Abstract
Background
Eukaryotic initiation factor 5 A (eIF5A) and hypusination-related disorders (eIF5A-HRD) are recently described diseases caused by pathogenic heterozygous variants in the translation factor EIF5A or biallelic variants in the two enzymes involved in the post-translational synthesis of hypusine in the eIF5A precursor, deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH), necessary for its activation. We review the current knowledge regarding eIF5A-HRD, and report the case of the sixth and oldest known patient with DOHH-related disorder (DOHH-D), aiming to expand and discuss the molecular basis and the general and epilepsy phenotypes of this group of diseases.
Results
Literature review yielded one paper describing 7 individuals with eIF5A-related disorders (eIF5A-D), one reporting 5 subjects with DHPS-related disorders (DHPS-D) and one characterizing 5 individuals with DOHH-D. Main phenotypic features consisted of prenatal issues, hypotonia, dysmorphisms, microcephaly, moderate-severe neurodevelopmental disorders/intellectual disability and behavioral disorders. We report the case of a 24-years-old male with DOHH-D manifesting as Dravet-like syndrome. He displays microcephaly and neurodevelopmental delay with attention deficit with hyperactivity disorder, along with a happy demeanor. Basic language skills and ambulation capacity with crouch gait are preserved. Onset of epilepsy was at 8 months with refractory temperature-triggered hemiclonic seizures and status epilepticus, followed by nocturnal tonic-clonic seizures from adolescence. Fenfluramine was the most effective approach, reducing seizure intensity, duration and frequency, and contributing to cognitive and behavior improvements. No patient with eIF5A-D presented seizures. Taking our patient into account, 4/5 and 4/6 reported individuals with DHPS-D and DOHH-D, respectively, presented epilepsy. Seven out of 8 epilepsy patients debuted between 2 and 5 years, most of them presented developmental and epileptic encephalopathies or generalized epilepsies (5/8 with temperature or infection-triggered seizures), and 4/8 were refractory. We hypothesize that dysregulation of IQSEC2 and SHANK3, among other genes, might contribute to the eIF5A-HRD phenotype.
Conclusions
eIF5A-HRD are recently described entities displaying neurodevelopmental disorders and microcephaly, and reported patients are scarce. More than 70% of DHPS-D and DOHH-D patients present epilepsy, 63% of them with temperature-triggered seizures. Valproic acid or fenfluramine may be effective. Rare homozygous or compound heterozygous missense variants in these genes should be screened in patients with encephalopathy and temperature-triggered seizures.
Keywords: Eukaryotic translation factors, DOHH, DHPS, EIF5A, Developmental and epileptic encephalopathy, Refractory epilepsy, Febrile seizures, Dravet syndrome, Fenfluramine
Background
Eukaryotic translation initiation factors (eIF) are a group of at least 12 proteins regulating global messenger RNA translation, along with eukaryotic translation elongation (eEF) and termination factors (eTF) [1]. These proteins display a direct role in cell physiology, development and response to stress, and its dysregulation has been linked to cancer, neurological and metabolic disorders [2, 3].
Eukaryotic initiation factor 5 A (eIF5A) and hypusination-related disorders (eIF5A-HRD) are recently-described diseases caused by variants in the genes coding for eIF5A or the enzymes involved in the post-translational synthesis of hypusine in the eIF5A precursor, deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH), necessary for its activation. eIF5A, is a key translation factor for cell proliferation and differentiation, apoptosis, autophagy, and development in eukaryotes [4].
eIF5A has been related to disorders such as cancer [5]diabetes [6] or retroviral infections [7]. De novo heterozygous variants in EIF5A (MIM # 600187) have been associated to neurodevelopmental disorder with microcephaly, the Faundes-Banka syndrome (MIM # 619376), in 7 patients [8]. Bi-allelic loss-of-function variants in DHPS (MIM # 600944) have been also associated to neurodevelopmental disorder with microcephaly and epilepsy (MIM # 618480) in 5 individuals [9]. Most recently, Ziegler et al. have described bi-allelic loss-of-function variants in DOHH (MIM # 611262) in 5 individuals with similar neurodevelopmental disorder with microcephaly and epilepsy (MIM # 620066) [10].
The genotypic and phenotypic characterization of these conditions is still in its initial steps. In this work, we review the current knowledge regarding eIF5A-HRD, and report the case of a patient with DOHH-related disorder (DOHH-D) manifesting as a fenfluramine-responsive Dravet-like syndrome. We aim to expand and discuss the molecular basis and the phenotype of this group of diseases, and to better define the epilepsy features of patients with eIF5A-HRD.
Methods
A literature review was carried out, including the descriptors “EIF5A”, “DHPS” and “DOHH” independently in the thesaurus of MEDLINE, as well as the PubMed search strategy “(“translation factor*”) AND (“neuro*” OR “epilep*” OR “seizure*” OR “Dravet” OR “fenfluramine”)”. Articles in English and Spanish up to June 2025, along with their references, were included and screened by a team of neurologists with expertise in neurodevelopmental disorders and genetics.
With respect to the case report, trio-whole exome sequencing (T-WES) was performed by BluePrint Genetics extracting genomic DNA from saliva samples of the proband and his parents. After initial non-diagnostic results, reanalysis was made 5 months later on the basis of the Variant Call Format (VCF) files of the previous sequencing (without additional sampling or re-sequencing), incorporating updated clinical evidence with further candidate genes and rare bi-allelic missense variants. Reanalysis process was physician-driven and the obtained results were validated by the laboratory. Variants were described according to the Human Genome Variation Society nomenclature recommendations. Phenotypic characteristics were collected from the patient’s medical records, and seizure data were assessed from diaries filled out by the patient’s parents.
Results
Literature review yielded just one original paper describing 7 individuals with eIF5A-related disorders (eIF5A-D), one reporting 5 subjects with DHPS-related disorders (DHPS-D) and one characterizing 5 individuals with DOHH-D. Table 1 details the phenotypic and genotypic features of our patient in comparison to the rest of patients reported with eIF5A-D, DHPS-D and DOHH-D.
Table 1.
Modified from Ziegler et al. [10]. General phenotype of patients diagnosed with eIF5A and hypusination-related disorders
| DOHH-D (Beltrán-Corbellini et al.) N = 1 | DOHH-D (Ziegler et al. [10]) N = 5 |
DHPS-D (Ganapathi et al. [9]) N = 5 |
EIF5A-D (Faundes V, et al. [8]) N = 7 |
|
|---|---|---|---|---|
| Sex | Male | 2/5 females | 4/5 females | 4/7 females |
| Variants |
Homozygous for c.455 C > T, p.(Pro152Leu) Ethnicity: Spanish (pat), Spanish (mat) |
7 variants: one stop gain, two frameshift, 4 missense; compound heterozygous | 4 variants: 1 missense, one start loss, one splice, one inframe deletion; compound heterozygous | 7 variants: 5 missense, 1 stop gain, 1 frameshift; de novo heterozygous |
| Prenatal | Mat. hypertension | 2/5 cardiac malformation | 4/5 preeclampsia | 5/6 (4 IUGR, 1 fetal ascites) |
| Gestational age | 39 weeks | 0/5 preterm or postterm | 2/5 preterm | N/S |
| Neonatal | Poor feeding | 2/5 poor feeding, 1/4 temperature instability, 3/5 hypotonia | 1/5 premature instability | 3/7 poor feeding |
| Birth weight | 3.100 Kg (−0.58 SD) | 0/5 underweight | 0/5 underweight | 3/7 underweight |
| Birth lenght | 51 cm (+ 0.33 SD) | 0/5 short stature | 0/4 short stature | 3/7 short stature |
| Birth HC | 36 cm (+ 1.03 SD) | 0/3 congenital microcephaly | 0/3 congenital microcephaly | 3/7 congenital microcephaly |
| Height or lenght at last evaluation | 178 cm (normal stature) | 4/5 short stature | 2/5 short stature | 2/7 short stature |
| Weight at last evaluation | 52.5 Kg (BMI 16.5, underweight) | 4/4 underweight | 0/5 underweight | 2/7 underweight |
| HC at last evaluation | 50 cm (< 2 SD, microcephaly) | 4/4 microcephaly | 3/4 microcephaly | 5/7 microcephaly |
| Sitting/walking/speaking | Yes/Yes/Yes | 3/5 sitting, 3/5 walking, 1/5 speaking |
5/5 sitting, 5/5 walking, 2/5 speaking |
6/7 sitting, 6/6 walking, 6/6 speaking |
| Sitting | 9 months | |||
| Walking | 18 months | |||
| Speaking | 3 years | |||
| Intellectual disability | Yes | 5/5 | 5/5 | 7/7 (1 mild) |
| Behavioral | Happy demeanor, ADHD | 3/5 happy demeanor | 1/5 autism, hand flapping 3/5 | 2/7 autism, 1/7 ADHD |
| Epilepsy | Yes | 3/5 | 4/5 | 0/7 |
| Tonus | Hypotonia | 5/5 hypotonia | 4/5 hypotonia, 1/5 spasticity | 1/5 hypotonia |
| Brain MRI | Normal | 5/5 abnormal, 4/5 cortical atrophy | 4/4 normal | 2/2 normal |
| Cardiac malformation | No | 3/5 | 0/5 | 3/4 |
| Visual impairment | No | 3/5 nystagmus, 1/4 cortical visual impairment | 0/5 | 3/7 strabismus, 1/7 glaucoma |
| Recurrent infections | No | 3/5 | 1/5 | 0/7 |
DOHH-D: DOHH-related disorders; DHPS-D: DHPS-related disorders; EIF5A-D: EIF5A-related disorders; pat: paternal; mat: maternal; IUGR: intrauterine growth restriction; Kg: kilograms; SD: standard deviation; cm: centimeters; HC: head circumference; ADHD: attention deficit with hyperactivity disorder
eIF5A-related disorders
eIF5A is key for synthesizing bonds between successive proline residues resolving ribosomal stalling in eukaryotic cells [11]among other functions. Increased somatic expression of EIF5A had been related to several cancers [12]. However, germline de novo heterozygous variants in EIF5A have been associated to neurodevelopmental disorder with microcephaly in up to 7 patients [8].
Faundes et al. [8]. describe a variable combination of moderate-severe neurodevelopmental delay (NDD) and/or intellectual disability (ID) (7/7), autism spectrum disorders (ASD − 2/7), congenital microcephaly (3/7) among other perinatal processes such as intrauterine growth restrictions (3/7), facial dysmorphisms including micrognathia (5/7), cardiac and central nervous system anomalies (ventriculomegaly, white matter hyperintensities), along with other medical issues like joint hypermobility or eye anomalies. No patient presented seizures. In 4/7 individuals, the initial clinical suspicions were Kabuki syndrome-like or mandibulofacial dysostosis-like conditions. The authors report de novo heterozygous missense (5/7), nonsense (1/7) and frameshift (1/7) likely pathogenic (LP) or pathogenic variants (P). The codon for Arg109 was affected in 3/7, perhaps due to the propensity of codon CGA to methylation, deamination and CG-TA transition.
The first de novo heterozygous frameshift variant in EIF5A was initially identified by the authors through trio-WES in an individual displaying ID, congenital microcephaly, micrognathia and a clinical suspicion of Kabuki Syndrome. Consequently, 6 additional individuals harboring de novo EIF5A variants were identified through Matchmaker Exchange and GeneMatcher. Rarity of truncating variants affecting the main EIF5A transcript reported in gnomAD, GeVIR metrics, the high evolutionary conservation of the residues altered by missense variants of the affected patients and in silico modelling of missense variants onto the structure of yeast eIF5A in complex with the 60 S ribosome showing that these variants involve residues exposed to the surface, supported the significance of the variants. After identifying the individuals on the basis of their genotypes, notable convergence of their clinical features through reverse phenotyping also contributed to suggest the causality of EIF5A variants for the described phenotypes.
Basing on several types of functional studies, they suggest that decreased or impaired eIF5A function in their patients depends on reduced eIF5A-ribosome interactions due to mutation-specific mechanisms. Growth potential in an eIF5A-deleted yeast strain was restored by introducing human EIF5A cDNA, and a significant reduction of EIF5A mRNA levels was found in lymphoblastoid cells of an individual harboring the p.R109Tfs*8 variant, suggesting nonsense mediated decay. Consistently, yeast colonies expressing p.R109fs*8 in human EIF5A as the sole source of eIF5A could not be obtained, with poor expression and hypusination when co-expressed with wild type (WT) revealed by western blotting (WB). Yeast cells containing the p.T48N and p.G106R human-identified variants showed slow growth, in contrast with p.E122K displaying no differences in growth in comparison to WT. Additionally, these 3 missense variants displayed impaired eIF5A-ribosome interaction demonstrated by aberrant polysome profiles (p.T48N showing normal eIF5A levels but reduced hypusination, and p.G106R and p.E122K reduced eIF5A levels and normal hypusination identified via WB). Finally, significantly reduced expression of 2 poly-proline tracts (PPT) reporters was found for p.T48N and p.G106R, and a tendency was observed for p.E122K in yeast through WB.
Regarding generation of specific phenotype features, taking microcephaly as the most consistent characteristic of their patients, they observed a statistically significant difference in the proportion of microcephaly-associated genes containing 1 or more PPT in comparison to all other human protein-coding genes, according to OMIM. Particularly, pathogenic variants in high poly-proline-content KMT2D and SF3B4 genes cause Kabuki Syndrome and acrofacial dysostosis 1, Nager type, showing notable phenotypic overlap with the reported eIF5A-D patients. Other genes with high content in polyproline, such as actin-encoding genes or those taking part in spliceosomal disorders, would also show overlapping craniofacial characteristics with eIF5A-D individuals when affected. The authors also discuss that eif5a/Eif5a mRNA is highly expressed in structures forming the brain and mouth in zebrafish and mouse embryos, similar to the most affected systems in the reported patients. Regarding the p.E122K variant reported by Faundes et al., they argue that although a reduction in function was observed, it was not statistically significant compared to other variants, consistently with the milder phenotype of that individual. Besides, they suggest that haploinsufficiency of EIF5A may account for the phenotype of 17p13.1 microdeletion syndrome.
No precision therapy is available for eIF5A-HRD. However, our colleagues also demonstrate partial improvement of eIF5A function and phenotypes over yeast and zebrafish models by spermidine, proposing a broader action of the former beyond hypusination, involving optimization of eIF5A-ribosome interaction [8].
As far as we know, up to June 2025, 40 LP/P variants affecting the EIF5A gene were reported in ClinVar: 7 missense, 1 nonsense, 3 frameshift, 2 splice site (Table 2), and 27 taking part in copy number variants (CNVs) including up to hundreds of genes (1 of them a deletion encompassing 15 genes and with Faundes-Banka Syndrome phenotype, 2 deletions of 25 and 71 genes with very long chain acyl-CoA dehydrogenase deficiency phenotype (autosomal recessive), 1 deletion of 68 genes with Li Fraumeni Syndrome phenotype and 1 deletion with Pierre-Robin Syndrome phenotype (autosomal recessive) -the rest not provided).
Table 2.
Pathogenic and likely pathogenic single nucleotide variants and indels reported in EIF5A, DHPS and DOHH genes in clinvar up to June 2025
| Gene | Variant | Phenotype | Affected status | Previous reports |
|---|---|---|---|---|
| EIF5A | c.77G > C p.R26P |
Entry 1: Neurodevelopmental disorder Entry 2: not provided |
Entry 1: yes Entry 2: yes |
No |
| EIF5A | c.124G > A p.E42K | Not provided | Yes | No |
| EIF5A | c.415_416delinsAT p.S139i | Faundes-Banka Syndrome | Yes | No |
| EIF5A | c.143 C > A p.T48N | Faundes-Banka Syndrome | Not provided | Faundes V et al. [8] |
| EIF5A | c.316G > A p.G106R | Not provided | Yes | Faundes V et al. [8] |
| EIF5A | c.325 C > G p.R109G | Faundes-Banka Syndrome | Not provided | Faundes V et al. [8] |
| EIF5A | c.343 C > T p.P115S | Not provided | Yes | Faundes V et al. [8] |
| EIF5A | c.325 C > T p.R109* |
Entry 1: Faundes-Banka Syndrome Entry 2: not provided Entry 3: Faundes-Banka Syndrome |
Entry 1: yes Entry 2: yes Entry 3: unknown |
Faundes V et al. [8] |
| EIF5A | c.191_192del p.F64fs | Faundes-Banka Syndrome | Yes | No |
| EIF5A | c.344_345del p.P115fs | Not provided | Yes | No |
| EIF5A | c.324dup p.Arg109fs | Faundes-Banka Syndrome | Not provided | Faundes V et al. [8] |
| EIF5A | c.165 + 1G > A | Not provided | Unknown | No |
| EIF5A | c.270 + 1G > A | Not provided | Yes | No |
| DHPS | c.518 A > G |
Entry 1: NDDSSWI Entry 2: not provided Entry 3: NDDSSWI Entry 4: DHSP-related condition Entry 5: NDDSSWI |
Entry 1: unknown Entry 2: yes Entry 3: yes Entry 4: unknown Entry 5: yes |
Ganapathi M et al. [9] |
| DHPS | c.216_217dup p.K73Rfs*16 | Not provided | Yes | No |
| DHPS | c.460_461del p.R154Gfs*11 |
Entry 1 (LP): NDDSSWI Entry 2 (VUS): not provided |
Entry 1: yes Entry 2: yes |
No |
| DHPS | c.912_917del p.Tyr305_Ile306del |
Entry 1: NDDSSWI Entry 2: DHPS-related condition |
Entry 1: unknown Entry 2: unknown |
Ganapathi M et al. [9] |
| DHPS | c.785-1G > C | Not provided | Yes | No |
| DOHH | c.746T > C p.I249T |
Entry 1: DOHH-related NDD Entry 2: NDDMCAVI |
Entry 1: yes Entry 2: not provided |
Ziegler A et al. [10] |
| DOHH | c.552 C > A p.N184K |
Entry 1: DOHH-related NDD Entry 2: NDDMCAVI |
Entry 1: yes Entry 2: not provided |
Ziegler A et al. [10] |
| DOHH | c.840T > A p.Y280* |
Entry 1: DOHH-related NDD Entry 2: NDDMCAVI |
Entry 1: yes Entry 2: not provided |
Ziegler A et al. [10] |
| DOHH | c.654_655insAACC p.E219fs |
Entry 1: NDDMCAVI Entry 2: NDDMCAVI Entry 3: NDDMCAVI |
Entry 1: yes Entry 2: unknown Entry 3: unknown |
Ziegler A et al. [10] |
| DOHH | c.304del p.E10fs |
Entry 1: DOHH-related NDD Entry 2: NDDMCAVI |
Entry 1: yes Entry 2: not provided |
Ziegler A et al. [10] |
NDDSSWI: neurodevelopmental disorder with seizures and speech and walking impairment. NDD: neurodevelopmental disorder. NDDMCAVI: neurodevelopmental disorder with microcephaly, cerebral atrophy, and visual impairment
DHPS-related disorders
DHPS is the first enzyme involved in the sequential post-translational synthesis of hypusine in the eIF5A precursor, necessary for its activation [4]. This enzyme transfers the 4-aminoutyl moiety from spermidine to Lys50 in eIF5A. Bi-allelic loss-of-function variants in DHPS have been associated to neurodevelopmental disorder with microcephaly and epilepsy in up to 5 individuals [9].
Ganapathi et al. [9]. describe an association of variable degrees of NDD/ID (5/5), ASD traits (4/5), hypotonia (4/5), preeclampsia (4/5) among other perinatal processes, microcephaly at last evaluation in 3/4 (but 0/3 congenital), dysmorphisms including deep set eyes (4/5) and normal brain MRI (4/4), along with other medical issues like skin conditions or low immunoglobulins levels. Moreover, 4/5 presented epilepsy.
The authors report 4 biallelic inherited LP/P variants, including 1 missense, 1 start loss, 1 inframe deletion and 1 splice site. The c.518 A > G (p.Asn173Ser) variant was found in trans with another variant in all 5 subjects, being suggested by the authors that this might be a founder variant. This variant, mainly reported in non-Finnish Europeans in gnomAD, was located on 2 distinct haplotypes throughout the 4 carrier parents: one discussed to represent a common ancestor older than 20 generations, and the other likely due to a recurrent mutation. The 4 carriers were reported to have Irish/English ethnicity. In this line, in affected individuals one allele resulted in complete lack of DHPS activity (p.Tyr305_Ile306del, c.1014 + 1G > A, p.Met1?), while the other allele showed significantly diminished function (p.Asn173Ser).
The first 2 affected individuals within the same family were identified by the authors via reanalysis of previous non-diagnostic clinical exome sequencing performed 4 years earlier. The reanalysis included aligning sequence to the GRCh37/hg19 version of the human reference genome and analyzing for sequence variants using a custom-developed analysis tool. DHPS variants were prioritized based on their inheritance pattern, low allele frequencies and the results of several in silico prediction tools. Later on, 3 additional families were identified via GeneMatcher.
Basing on functional studies, the authors propose that the reported genotypes lead to a decreased DHPS activity and reduced eIF5A hypusination. Transcript analysis from blood samples of family 1, revealed that translation of c.1014 + 1G > A variant leads to a DHPS truncated protein lacking the active site of the enzyme. On the other hand, p.Y305_I306del led to absent enzyme activity and p.N173S to partial (approximately 20%) activity through in vitro assays of recombinant enzymes expressed in E. coli, respectively exhibiting significant and partial impairment of eIF5A hypusination via co-transfection experiments in HEK293T cells. In the case of DHPS-D and opposite to eIF5A-D, inheritance patterns and data from population databases points towards tolerance of DHPS haploinsufficiency in humans. In addition to the rationale for phenotype generation in eIF5A-D, Ganapathi et al. also discuss that observed dermatological and immunological manifestations are consistent with the broad expression pattern of EIF5A and DHPS in other cells and tissues.
As far as we know, up to June 2025, 24 LP/P variants affecting the DHPS gene were reported in ClinVar: 1 missense, 2 frameshift, 1 inframe deletion, 1 splice site (Table 2), and 19 taking part in CNVs including up to hundreds of genes (1 deletion of 34 genes with “developmental and epileptic encephalopathy, 42” phenotype and unknown affected status, 1 deletion of 31 genes − 1 entry with “developmental and epileptic encephalopathy, 42” phenotype, 1 with “glutaric aciduria, type 1” phenotype (autosomal recessive) and 1 with “Aicardi-Goutieres Syndrome 4” phenotype (autosomal recessive) with unknown affected status, and the rest with not provided phenotypes).
DOHH-related disorders
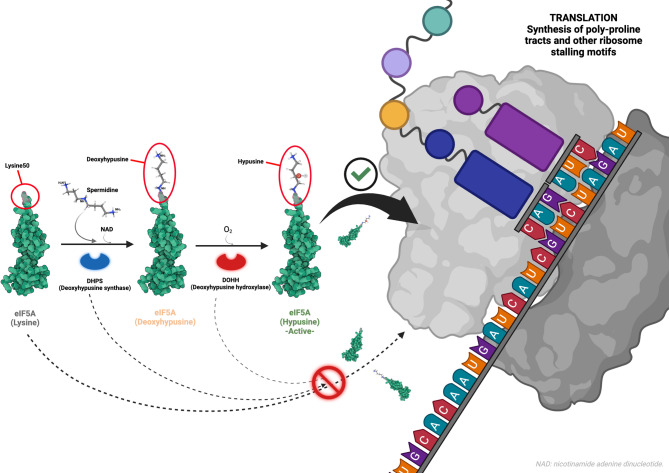
DOHH is the second enzyme involved in the sequential post-translational synthesis of hypusine in the eIF5A precursor [4]. This enzyme hydroxylates the residue created in Lys50 by DHPS, finishing the synthesis of hypusine and activating eIF5A (Fig. 1 summarizes the activation pathway and main function of eIF5A). Bi-allelic loss-of-function variants in DOHH have been described in up to 5 individuals with similar neurodevelopmental disorder with microcephaly and epilepsy [10].
Fig. 1.
Activation pathway and main function of eIF5A. Hypusine synthesis over the Lys50 residue of eIF5A is necessary for its activation and correct function. In a first step, DHPS enzyme transfers the 4-aminoutyl moiety from spermidine to Lys50 in eIF5A, synthesizing deoxyhypusine. In a second step, DOHH enzyme hydroxylates the deoxyhypusine created by DHPS, finishing the synthesis of hypusine and activating eIF5A. Active eIF5A is key for synthesizing bonds between successive proline residues resolving ribosomal stalling during translation in eukaryotic cells. Neurodevelopmental disorders caused by alteration of EIF5A, DHPS and DOHH genes have been proposed to account for the impaired synthesis of specific proteins rich in poly-proline tracts. Crystal structure of eIF5A: PDB 3CPF (Tong et al. [13]). Structure of spermidine: PDB 8A0E, ligand (Wator et al. [14]). Created in https://BioRender.com
Ziegler et al. [10]. also describe a combination of NDD/ID (5/5), happy demeanor (3/5), hypotonia (5/5), variable visual impairment (4/5), microcephaly in 4/4 (but 0/3 congenital), brain MRI showing cortical atrophy (4/5), cardiac malformations (3/5), nystagmus (3/5), cortical visual impairment (1/4) and recurrent infections (3/5). In this case, 3/5 also presented epilepsy. The authors report 7 biallelic inherited LP/P variants, including 4 missense, 2 frameshift and 1 stop gain. In line with the lethality of homozygous knockout mice, every individual was thought to carry at least 1 variant with residual DOHH activity.
The first individual harboring bi-allelic variants in DOHH was identified by the authors via exome sequencing. DOHH variants were prioritized based on their inheritance pattern, low allele frequencies and the results of several in silico prediction tools. The following 4 individuals were identified through GeneMatcher, all via exome sequencing after normal molecular karyotypes in the context of different national or regional clinical research programs.
Based on several types of functional studies, our colleagues suggest that the assessed genotypes lead to a decreased DOHH activity with an increased accumulation of unhydroxylated eIF5A. Particularly, they suggest that the limitation of DOHH activity in individuals with missense variants is more likely accounted for by reduced stability rather than loss of activity, and propose that the variable residual activity of eIF5A across the three eIF5A-HRD, might explain the less severe phenotypes of eIF5A-D in comparison to DHPS-D or DOHH-D. Functional studies consisted of examining the amount of DOHH in fibroblasts from 3 affected individuals of their sample and 3 controls by immunoblotting, showing notable reduction in cases, although with limitations regarding the assessment of the C terminal truncated variants in 2 of them. Additionally, the authors evaluated the effects of the variants on expression and activity by using GST-DOHH recombinant proteins produced in E. coli, showing very low expression of 1 truncated variant and normal expression of other 2 truncated and 3 missense variants (supporting the hypothesis of diminished activity due to reduced stability in missense variants). Truncated variants lacking some of the HE critical motifs were inactive. On the contrary, the truncated variant containing all of them (p.Tyr280Ter), displayed low partial activity. Finally, through two-dimensional gel electrophoresis of proteins of fibroblast from 3 affected and control individuals, they demonstrated an increased accumulation of the unhydroxylated, deoxyhypusine-containing form of eIF5A as well as reduced levels of hypusinated eIF5A in cases in comparison to controls.
The authors also add further discussion with respect to phenotype generation mechanisms, arguing that microcephaly in eIF5A-HRD may account for increased neuronal cells death. Moreover, they suggest that the reduction of a myogenic transcription factor (MyoD) whose expression is stimulated by activated eIF5A in mice, might explain the heart malformations observed in several individuals with eIF5A-HRD. They state that on the contrary of eIF5A-D and DOHH-D, the absence of ophthalmologic impairment reported in DHPS-D could be due to small sample, ascertainment bias and incomplete penetrance, and that anomalies in brain MRI in DOHH-D patients may be explained by different ages at imaging acquisition in comparison to patients with the other disorders.
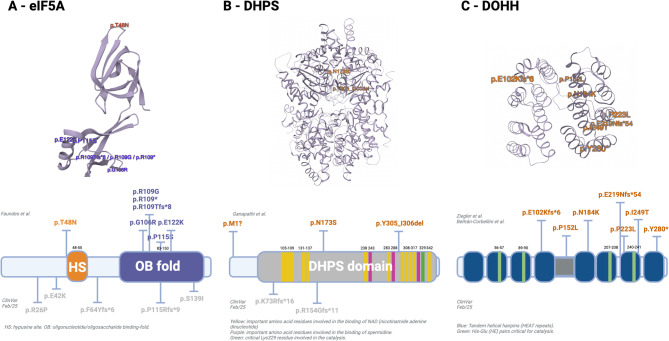
As far as we know, up to June 2025, 23 LP/P variants affecting the DOHH gene were reported in ClinVar: 2 missense, 1 nonsense, 2 frameshift (Table 2), and 18 taking part in CNVs including up to hundreds of genes with not provided phenotypes. Figure 2 illustrates the localization of P and LP variants in the tertiary and primary structures of the eIF5A, DHPS and DOHH proteins.
Fig. 2.
Localization of P and LP SNVs and indel variants in eIF5A, DHPS and DOHH proteins. (A) Structure of eIF5A protein. Top: tertiary structure (crystal structure, PDB 3CPF, Tong et al. [13]) Bottom: primary structure (modified from Faundes et al. [8]) Variants in color: published by Faundes et al. [8] variants in grey: ClinVar variants. (B) Structure of DHPS protein. Top: tertiary structure (crystal structure, PDB 1RQD, Umland et al. [15]) Bottom: primary structure (modified from Ganapathi et al. [9]) Variants in color: published by Ganapathi et al. [9] variants in grey: ClinVar variants. (C) Structure of DOHH protein. Top: tertiary structure (crystal structure, PDB 4D4Z, Han et al. [16]) Bottom: primary structure (modified from Ziegler et al. [10]) Variants in color: published by Ziegler et al. [10] and Beltrán-Corbellini et al. Created in https://BioRender.com
Case report
We report a 24-years-old male diagnosed with DOHH-related encephalopathy. Main clinical features consist of maternal hypertension during pregnancy, normal birth weight, length and head circumference, poor feeding during the first months, neurodevelopmental disorder with moderate-severe ID, attention deficit with hyperactivity disorder (ADHD), and refractory epilepsy with temperature and excitement-triggered seizures, diagnosed from the 8th month. Ambulation capacity was acquired, manifesting hypotonia, dystaxic and crouch gait, and varus knee. Speaking is limited to short simple 5–6 words sentences, with dysarthria. Behavior is characterized by a happy demeanor, with sporadic disruptions from adolescence. Physical examination at last evaluation (24 years-old), revealed normal stature, low body mass index, and microcephaly (known from the age of 4 years). Brain MRI showed no significant anomalies.
He presented his first seizure at eight months. Between month 8 and the age of 7 years, he displayed both typical and lateralization-alternating hemiclonic febrile seizures with a frequency of four per year, including one episode of status epilepticus while in the swimming pool at the age of four years, characterized by sequential unrecovered generalized tonic-clonic seizures, and after which a routine EEG showed focal left frontal interictal epileptiform activity for the first time. Baseline EEGs during his first years were normal.
Between seven and 16-years-old, afebrile focal unaware motor seizures appeared along with tonic seizures and falls. Seizures occurred during wakefulness and sleep, occasionally in clusters, and frequently triggered by temperature changes, excitement or physical activity. EEGs showed either focal left frontal or right posterior quadrant interictal activity. From 16-years-old onwards, seizures became mainly generalized tonic-clonic during sleep with a frequency of 4–5/month. Between 20 and 23-years old, EEGs revealed multifocal interictal activity. Nonetheless, last 24-hour video-EEG monitoring from 24-years-old onwards was unremarkable.
Clobazam and levetiracetam resulted in partial improvements in seizure frequency, but provoked marked irritability. Clusters responded well to oral midazolam. Valproic acid contributed to behavior improvement. Lacosamide was associated with mild improvement in nocturnal tonic seizures. Fenfluramine was the most effective drug, reducing seizure intensity, duration and frequency by 50% from 20-years old onwards, contributing to the cessation of clusters, along with cognitive and behavior improvements. Lamotrigine, oxcarbazepine, perampanel and methylphenidate showed no effectiveness. Currently, the patient is on fenfluramine, clobazam, valproic acid and lacosamide.
Regarding diagnostic work-up, he underwent SCN1A gene sequencing at 10-years-old with negative results. When he was 16-years-old, an epilepsy gene panel (200 genes) was also unremarkable. T-WES was performed when he was 24-years-old, displaying again initial non-diagnostic results. Nonetheless, 5 months later, a physician-driven T-WES reanalysis process was carried out by a team of epileptologists with expertise in epilepsy genetics along with geneticists of BluePrint Genetics, on the basis of the VCF files of the initial exome sequencing (without additional sampling or re-sequencing). Updated clinical evidence with further candidate genes and rare bi-allelic missense variants were included. On this occasion, a likely pathogenic homozygous variant in DOHH (c.455 C > T, p.Pro152Leu) was found, then validated by the geneticists of the laboratory. DOHH variant was prioritized according to its low allele frequencies, inheritance pattern, outcomes of in silico prediction tools and phenotype concordance. The classification of pathogenicity was based on the association between the gene and the patient’s phenotype, the variant’s rarity in control populations (at that moment, 36 heterozygous and 0 homozygous individuals in gnomAD), prediction of pathogenicity by multiple in silico tools (mainly Polyphen, SIFT and MutTaster) and high conservation of the affected amino acid residue in most species. Significantly, as previously mentioned and later discussed, this variant had already been reported as LP in trans with another missense variant (p.Asn184Lys) by Ziegler et al. in an affected individual, providing functional evidence of reduction of the amount of DOHH protein and increased accumulation of the unhydroxylated, deoxyhypusine-containing form of eIF5A in fibroblasts of this patient. Moreover, the variant was also independently submitted to ClinVar as LP in other affected individual. Both parents are non-consanguineous asymptomatic heterozygous carriers with Spanish ethnicity, displaying no other relevant phenotypic features. Family history was unremarkable.
Phenotype comparison in eIF5A-HRD
Concerning our patient, his mother manifested hypertension during the third trimester of pregnancy. No other reported individual with DOHH-D displayed maternal hypertension. However, 4/5 mothers of DHPS-D individuals were diagnosed with preeclampsia. Basic speaking was acquired, on the contrary to most DOHH-D and DHPS-D, but similar to EIF5A-D cases. Hypotonia was present as in most DOHH-D and DHPS-D, along with dystaxic and crouch gait. No ADHD was described among DOHH-D patients, but happy demeanor was also reported. Brain MRI (9-years-old) was unremarkable, on the contrary to all DOHH-D individuals (cortical atrophy). No cardiac malformations, visual impairment or recurrent infections were present, opposite to most DOHH-D individuals (Table 1).
Table 3 shows the description of the epilepsy features of our patient, and the rest of individuals diagnosed with hypusination-related disorders. No patient with EIF5A-D was diagnosed with epilepsy (it has been proposed that heterozygous variants in EIF5A-D allow higher residual activity of eIF5A, giving rise to milder phenotypes [10]). Our patient presented with a phenotype resembling Dravet syndrome, including early hemiclonic, focal, tonic and tonic-clonic seizures, triggered by temperature elevation, infections and excitement, refractory to antiseizure medication, evolving to developmental and epileptic encephalopathy, and occurrence of tonic-clonic seizures during sleep after adolescence, along with posterior-predominant multifocal interictal activity. DOHH-D reported patients presented later epilepsy onsets, displaying mainly generalized epilepsies with 2/3 manifesting focal and generalized fever-triggered seizures. DHPS-D patients also presented with later epilepsy onsets, displaying mainly generalized epilepsies (2/4 with continuous spike-wave during sleep patterns) with 2/4 manifesting focal and generalized fever or infection-triggered seizures, along with posterior-predominant focal or multifocal interictal activity in 2/4. Regarding response to therapies, our patient is partially responsive to valproic acid, and 50% responder to fenfluramine along with cognitive and behavioral improvements, without response to several sodium channel blockers, except for low dose lacosamide used to improve nocturnal seizures [17]. Among the rest of DOHH-D reported patients, 2/3 were refractory and 1/3 controlled with unspecified anti-seizure medications. One out of 4 individuals with DHPS-D was reported to be refractory, the rest showing responses to oxcarbazepine, valproic acid and low-carbohydrate diet.
Table 3.
Epilepsy phenotype of hypusination disorders
| DOHH-D (Beltrán-Corbellini et al.) N = 1 | DOHH-D (Ziegler et al. [10]) N = 5 |
DHPS-D (Ganapathi et al. [9]) N = 5 |
||||||
|---|---|---|---|---|---|---|---|---|
| Age at epilepsy onset | Individual 2 | Individual 3 | Individual 5 | Individual 1 | Individual 2 | Individual 4 | Individual 5 | |
| 8 months | 5 years | 3 years | NS | 2 years | 2.5 years (unclear episodes before) | 5 years | 5 years | |
| Seizure type |
8 months-7 years: -Typical and hemiclonic febrile seizures -Status epilepticus while in the swimming pool 7–16 years -Focal unaware motor seizures -Tonic seizures -During wakefulness and sleep, occasionally in clusters, triggered by temperature changes, excitement or physical activity 16 years-onwards: -GTCS -Predominantly during sleep |
Fever-triggered GTCS | Drop attacks at the age of 11 years, myoclonic seizures at the age of 15 years, fever-triggered focal and generalized seizures at the age of 16 years | Hyperextension of upper limbs | Absences | Single infection-triggered GTCS (staring episodes before) | Fever-triggered focal seizure at the age of 5 years, infection-triggered generalized tonic status epilepticus at the age of 7 years | Absences and GTCS |
| Epilepsy syndrome | DEE | NS | GE | GE | CSWS | NS | NS | NS |
| EEG |
8 months-4 years: -Unremarkable routine EEG 4–20 years: −24-hour video-EEG showing focal left frontal or right posterior quadrant interictal activity. 20–23 years: −24-hour video-EEG showing multifocal interictal activity 23 years-onwards: -Unremarkable 24-hour video-EEG |
NS | NS | Normal | Posterior temporal and parietal interictal activity | Focal bilateral temporal-occipital interictal activity | Multifocal interictal activity | NS |
| Response to therapy and evolution |
VPA: behavior improvement) LEV: partial improvement in seizure frequency, but irritability. CLB: partial improvement in seizure frequency FFN: 50% responder, with improvement in intensity and duration and cessation of clusters, along with cognitive and behavior improvements. LTG, OXC, PER: no effectivity. Methylphenidate: no effectivity. |
Controlled by ASMs, and stopping at the age of 11 | NS | Sulthiame led to a 25% reduction in nocturnal interictal activity. CBD/THC high ratio improved receptivity and sleep. Unresponsive to LEV, solumedrol, CLB, CLN, VPA, KD, prednisone. | KD markedly improved language skills, stimulants improved ADHD | VPA led to a good seizure control | Responder to low carbohydrate diet and OXC | |
DOHH-D: DOHH-related disorders; DHPS-D: DHPS-related disorders; NS: not specified; GTCS: generalized tonic-clonic seizures; DEE: developmental and epileptic encephalopathy; GE: generalized epilepsy; CSWS: continuous spike-wave during sleep; VPA: valproic acid; LEV: levetiracetam; CLB: clobazam; FFN: fenfluramine; LTG: lamotrigine; OXC: oxcarbazepine; PER: perampanel; ASM: anti-seizure medication; CBD/THC: cannabidiol/tetrahydrocannabinol; CLN: clonazepam; KD: ketogenic diet; ADHD: attention deficit with hyperactivity disorder
Discussion
Pathogenic variants in other eIF, eEF and closely related proteins have been previously identified as the cause of several neurological and developmental disorders. Variants in eIF2B subunits (MIM # 603896) [18] have been related to leukoencephalopathy with vanishing white matter, a chronic-progressive disease with episodes of acute worsening following intercurrent events. In this line, variants in EIF2S3 (MIM # 300148) [19] have been associated to ID and microcephaly, in EIF3F (MIM # 618295) [20] to ID, behavioral disorders, epilepsy and sensorineural hearing loss; in EIF4E (MIM # 615091) [21] to ASD, and in EIF4G1 (MIM # 614251) [22] to familial parkinsonism and idiopathic Lewy body disease.
Regarding eEF factors, variants in EEF1A2 (MIM # 602959) have been linked to ID, ASD, epilepsy and ataxia, and in EEF2 and other proteins of the same pathway (MIM # 130610) to spinocerebellar ataxia, ID, central nervous system malformations and craniofacial abnormalities [23]. Additionally, variants in related proteins such as alanyl tRNA synthetase (AARS -MIM # 616339) have been associated to microcephaly, hypomyelination and epilepsy, and in ribosomal proteins such as RPS23 (MIM # 617412) to ID, brachycephaly, trichomegaly and hearing loss, or in RPL10 (MIM # 300998) to ID, ASD, and cerebellar hypoplasia [23].
The phenotype of our patient seems to be milder than the described for most of the rest of DOHH-D cases. Variant c.455 C > T (p.Pro152Leu) in DOHH was previously reported by Ziegler et al. [10] in their individual 4, along with other missense variant. Basing on several functional approaches, they describe a drastical reduction in the amount of DOHH protein in individual 4 (20% of controls), accounting it for protein instability rather than the loss of activity, along with an accumulation of the inactive unhydroxylated deoxyhypusine-containing eIF5A. Individual 4 of Ziegler et al. (2 missense variants, as our patient), was the only one acquiring speaking capacity (as our patient). However, he did not develop epilepsy, and did show cortical atrophy, cardiac malformations and horizontal nystagmus (opposite to our patient). This might indicate that homozygous or compound heterozygous missense variants could give rise to a milder neurodevelopmental phenotype, warranting future confirmation.
Taking our patient into account, among DOHH-D and DHPS-D reported individuals with epilepsy, 5/8 displayed fever or infection-triggered seizures, in some cases resembling a wider Dravet-like phenotype. eIF5A has been reported as key for synthesizing bonds between successive proline residues during the elongation phase of translation process [11]. Thus, Faundes et al. have proposed that phenotypes of eIF5A-D patients could be explained by impaired synthesis of specific proteins rich in PPT [8].
Following the methodology employed by Faundes et al. to unravel the mechanisms for microcephaly in eIF5A-D, we evaluated if disrupted synthesis of proteins with PPT may contribute to explain epilepsy in eIF5A-HRD patients. We also used a catalogue of all known human epilepsy genes according to OMIM and of additional potential epilepsy-associated genes according to HGMD reports [24, 25] assessing their PPT content [26]. Analysis showed that 408/1360 (30%) epilepsy genes and 4156/17,306 (24%) of all other human protein-coding genes have 1 or more PPT (χ2 24.45; OR 1.36, 95% CI 1.2–1.53; p < 0.001). If within the OMIM epilepsy genes we include just the genes associated with neurodevelopmental disorders as the main symptom and epilepsy, excluding those associated with epilepsies as core symptom or those associated with systemic anomalies and epilepsy as a secondary manifestation, we obtain that 109/287 (38%) of these genes and 4455/18,379 (24.2%) of all other human protein-coding genes have 1 or more PPT (χ2 28.88; OR 1.91, 95% CI 1.5–2.43; p < 0.001). If OMIM epilepsy genes and other additional potential epilepsy-associated genes are taken into account together, significance maintains with 812/2687 (30.2%) of these genes and 3752/15,979 (23.5%) of all other human protein-coding genes having 1 or more PPT (χ2 56.55; OR 1.41, 95% CI 1.29–1.54; p < 0.001) [25].
Moreover, we also ranked epilepsy genes (OMIM) according to the content of proline in PPT [26] (Fig. 3). First, it is worth to mention that there are genes that appear both in our “top 10” ranking and in the “top 10” ranking described by Faundes et al. for microcephaly, such as IQSEC2, DIAPH1, SRCAP4 or PCLO. In the case of our analysis, FMN2 was ranked as #1. Biallelic loss-of-function variants in FMN2 cause intellectual developmental disorder 47 (MIM # 616193), displaying controlled focal epilepsy in 2/5 patients [27]. On the other hand, loss-of-function variants in WASF1 (#5) cause neurodevelopmental disorder with absent language and variable seizures (MIM # 618707). In one series, 4/5 patients presented epilepsy (2/4 with onset at 6 and 8 years and 3/4 with generalized seizure types) [28]. In other series, 5/6 patients presented epilepsy (4/5 with generalized seizure types and 1/5 with reflex seizures) [29]. Importantly, loss-of-function variants in IQSEC2 (#9) cause IQSEC2-related encephalopathy (MIM # 309530), displaying refractory epilepsy with variable age of onset, mainly with generalized seizure types and fever-triggered seizures [30–32]. Finally, pathogenic variants in SHANK3 (#10) causes Phelan-McDermid Syndrome-SHANK 3 related (MIM # 606232), with more than 60% of patients displaying epilepsy with variable age of onset and seizure types, including febrile seizures [33].
Fig. 3.

“Top 10” epilepsy genes according to the content of proline in their poly-proline tracts. Regarding epilepsy, IQSEC2 and SHANK3-related disorders share phenotypic characteristics with DHPS-D and DOHH-D [30–33]
Furthermore, loss-of-function variants in the widely-studied Proline-Rich Transmembrane Protein 2 (PRRT2), were identified in up to 18% of a cohort of 136 patients with febrile seizures (among other neurological and epilepsy syndromes), including febrile seizures plus, generalized epilepsy with febrile seizure plus and Dravet syndrome [34]. Moreover, PRRT2 has been demonstrated as an important negative modulator of sodium channels Nav1.2 (SCN2A gene) and Nav1.6 (SCN8A gene), leading to their hyperactivity if lacking [35]. Further, gain-of-function variants in SCN2A and SCN8A have been linked to Dravet-like phenotypes [36]. Although PRRT2 does not codify for long PPT, it does include several proline-proline, and several proline-X-proline sequences in its proline-rich domain, and it could be hypothesized that febrile seizures in patients with eIF5A-HRD might be explained, at least partially, by a loss of function of PRRT2.
On the other hand, eukaryotic elongation factor 2 kinase (EEF2K) phosphorylates and inactivates eukaryotic elongation factor 2 (EEF2, which catalyzes translation elongation), and thus, reducing the rate of protein synthesis. It has been observed that an increase in EEF2 function cooperates with the role of EIF5A during the elongation step of protein synthesis [37]. Furthermore, it has been reported that EEF2K knock out mice display enhanced GABAergic transmission, being less susceptible to seizures, and that EEF2K is upregulated in murine models of Dravet Syndrome, inactivating EEF2 [38]. Taking this into consideration, it could be also hypothesized that decreased activity of EIF5A due to variants in DHPS and DOHH, might lead to a parallel inactivation of EEF2, and thus contributing to the arising of a fenfluramine-responsive Dravet-like phenotype. Finally, it has been reported that EIF5A point mutated form V81G in a certain strain of the yeast Saccharomyces cerevisiae, led to a strongly temperature-sensitive phenotype [39]. Overall, the genetics of Dravet Syndrome without mutations in SCN1A [40, 41] and of epilepsies with fever-triggered seizures [42] is complex and remains to be clarified. In this context, these premises might establish an initial link associating eIF5A-HRD to these described neurodevelopmental and epilepsy phenotypes. However, the suggested pathophysiological proposals arise from literature review and hypothetical links among different molecular pathways, thus remaining speculative and definitely warranting further insight from future well-designed specific functional and clinical studies.
The diagnosis of recently described ultra-rare genetic disorders, such as eIF5A-HRD, presents some challenges. In this scenario, gene panels or clinical exome sequencing may be insufficient even if allegedly updated, since scarcely or initially described gene-disease associations may still not be curated by laboratories or prioritized by the main analysis softwares. Hence, whole exome or whole genome sequencing may show higher diagnostic yields in this situation, especially if trio studies are performed, given their advantages for de novo and compound heterozygous variants detection in autosomal recessive disorders. Moreover, training and implication of specialized clinicians along with geneticists and variant analysts in deep phenotyping of families, variant prioritization and interpretation and reanalysis processes, could also improve the diagnostic yield of these tests, since updates in medical literature or in phenotype have been proven among the main reasons for new diagnoses in reanalysis after initial non-diagnostic WES [43].
As a literature review and a case report with hypotheses based on a small sample, this and other mentioned studies have limitations, limiting the generalizability of their conclusions. Further investigation will be needed in order to define the precise phenotype, the response to treatment -including the efficacy of fenfluramine, and the molecular basis of eIF5A-HRD.
Conclusions
eIF5A-HRD are recently described entities and reported patients are scarce. Main phenotypic features consist of prenatal issues, hypotonia, dysmorphisms, microcephaly, moderate-severe NDD/ID and behavioral disorders. More than 70% of DHPS-D and DOHH-D patients present epilepsy, 63% of them with temperature-triggered seizures, and half of them refractory. Valproic acid or fenfluramine may be effective. Rare homozygous or compound heterozygous missense variants in these genes should be screened in patients with encephalopathy and temperature-triggered seizures. T-WES should be periodically reanalyzed in multidisciplinary teams in order to optimize diagnostic yield.
Acknowledgements
We thank the patient’s family for their collaboration and consent for publishing this work. We also thank Ana Mingorance for her cooperation and expertise.
Abbreviations
- eIF
Eukaryotic translation initiation factors
- eEF
Eukaryotic translation initiation factors
- eTF
Eukaryotic translation termination factors
- eIF5A
Eukaryotic initiation factor 5 A (protein)
- eIF5A-HRD
eIF5A and hypusination-related disorders
- DHPS
Deoxyhypusine synthase
- DOHH
Deoxyhypusine hydroxylase
- EIF5A
Eukaryotic initiation factor 5 A (gene)
- DOHH-D
DOHH-related disorders
- T-WES
Trio-exome sequencing
- eIF5A-D
eIF5A-related disorders
- DHPS-D
DHPS-related disorders
- NDD
Neurodevelopmental delay
- ID
Intellectual disability
- ASD
Autism-spectrum disorders
- LP
Likely pathogenic
- P
Pathogenic
- MRI
Magnetic resonance imaging
- ADHD
Attention deficit with hyperactivity disorder
- EEG
Electroencephalogram
- PRRT2
Proline-Rich Transmembrane Protein 2
- EEF2K
Eukaryotic elongation factor 2 kinase
- EEF2
Eukaryotic elongation factor 2
- VCF
Variant Call Format
- GeVIR
Gene Variation Intolerance Rank
- mRNA
Messenger RNA
- WT
Wild type
- WB
Western blotting
- PPT
Poly-proline tracts
- OMIM
Online Mendelian Inheritance in Man
- NDSSWI
Neurodevelopmental disorder with seizures and speech and walking impairment DRND: DOHH-related neurodevelopmental disorder
- HGMD
Human Gene Mutation Database
- SNVs
Single nucleotide variants
Author contributions
ABC: conceptualization; data curation; formal analysis; investigation; methodology; resources; supervision; visualization; original draft preparation; review and editing. AVC: data curation; formal analysis; resources; review and editing. RT: investigation; review and editing. IGM: investigation; review and editing. ISMR: investigation; review and editing. AGN: conceptualization; methodology; supervision; visualization; original draft preparation; review and editing. All authors read and approved the final manuscript.
Funding
This work has received no funding.
Data availability
The exome-sequencing data supporting this work have not been included in a public repository due to ethical restrictions, but are available from the corresponding author (ABC) on reasonable request. The rest of the data are available upon request.
Declarations
Ethics approval and consent to participate
The local ethics committee waived the need for approval regarding this case report.
Consent for publication
Written consent for publication was obtained from the parents of the patient.
Competing interests
ABC, RT, IGM, ISMR and AGN have received support from, and served as paid consultants for UCB Pharma. AVC has no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verma M, Choi J, Cottrell KA, Lavagnino Z, Thomas EN, Pavlovic-Djuranovic S, et al. A short translational ramp determines the efficiency of protein synthesis. Nat Commun. 2019;10(1):5774. 10.1038/s41467-019-13810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio A, Garland GD, Sfakianos A, Harvey RF, Willis AE. Aberrant protein synthesis and cancer development: the role of canonical eukaryotic initiation, elongation and termination factors in tumorigenesis. Semin Cancer Biol. 2022;86(Pt 3):151–65. 10.1016/j.semcancer.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Hanson FM, Hodgson RE, de Oliveira MIR, Allen KE, Campbell SG. Regulation and function of elF2B in neurological and metabolic disorders. Biosci Rep. 2022;42(6): BSR20211699. 10.1042/BSR20211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park MH, Kar RK, Banka S, Ziegler A, Chung WK. Post-translational formation of hypusine in eIF5A: implications in human neurodevelopment. Amino Acids. 2022;54(4):485–99. 10.1007/s00726-021-03023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi S, Cleveland JL. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids. 2016;48(10):2353–62. 10.1007/s00726-016-2275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colvin SC, Maier B, Morris DL, Tersey SA, Mirmira RG. Deoxyhypusine synthase promotes differentiation and proliferation of T helper type 1 (Th1) cells in autoimmune diabetes. J Biol Chem. 2013;288(51):36226–35. 10.1074/jbc.M113.473942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauber I, Bevec D, Heukeshoven J, Krätzer F, Horn F, Choidas A, et al. Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy. J Clin Invest. 2005;115(1):76–85. 10.1172/JCI21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faundes V, Jennings MD, Crilly S, Legraie S, Withers SE, Cuvertino S, et al. Impaired eIF5A function causes a Mendelian disorder that is partially rescued in model systems by spermidine. Nat Commun. 2021;12(1): 833. 10.1038/s41467-021-21053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganapathi M, Padgett LR, Yamada K, Devinsky O, Willaert R, Person R, et al. Recessive rare variants in deoxyhypusine synthase, an enzyme involved in the synthesis of hypusine, are associated with a neurodevelopmental disorder. Am J Hum Genet. 2019;104(2):287–98. 10.1016/j.ajhg.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler A, Steindl K, Hanner AS, Kar RK, Prouteau C, Boland A, et al. Bi-allelic variants in DOHH, catalyzing the last step of hypusine biosynthesis, are associated with a neurodevelopmental disorder. Am J Hum Genet. 2022;109(8):1549–58. 10.1016/j.ajhg.2022.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51(1):35–45. 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews MB, Hershey JW. The translation factor eIF5A and human cancer. Biochim Biophys Acta. 2015;1849(7):836–44. 10.1016/j.bbagrm.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong Y, Park I, Hong BS, Nedyalkova L, Tempel W, Park HW. Crystal structure of human eIF5A1: insight into functional similarity of human eIF5A1 and eIF5A2. Proteins. 2009;75(4):1040–5. 10.1002/prot.22378. [DOI] [PubMed] [Google Scholar]
- 14.Wątor E, Wilk P, Biela A, Rawski M, Zak KM, Steinchen W, et al. Cryo-EM structure of human eIF5A-DHS complex reveals the molecular basis of hypusination-associated neurodegenerative disorders. Nat Commun. 2023;14(1):1698. 10.1038/s41467-023-37305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umland TC, Wolff EC, Park MH, Davies DR. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme.NAD.inhibitor ternary complex. J Biol Chem. 2004;279(27):28697–705. 10.1074/jbc.M404095200. [DOI] [PubMed] [Google Scholar]
- 16.Han Z, Sakai N, Böttger LH, Klinke S, Hauber J, Trautwein AX. er al. Crystal Structure of the Peroxo-diiron(III) Intermediate of Deoxyhypusine Hydroxylase, an Oxygenase Involved in Hypusination. Structure. 2015;23(5):882–892. 10.1016/j.str.2015.03.002 [DOI] [PubMed]
- 17.García-Morales I, Delgado RT, Falip M, Campos D, García ME, Gil-Nagel A. Early clinical experience with lacosamide as adjunctive therapy in patients with refractory focal epilepsy and nocturnal seizures. Seizure. 2011;20(10):801–4. 10.1016/j.seizure.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Leegwater PA, Vermeulen G, Könst AA, Naidu S, Mulders J, Visser A, et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet. 2001;29(4):383–8. 10.1038/ng764. [DOI] [PubMed] [Google Scholar]
- 19.Borck G, Shin BS, Stiller B, Mimouni-Bloch A, Thiele H, Kim JR, et al. eIF2γ mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol Cell. 2012;48(4):641–6. 10.1016/j.molcel.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin HC, Jones WD, McIntyre R, Sanchez-Andrade G, Sanderson M, Stephenson JD, et al. Quantifying the contribution of recessive coding variation to developmental disorders. Science. 2018;362(6419):1161–4. 10.1126/science.aar6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gkogkas CG, Khoutorsky A, Ran I, Rampakakis E, Nevarko T, Weatherill DB, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493(7432):371–7. 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chartier-Harlin MC, Dachsel JC, Vilariño-Güell C, Lincoln SJ, Leprêtre F, Hulihan MM, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89(3):398–406. 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapur M, Ackerman SL. MRNA translation gone awry: translation fidelity and neurological disease. Trends Genet. 2018;34(3):218–31. 10.1016/j.tig.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: online Mendelian inheritance in man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–98. 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang MW, Liang XY, Wang J, Gao LD, Liao HJ, He YH, et al. Epilepsy-associated genes: an update. Seizure. 2024;116:4–13. 10.1016/j.seizure.2023.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Morgan AA, Rubenstein E. Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS One. 2013;8(1): e53785. 10.1371/journal.pone.0053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law R, Dixon-Salazar T, Jerber J, Cai N, Abbasi AA, Zaki MS, et al. Biallelic truncating mutations in FMN2, encoding the actin-regulatory protein formin 2, cause nonsyndromic autosomal-recessive intellectual disability. Am J Hum Genet. 2014;95(6):721–8. 10.1016/j.ajhg.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito Y, Carss KJ, Duarte ST, Hartley T, Keren B, Kurian MA, et al. De novo truncating mutations in WASF1 cause intellectual disability with seizures. Am J Hum Genet. 2018;103(1):144–53. 10.1016/j.ajhg.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava S, Macke EL, Swanson LC, Coulter D, Klee EW, Mullegama SV, et al. Expansion of the genotypic and phenotypic spectrum of WASF1-related neurodevelopmental disorder. Brain Sci. 2021;11(7): 931. 10.3390/brainsci11070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zerem A, Haginoya K, Lev D, Blumkin L, Kivity S, Linder I, et al. The molecular and phenotypic spectrum of IQSEC2-related epilepsy. Epilepsia. 2016;57(11):1858–69. 10.1111/epi.13560. [DOI] [PubMed] [Google Scholar]
- 31.Mignot C, McMahon AC, Bar C, Campeau PM, Davidson C, Buratti J, et al. Iqsec2-related encephalopathy in males and females: a comparative study including 37 novel patients. Genet Med. 2019;21(4):837–49. 10.1038/s41436-018-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastrangelo M, Greco C, Tolve M, Bartolini E, Russo A, Nicita F, et al. Epilepsy phenotypes across the different age-ranges in IQSEC2-related encephalopathy: an Italian multicentre retrospective cohort study. Seizure. 2024;119:119–27. 10.1016/j.seizure.2024.06.002. [DOI] [PubMed] [Google Scholar]
- 33.de Coo IFM, Jesse S, Le TL, Sala C, European Phelan-McDermid syndrome consortium. Consensus recommendations on epilepsy in Phelan-McDermid syndrome. Eur J Med Genet. 2023;66(6): 104746. 10.1016/j.ejmg.2023.104746. [DOI] [PubMed] [Google Scholar]
- 34.He ZW, Qu J, Zhang Y, Mao CX, Wang ZB, Mao XY, et al. PRRT2 mutations are related to febrile seizures in epileptic patients. Int J Mol Sci. 2014;15(12):23408–17. 10.3390/ijms151223408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fruscione F, Valente P, Sterlini B, Romei A, Baldassari S, Fadda M, et al. PRRT2 controls neuronal excitability by negatively modulating Na + channel 1.2/1.6 activity. Brain. 2018;141(4):1000–16. 10.1093/brain/awy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangano GD, Fontana A, Antona V, Salpietro V, Mangano GR, Giuffrè M, et al. Commonalities and distinctions between two neurodevelopmental disorder subtypes associated with SCN2A and SCN8A variants and literature review. Mol Genet Genomic Med. 2022;10(5):e1911. 10.1002/mgg3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias CA, Gregio AP, Rossi D, Galvão FC, Watanabe TF, Park MH, et al. eIF5A interacts functionally with eEF2. Amino Acids. 2012;42(2–3):697–702. 10.1007/s00726-011-0985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beretta S, Gritti L, Ponzoni L, Scalmani P, Mantegazza M, Sala M, et al. Rescuing epileptic and behavioral alterations in a Dravet syndrome mouse model by inhibiting eukaryotic elongation factor 2 kinase (eEF2K). Mol Autism. 2022;13(1): 1. 10.1186/s13229-021-00484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrader R, Young C, Kozian D, Hoffmann R, Lottspeich F. Temperature-sensitive eIF5A mutant accumulates transcripts targeted to the nonsense-mediated decay pathway. J Biol Chem. 2006;281(46):35336–46. 10.1074/jbc.M601460200. [DOI] [PubMed] [Google Scholar]
- 40.Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Møller RS, Hjalgrim H, et al. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology. 2014;82(14):1245–53. 10.1212/WNL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann C, von Spiczak S, Suls A, Weckhuysen S, Buyse G, Vilain C, et al. Investigating the genetic basis of fever-associated syndromic epilepsies using copy number variation analysis. Epilepsia. 2015;56(3):e26-32. 10.1111/epi.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson HE, Poduri A. Towards understanding genetic risk in febrile seizures: innate immunity and neuronal excitability. Brain. 2022;145(2):416–7. 10.1093/brain/awac036. [DOI] [PubMed] [Google Scholar]
- 43.Dai P, Honda A, Ewans L, McGaughran J, Burnett L, Law M, et al. Recommendations for next generation sequencing data reanalysis of unsolved cases with suspected Mendelian disorders: a systematic review and meta-analysis. Genet Med. 2022;24(8):1618–29. 10.1016/j.gim.2022.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The exome-sequencing data supporting this work have not been included in a public repository due to ethical restrictions, but are available from the corresponding author (ABC) on reasonable request. The rest of the data are available upon request.