and therapeutic targets of tyrosine kinase inhibitors in Triple-Negative breast cancer. Cancer Res Treat. 2020;52:973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang W, Li Q, Takeuchi S, Yamada T, Koizumi H, Nakamura T, Matsumoto K, Mukaida N, Nishioka Y, Sone S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18:1663–71. [DOI] [PubMed] [Google Scholar]
- 227.Cao L, Liu P, Yang P, Gao Q, Li H, Sun Y, Zhu L, Lin J, Su D, Rao Z, Wang X. Structural basis for neutralization of hepatitis A virus informs a rational design of highly potent inhibitors. PLoS Biol. 2019;17:e3000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Molife LR, Dean EJ, Blanco-Codesido M, Krebs MG, Brunetto AT, Greystoke AP, Daniele G, Lee L, Kuznetsov G, Myint KT, et al. A phase I, dose-escalation study of the multitargeted receptor tyrosine kinase inhibitor, golvatinib, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:6284–94. [DOI] [PubMed] [Google Scholar]
- 229.Claridge S, Raeppel F, Granger MC, Bernstein N, Saavedra O, Zhan L, Llewellyn D, Wahhab A, Deziel R, Rahil J, et al. Discovery of a novel and potent series of thieno[3,2-b]pyridine-based inhibitors of c-Met and VEGFR2 tyrosine kinases. Bioorg Med Chem Lett. 2008;18:2793–8. [DOI] [PubMed] [Google Scholar]
- 230.Hong DS, Cappuzzo F, Chul Cho B, Dowlati A, Hussein M, Kim DW, Percent I, Christensen JG, Morin J, Potvin D, et al. Phase II study investigating the efficacy and safety of glesatinib (MGCD265) in patients with advanced NSCLC containing MET activating alterations. Lung Cancer. 2024;190:107512. [DOI] [PubMed] [Google Scholar]
- 231.Zhang Y, Kaplan-Lefko PJ, Rex K, Yang Y, Moriguchi J, Osgood T, Mattson B, Coxon A, Reese M, Kim TS, et al. Identification of a novel recepteur d’origine nantais/c-met small-molecule kinase inhibitor with antitumor activity in vivo. Cancer Res. 2008;68:6680–7. [DOI] [PubMed] [Google Scholar]
- 232.Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV, Gibbs JB, Gil S, Hang G, Hatch H, et al. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010;70:1524–33. [DOI] [PubMed] [Google Scholar]
- 233.Northrup AB, Katcher MH, Altman MD, Chenard M, Daniels MH, Deshmukh SV, Falcone D, Guerin DJ, Hatch H, Li C, et al. Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A specific c-Met/Ron dual kinase inhibitor with Preferential affinity for the activated state of c-Met. J Med Chem. 2013;56:2294–310. [DOI] [PubMed] [Google Scholar]
- 234.Keedy VL, Lenz HJ, Saltz L, Whisenant JG, Berlin JD, Camacho LH. First-in-human phase I dose escalation study of MK-8033 in patients with advanced solid tumors. Invest New Drugs. 2018;36:860–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Raeppel SL, Raeppel F, Therrien E. Design and synthesis of close analogs of LCRF-0004, a potent and selective RON receptor tyrosine kinase inhibitor. Bioorg Med Chem Lett. 2015;25:2527–31. [DOI] [PubMed] [Google Scholar]
- 236.O’Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M, Gottfredsen KJ, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–70. [DOI] [PubMed] [Google Scholar]
- 237.Gunes Z, Zucconi A, Cioce M, Meola A, Pezzanera M, Acali S, Zampaglione I, De Pratti V, Bova L, Talamo F, et al. Isolation of fully human antagonistic RON antibodies showing efficient block of downstream signaling and cell migration. Transl Oncol. 2011;4:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yao HP, Zhou YQ, Ma Q, Guin S, Padhye SS, Zhang RW, Wang MH. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. Mol Cancer. 2011;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Feng L, Yao HP, Wang W, Zhou YQ, Zhou J, Zhang R, Wang MH. Efficacy of anti-RON antibody Zt/g4-drug maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for targeted colorectal cancer therapy. Clin Cancer Res. 2014;20:6045–58. [DOI] [PubMed] [Google Scholar]
- 240.Yao HP, Feng L, Zhou JW, Zhang RW, Wang MH. Therapeutic evaluation of monoclonal antibody-maytansinoid conjugate as a model of RON-targeted drug delivery for pancreatic cancer treatment. Am J Cancer Res. 2016;6:937–56. [PMC free article] [PubMed] [Google Scholar]
- 241.Tong XM, Feng L, Suthe SR, Weng TH, Hu CY, Liu YZ, Wu ZG, Wang MH, Yao HP. Therapeutic efficacy of a novel humanized antibody-drug conjugate recognizing plexin-semaphorin-integrin domain in the RON receptor for targeted cancer therapy. J Immunother Cancer. 2019;7:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fujita K, Yamada M, Morishita A, Ono M, Himoto T, Kobara H, Masaki T. Cabozantinib inhibits the growth of lenvatinib-resistant hepatoma cells restoring FTCD expression. Biochem Pharmacol. 2024;226:116321. [DOI] [PubMed] [Google Scholar]
- 243.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xiang Q, Chen W, Ren M, Wang J, Zhang H, Deng DY, Zhang L, Shang C, Chen Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual Blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20:2959–70. [DOI] [PubMed] [Google Scholar]
- 245.Matsuda T, Kaji K, Nishimura N, Asada S, Koizumi A, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, et al. Cabozantinib prevents the progression of metabolic dysfunction-associated steatohepatitis by inhibiting the activation of hepatic stellate cell and macrophage and attenuating angiogenic activity. Heliyon. 2024;10:e38647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Funato K, Miyake N, Sekiba K, Miyakawa Y, Seimiya T, Shibata C, Kishikawa T, Otsuka M. Cabozantinib inhibits HBV-RNA transcription by decreasing STAT3 binding to the enhancer region of CccDNA. Hepatol Commun. 2023;7:e0313. [DOI] [PMC free article] [PubMed]
- 247.Reichinger A, Essl L, Kerschner P, Burghofer J, Webersinke G, Rumpold H, Doleschal B. Exceptional sustained long-term complete response to Tepotinib in a MET-amplified advanced intrahepatic biliary tract cancer failing durvalumab plus cisplatin and gemcitabine. Oncologist. 2024;29:1090–4. [DOI] [PMC free article] [PubMed]
- 248.Santoro A, Simonelli M, Rodriguez-Lope C, Zucali P, Camacho LH, Granito A, Senzer N, Rimassa L, Abbadessa G, Schwartz B, et al. A Phase-1b study of Tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer. 2013;108:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Qin S, Chan SL, Sukeepaisarnjaroen W, Han G, Choo SP, Sriuranpong V, Pan H, Yau T, Guo Y, Chen M, et al. A phase II study of the efficacy and safety of the MET inhibitor Capmatinib (INC280) in patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2019;11:1758835919889001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Santoro A, Assenat E, Yau T, Delord JP, Maur M, Knox J, Cattan S, Lee KH, Del Conte G, Springfeld C, et al. A phase ib/ii trial of Capmatinib plus Spartalizumab vs. Spartalizumab alone in patients with pretreated hepatocellular carcinoma. JHEP Rep. 2024;6:101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jia H, Dai G, Weng J, Zhang Z, Wang Q, Zhou F, Jiao L, Cui Y, Ren Y, Fan S, et al. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem. 2014;57:7577–89. [DOI] [PubMed] [Google Scholar]
- 252.Yan N, Zhang Z, Guo S, Shen S, Li X. Advanced HCC with amplified mesenchymal epithelial transition factor receptor responds well to savolitinib: a case report. Front Med (Lausanne). 2023;10:1130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Huether A, Höpfner M, Sutter AP, Schuppan D, Scherübl H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J Hepatol. 2005;43:661–9. [DOI] [PubMed] [Google Scholar]
- 254.Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657–63. [DOI] [PubMed] [Google Scholar]
- 255.Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, Rashid A, Dancey J, Abbruzzese JL. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059–67. [DOI] [PubMed] [Google Scholar]
- 256.Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–50. [DOI] [PubMed] [Google Scholar]
- 257.Gan CJ, Li WF, Li CN, Li LL, Zhou WY, Peng XM. EGF receptor inhibitors comprehensively suppress hepatitis B virus by downregulation of STAT3 phosphorylation. Biochem Biophys Rep. 2020;22:100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T, et al. Epidermal growth factor receptor Inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Höpfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherübl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008–16. [DOI] [PubMed] [Google Scholar]
- 260.Ueda S, Basaki Y, Yoshie M, Ogawa K, Sakisaka S, Kuwano M, Ono M. PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to Inhibition by gefitinib. Cancer Res. 2006;66:5346–53. [DOI] [PubMed] [Google Scholar]
- 261.Gu HR, Park SC, Choi SJ, Lee JC, Kim YC, Han CJ, Kim J, Yang KY, Kim YJ, Noh GY, et al. Combined treatment with Silibinin and either Sorafenib or gefitinib enhances their growth-inhibiting effects in hepatocellular carcinoma cells. Clin Mol Hepatol. 2015;21:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Finlay MR, Anderton M, Ashton S, Ballard P, Bethel PA, Box MR, Bradbury RH, Brown SJ, Butterworth S, Campbell A, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57:8249–67. [DOI] [PubMed] [Google Scholar]
- 263.Huang Q, He S, Zhan D. Osimertinib is a dual inhibitor of hepatocellular carcinoma and angiogenesis in an EGFR-independent manner, and synergizes with venetoclax. J Cancer Res Clin Oncol. 2023;149:10727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–15. [DOI] [PubMed] [Google Scholar]
- 265.Chen Y, Chen X, Ding X, Wang Y. Afatinib, an EGFR inhibitor, decreases EMT and tumorigenesis of Huh–7 cells by regulating the ERK–VEGF/MMP9 signaling pathway. Mol Med Rep. 2019;20:3317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yu C, Zhang X, Wang M, Xu G, Zhao S, Feng Y, Pan C, Yang W, Zhou J, Shang L, Ma Y. Afatinib combined with anti-PD1 enhances immunotherapy of hepatocellular carcinoma via ERBB2/STAT3/PD-L1 signaling. Front Oncol. 2023;13:1198118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kim H, Lim HY. Novel EGFR-TK inhibitor EKB-569 inhibits hepatocellular carcinoma cell proliferation by AKT and MAPK pathways. J Korean Med Sci. 2011;26:1563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lee S, Kang E, Lee U, Cho S. Role of Pelitinib in the regulation of migration and invasion of hepatocellular carcinoma cells via Inhibition of Twist1. BMC Cancer. 2023;23:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Huether A, Höpfner M, Baradari V, Schuppan D, Scherübl H. EGFR Blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem Pharmacol. 2005;70:1568–78. [DOI] [PubMed] [Google Scholar]
- 270.Chen W, Shen X, Xia X, Xu G, Ma T, Bai X, Liang T. NSC 74859-mediated Inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinoma. Liver Int. 2012;32:70–7. [DOI] [PubMed] [Google Scholar]
- 271.Geng J, Li X, Lang X, Qiao C, Hu M, Yang J, Feng J, Lv M. Combination of cetuximab and Rapamycin enhances the therapeutic efficacy in hepatocellular carcinoma. Technol Cancer Res Treat. 2014;13:377–85. [DOI] [PubMed] [Google Scholar]
- 272.Huang S, He R, Rong M, Dang Y, Chen G. Synergistic effect of MiR-146a mimic and cetuximab on hepatocellular carcinoma cells. Biomed Res Int. 2014; 2014:384121. [DOI] [PMC free article] [PubMed]
- 273.Sohn J, Liu S, Parinyanitikul N, Lee J, Hortobagyi GN, Mills GB, Ueno NT, Gonzalez-Angulo AM. cMET activation and EGFR-Directed therapy resistance in Triple-Negative breast cancer. J Cancer. 2014;5:745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li WY, Li Q, Jing L, Wu T, Han LL, Wang Y, Yu SZ, Nan KJ, Guo H. P57-mediated autophagy promotes the efficacy of EGFR inhibitors in hepatocellular carcinoma. Liver Int. 2019;39:147–57. [DOI] [PubMed] [Google Scholar]
- 275.Mahgoub S, Abosalem H, Emara M, Kotb N, Maged A, Soror S. Restoring NK cells functionality via cytokine activation enhances cetuximab-mediated NK-cell ADCC: A promising therapeutic tool for HCC patients. Mol Immunol. 2021;137:221–27. [DOI] [PubMed] [Google Scholar]
- 276.Taha AM, Aboulwafa MM, Zedan H, Helmy OM. Ramucirumab combination with Sorafenib enhances the inhibitory effect of Sorafenib on HepG2 cancer cells. Sci Rep. 2022;12:17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang Y, Zhang M, Gong Y, Wu Q, Zhang L, Jiao S. Bioinformatic analysis of hepatocellular carcinoma cell lines to the efficacy of nimotuzumab. Int J Gen Med. 2021;14:2611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Susutlertpanya W, Wakuda H, Otani N, Kuramoto T, Li L, Kuranari M, Sekiguchi A, Kudo H, Uchida T, Imai H, Uemura N. Histological evaluation of nintedanib in non-alcoholic steatohepatitis mice. Life Sci. 2019;228:251–57. [DOI] [PubMed] [Google Scholar]
- 279.Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, Gantumur D, Handa T, Ishii N, Tsukagoshi M, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wind S, Schmid U, Freiwald M, Marzin K, Lotz R, Ebner T, Stopfer P, Dallinger C. Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin Pharmacokinet. 2019;58:1131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, Lederle W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–31. [DOI] [PubMed] [Google Scholar]
- 282.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, Mazzaferro V, Wiest R, Reig M, Wagner A, Bolondi L. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–9. [DOI] [PubMed] [Google Scholar]
- 283.Tai WT, Chu PY, Shiau CW, Chen YL, Li YS, Hung MH, Chen LJ, Chen PL, Su JC, Lin PY, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20:5768–76. [DOI] [PubMed] [Google Scholar]
- 284.Trojan J, Waidmann O. Role of regorafenib as second-line therapy and landscape of investigational treatment options in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;3:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on Sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 286.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- 287.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. [DOI] [PubMed] [Google Scholar]
- 288.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, et al. Phase II study of Sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300. [DOI] [PubMed] [Google Scholar]
- 289.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, et al. Efficacy and safety of Sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Himmelsbach K, Sauter D, Baumert TF, Ludwig L, Blum HE, Hildt E. New aspects of an anti-tumour drug: Sorafenib efficiently inhibits HCV replication. Gut. 2009;58:1644–53. [DOI] [PubMed] [Google Scholar]
- 291.Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, Fukuda JY, Chu JY, Nematalla A, Wang X, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46:1116–9. [DOI] [PubMed] [Google Scholar]
- 292.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, et al. Efficacy, safety, and potential biomarkers of Sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S, et al. Safety and efficacy of Sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. [DOI] [PubMed] [Google Scholar]
- 294.Zhu AX, Duda DG, Sahani DV, Jain RK. Development of Sunitinib in hepatocellular carcinoma: rationale, early clinical experience, and correlative studies. Cancer J. 2009;15:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. [DOI] [PubMed] [Google Scholar]
- 296.Brezgin SA, Kostyusheva AP, Ponomareva NI, Gegechkori VI, Kirdyashkina NP, Ayvasyan SR, Dmitrieva LN, Kokoreva LN, Chulanov VP, Kostyushev DS. [HBx protein potentiates hepatitis B virus reactivation]. Mol Biol (Mosk). 2022;56:783–94. [DOI] [PubMed] [Google Scholar]
- 297.Yau T, Chen PJ, Chan P, Curtis CM, Murphy PS, Suttle AB, Gauvin J, Hodge JP, Dar MM, Poon RT. Phase I dose-finding study of pazopanib in hepatocellular carcinoma: evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res. 2011;17:6914–23. [DOI] [PubMed] [Google Scholar]
- 298.Zhu XD, Zhang JB, Fan PL, Xiong YQ, Zhuang PY, Zhang W, Xu HX, Gao DM, Kong LQ, Wang L, et al. Antiangiogenic effects of pazopanib in xenograft hepatocellular carcinoma models: evaluation by quantitative contrast-enhanced ultrasonography. BMC Cancer. 2011;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Scott LJ. Lenvatinib: first global approval. Drugs. 2015;75:553–60. [DOI] [PubMed] [Google Scholar]
- 300.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K, Kumada H. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22:1385–94. [DOI] [PubMed] [Google Scholar]
- 301.Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Al-Salama ZT, Syed YY, Scott LJ, Lenvatinib. A review in hepatocellular carcinoma. Drugs. 2019;79:665–74. [DOI] [PubMed] [Google Scholar]
- 303.Shirley M, Olaratumab. First global approval. Drugs. 2017;77:107–12. [DOI] [PubMed] [Google Scholar]
- 304.Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human Immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116:1018–26. [DOI] [PubMed] [Google Scholar]
- 305.Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503–13. [DOI] [PubMed] [Google Scholar]
- 306.Xie M, Sun M, Ji X, Li D, Chen X, Zhang B, Huang W, Zhang T, Wang Y, Tian D, Xia L. Overexpression of BACH1 mediated by IGF2 facilitates hepatocellular carcinoma growth and metastasis via IGF1R and PTK2. Theranostics. 2022;12:1097–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.D’Alessandro R, Refolo MG, Lippolis C, Carella N, Messa C, Cavallini A, Carr BI. Strong enhancement by IGF1-R antagonists of hepatocellular carcinoma cell migration Inhibition by Sorafenib and/or vitamin K1. Cell Oncol (Dordr). 2018;41:283–96. [DOI] [PubMed] [Google Scholar]
- 308.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, Hillerman S, Cao C, Cantor GH, Dell-John J, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. [DOI] [PubMed] [Google Scholar]
- 309.Leung PKH, Das B, Cheng X, Tarazi M. Prognostic and predictive utility of GPD1L in human hepatocellular carcinoma. Int J Mol Sci. 2023;24:13113. [DOI] [PMC free article] [PubMed]
- 310.Höpfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherübl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435–48. [DOI] [PubMed] [Google Scholar]
- 311.García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.