Abstract
Objective
To evaluate the short- and long-term outcomes of totally laparoscopic, laparoscopic-assisted, and open total gastrectomy in gastric cancer patients after neoadjuvant therapy.
Methods
This multicenter retrospective cohort study was conducted to collect clinical data from 289 patients who underwent total radical gastrectomy after neoadjuvant therapy at five centers. The patients were divided into three groups according to the surgical method they received: totally laparoscopic, laparoscopic-assisted, and open groups. The general baseline data, intraoperative and postoperative conditions, postoperative histopathological results, related complications, and survival outcomes of the three groups were compared.
Results
The totally laparoscopic group had a longer operation time and more intraoperative blood loss than the laparoscopic-assisted and open groups. However, the first postoperative bowel movement, first postoperative defecation, first postoperative meal, and removal of the postoperative drainage tube occurred earlier, and the total number of lymph node dissections was higher in totally laparoscopic group. The intraoperative blood transfusion rate, postoperative intensive care unit admission rate, maximum tumor diameter, positive lymph node dissection number, tumor staging, and hospitalization time did not differ significantly among the three groups, nor did the total incidence of postoperative complications, Clavien-Dindo classification, 30- and 90-day readmission rates and survival outcomes (all p > 0.05).
Conclusion
For patients with gastric cancer treated with neoadjuvant therapy, the three surgical approaches showed no significant differences in both short- and long-term outcomes.Although totally laparoscopic total gastrectomy has a longer operation time, it has the advantages of faster postoperative recovery and earlier food intake.
Keywords: Gastric cancer, Gastrointestinal, Stomach, Outcomes
Introduction
Gastric cancer(GC) is one of the most common malignant tumors worldwide, and its incidence and mortality rates remain relatively high [1]. Although neoadjuvant therapy (NAT) has been widely used for advanced GC and has greatly improved patient prognosis [2, 3], surgical operations still play an important role as a key link in the treatment of advanced GC [4].
With the continuous development of medical technology, laparoscopic surgery has gradually become the preferred method for many GC surgeries because of its minimally invasive advantages and rapid recovery characteristics [5, 6]. However, NAT can lead to tissue edema, fibrosis, and disruption of anatomical planes, making surgery more difficult, and the deterioration of nutritional and immune status in patients with advanced GC further affects surgical safety [7, 8]. Previous studies have reported on the safety and effectiveness of laparoscopic-assisted distal [8] and total gastrectomy [9–11] after neoadjuvant chemotherapy (NACT). Although some retrospective studies have confirmed the efficacy of the fully laparoscopic technique in total gastrectomy [10, 11], in the absence of prospective studies, multi-center cohort studies are needed to verify its safety.
Patients and methods
We retrospectively collected the clinical data of patients who underwent radical total gastrectomy after NAT from the First Affiliated Hospital of Air Force Medical University from December 2018 to December 2023 and from Yuncheng Central Hospital in Shanxi Province, People’s Hospital of Qinghai Province, Changzhi Medical College Affiliated Heji Hospital in Shanxi Province, and Air Force 986th Hospital, Fourth Military Medical University from January 2020 to December 2023. Before the study, all surgeons in each center had completed the standardized training of totally laparoscopic surgery and independently performed more than 50 total gastrectomy cases and/or more than 30 totally laparoscopic gastrectomy cases. The enrollment process is illustrated in Fig. 1. The inclusion criteria were as follows: (1) age 18–80 years; (2) D2 radical total gastrectomy after NAT; (3) pathologically confirmed gastric adenocarcinoma; and (4) family members or patients provided informed consent. The exclusion criteria were as follows: (1) incomplete clinical and pathological data; (2) patients undergoing palliative total gastrectomy or converted procedures; (3) presence of severe comorbidities; (4) multivisceral resection other than the gallbladder; (5) gastric stump cancer or emergency surgery; (6) Siewert type I esophagogastric junction cancer; (7)positive margins; (8)Patients who did not undergo postoperative adjuvant therapy.
Fig. 1.
Flow diagram of patient enrollment. NACT: Neoadjuvant chemotherapy
Surgical procedures
The selection of surgical procedures was determined by the operating surgeon based on patient-specific factors and institutional protocols, rather than randomization.According to the English version of the Japanese Classification of GC [12] and the 2014 Japanese GC Treatment Guidelines [13], all patients underwent standard D2 or D2 + lymph node dissections (1, 2, 3, 4sa, 4sb, 4 d, 5, 6, 7, 8a, 9, 11p, 11 d, 12a). No. 10 lymph node dissection was performed only when the tumor was on the greater curvature side. Trocars were performed according to previous literature descriptions [7]. After lymphadenectomy, all reconstruction procedures were completed intracorporeally using endoscopic linear staplers in the total laparoscopic group, and a small vertical incision was extended below the umbilicus for specimen removal. All patients who underwent total gastrectomy in the three groups underwent standard Roux-en-Y reconstruction.
Clinical data and surgical complications
Data on the following were collected at baseline: sex age, body mass index (BMI), American Society of Anesthesiologists physical status classification system (ASA), tumor involvement of the dentate line, perioperative blood biochemical indicators, preoperative imaging staging of the tumor, adjuvant therapy regimen, and chemotherapy cycle. The following intraoperative and postoperative conditions were recorded: duration of surgery, intraoperative blood loss, intraoperative transfusion rate, postoperative admission to the ICU, maximum tumor diameter, postoperative first bowel movement time, postoperative first defecation time, postoperative first feeding time, lymph node clearance (total and positive), postoperative extubation time, and postoperative hospital stay. Data on postoperative complications, Clavien-Dindo classification of complications [14], and postoperative management of complications were also collected. Follow-up was conducted via telephone or outpatient visits to assess readmission due to complications at 30 and 90 days after surgery.Histopathological conditions, including the postoperative pathological TNM staging, degree of tumor differentiation, vascular invasion, and nerve invasion, were recorded.
Follow-up
Among these five centers, all institutions implemented standardized postoperative monitoring through telephone follow-ups and outpatient examinations in accordance with guideline-specified protocols: quarterly (every 3 months) during the initial 24-month period, semiannually (every 6 months) for the subsequent 36 months, and annually (every 12 months) after completing 60 months of follow-up. All enrolled patients were tracked until either death or the study cutoff date (November 2024), with follow-up durations ranging from 1 to 72 months across cases.
Statistical analysis
Normally distributed data are expressed as the mean ± standard deviation (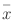 ± s), and intergroup comparisons were performed using analysis of variance. Non-normally distributed measurement data are expressed as the median (first quartile, third quartile) (M (Q1, Q3)), and intergroup comparisons were performed using the Kruskal–Wallis H test. Count data are expressed as cases (percentage), and intergroup comparisons were performed using the chi-square test or Fisher’s exact test.p < 0.05 indicated statistical significance.Data were analyzed using GraphPad Prism 8statistical software. OmicShare Tools for survival data analysis.
± s), and intergroup comparisons were performed using analysis of variance. Non-normally distributed measurement data are expressed as the median (first quartile, third quartile) (M (Q1, Q3)), and intergroup comparisons were performed using the Kruskal–Wallis H test. Count data are expressed as cases (percentage), and intergroup comparisons were performed using the chi-square test or Fisher’s exact test.p < 0.05 indicated statistical significance.Data were analyzed using GraphPad Prism 8statistical software. OmicShare Tools for survival data analysis.
Results
Clinical characteristics
A total of 289 patients were enrolled, comprising 241 men (83.4%) and 48 women (16.6%). The average age of the patients was 62.41 ± 7.77 years, and the average BMI was 22.8 ± 2.91 kg/m2. Among the included patients, 80 underwent totally laparoscopic total gastrectomy (TLTG), 96 underwent laparoscopically assisted total gastrectomy (LATG), and 113 underwent open total gastrectomy (OTG). The baseline data did not differ significantly among the three groups (all p > 0.05), as shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study population
| Baseline date | TLTG group (n = 80) |
LATG group (n = 96) |
OTG group (n = 113) |
F/H/X2 value | P-value |
|---|---|---|---|---|---|
| Sex | 1.051 | 0.596 | |||
| Male | 68 (85.0%) | 77 (80.2%) | 96 (84.9%) | ||
| Female | 12 (15.0%) | 19 (19.8%) | 17 (15.0%) | ||
| Age ( ‾x ± s ) (year) | 62.49 ± 7.33 | 62.36 ± 7.54 | 62.38 ± 8.46 | 0.408 | 0.665 |
| BMI (‾x ± s) (kg/m2 ) | 22.86 ± 2.74 | 22.82 ± 3.19 | 22.68 ± 2.80 | 2.352 | 0.095 |
| ASA score | 3.112 | 0.544 | |||
| I | 2 (2.5%) | 6 (6.3%) | 8 (7.1%) | ||
| II | 52 (65%) | 62 (64.6%) | 65 (57.5%) | ||
| III | 26 (32.5%) | 28 (29.2%) | 40 (35.4%) | ||
| Hemoglobin (‾ x ± s ) (g/L) | 121.24 ± 19.02 | 121.56 ± 19.32 | 121.20 ± 18.69 | 1.301 | 0.272 |
| Albumin (‾ x ± s ) (g/L) | 36.73 ± 5.79 | 36.77 ± 5.38 | 36.70 ± 4.56 | 0.200 | 0.818 |
| CT stagea | 1.682 | 0.817 | |||
| T2 | 3 (3.8%) | 2 (2.1%) | 2 (1.8%) | ||
| T3 | 18 (22.5%) | 19 (19.8%) | 28 (24.8%) | ||
| T4 | 59 (73.7%) | 75 (78.1%) | 83 (73.4%) | ||
| cN stagea | 1.031 | 0.929 | |||
| N1 | 3 (3.8%) | 2 (2.1%) | 3 (2.7%) | ||
| N2 | 33 (41.3%) | 45 (46.9%) | 50 (44.2%) | ||
| N3 | 44 (55.0%) | 49 (51.0%) | 60 (53.1%) | ||
| Tumor invading the dentate line of the gastric cardia | 0.999 | 0.620 | |||
| Yes | 26 (32.5%) | 27 (28.1%) | 39 (34.5%) | ||
| No | 54 (67.5%) | 69 (71.9%) | 74 (65.5%) | ||
| Cycle completed (‾ x ± s ) | 3.58 ± 0.90 | 3.70 ± 1.04 | 3.59 ± 1.08 | 1.424 | 0.241 |
|
Neoadjuvant chemotherapy plus immunotherapy |
1.136 | 0.577 | |||
| Yes | 32 (40%) | 46 (47.9%) | 49 (43.4%) | ||
| No | 48 (60%) | 50 (52.1%) | 64 (56.6%) | ||
| Chemotherapy–surgical procedure interval ( ‾x ± s ) (day) | 34.01 ± 9.73 | 34.65 ± 10.18 | 34.30 ± 9.71 | 2.952 | 0.052 |
| Tumor regression grade | 4.488 | 0.620 | |||
| 0 | 12 (15.0%) | 8 (8.3%) | 9 (8.0%) | ||
| 1 | 58 (72.5%) | 78 (81.3%) | 86 (76.1%) | ||
| 2 | 6 (7.5%) | 6 (6.3%) | 10(8.8%) | ||
| 3 | 4 (5.0%) | 4 (4.2%) | 8 (7.1%) |
a Fisher’s exact test was used
Operative and oncological outcomes
The operation time was longer in the total laparoscopic group than in the laparoscopic-assisted and open groups [(270.38 ± 39.02) min vs. (247.64 ± 29.21) min vs. (223.55 ± 61.61) min, p < 0.001]. The intraoperative blood loss was greater in the laparoscopic-assisted and open groups than in the total laparoscopic group [200 (100, 300) mL vs. 160 (100, 200) mL vs. 150 (100, 200) mL, p < 0.001]. However, in total laparoscopic group, the postoperative first exhaust time [(3.23 ± 0.91) d vs. (3.48 ± 1.00) d vs. (3.78 ± 1.09) d, p < 0.001], postoperative first defecation time [(3.92 ± 0.82) d vs. (4.21 ± 1.02) d vs. (4.54 ± 1.23) d, p < 0.001], postoperative first eating time [(5.77 ± 2.08) d vs. (5.97 ± 0.90) d vs. (6.11 ± 1.32) d, p = 0.037], and postoperative extubation time [(7.72 ± 1.88) d vs. (8.04 ± 1.44) d vs. (8.53 ± 1.94) d, p < 0.001] were earlier, and the total number of lymph node dissections was greater than those in the laparoscopic-assisted and open groups [(25.30 ± 7.52) min vs. (24.95 ± 6.34) min vs. (23.95 ± 7.50) min, p < 0.001]. The intraoperative blood transfusion rate, postoperative ICU monitoring rate, maximum tumor diameter, positive lymph node dissection number, TNM staging, and hospitalization time did not differ significantly among the three groups (all p > 0.05) (Table 2).
Table 2.
Surgical and postoperative recovery characteristics of the study population
| Item | TLTG group (n = 80) |
LATG group (n = 96) |
OTG group (n = 113) |
F/H/X2value | P-value |
|---|---|---|---|---|---|
| Operation time (‾x ± s) (min) | 270.38 ± 39.02 | 247.64 ± 29.21 | 223.55 ± 61.61 | 12.504 | <0.001 |
| Intraoperative blood loss | 200 (100, 300) | 160 (100, 200) | 150 (100, 200) | 52.241 | <0.001 |
| Intraoperative transfusion | 13 (10.4%) | 15 (15.6%) | 23 (20.4%) | 0.947 | 0.649 |
| Number of retrieved lymph nodes | |||||
| Total number | 25.30 ± 7.52 | 24.95 ± 6.34 | 23.95 ± 7.50 | 20.879 | <0.001 |
| Positive count | 10 (7, 14) | 5 (1, 14) | 2 (0, 10) | 0.204 | 0.903 |
| Maximum tumor diameter | 0.267 | 0.884 | |||
| ≤ 3 cm | 18 (22.5%) | 24 (25.0%) | 29 (25.7%) | ||
| > 3 cm | 62 (77.5%) | 72 (75.0%) | 84 (74.3%) | ||
| Postoperative ICU care (yes)a | 18 (22.5%) | 23 (24.0%) | 35 (31.0%) | 2.141 | 0.358 |
| Time to first (‾ x ± s )(d) | |||||
| Aerofluxus | 3.23 ± 0.91 | 3.48 ± 1.00 | 3.78 ± 1.09 | 22.635 | <0.001 |
| Defecation | 3.92 ± 0.82 | 4.21 ± 1.02 | 4.54 ± 1.23 | 32.112 | <0.001 |
| Liquid diet | 5.77 ± 2.08 | 5.97 ± 0.90 | 6.11 ± 1.32 | 3.294 | 0.037 |
| Extubation | 7.72 ± 1.88 | 8.04 ± 1.44 | 8.53 ± 1.94 | 39.879 | <0.001 |
| Postoperative length of stay | 9.39 ± 5.72 | 9.38 ± 5.88 | 9.85 ± 5.42 | 1.936 | 0.145 |
The postoperative pathological T- and N-stages differed significantly among the three groups (all p < 0.05), with the OTG group having a later tumor stage than the TLTG and LATG groups. The Lauren classification, Borrmann classification, tumor differentiation, vascular invasion, and neural invasion did not differ significantly among the three groups (all p > 0.05) (Table 3).
Table 3.
Pathological results
| Item | TLTG group (n = 80) |
LATG group (n = 96) |
OTG group (n = 113) |
F/H/X2 value | P-value |
|---|---|---|---|---|---|
| ypT stageb | 13.613 | 0.092 | |||
| ypT0 | 12(15.0%) | 8(8.3%) | 10(8.8%) | ||
| ypT1 | 10 (12.5%) | 4 (4.2%) | 6 (5.3%) | ||
| ypT2 | 11 (13.8%) | 12 (12.5%) | 16 (14.2%) | ||
| ypT3 | 31 (38.7%) | 37 (38.5%) | 40(35.4%) | ||
| ypT4 | 16 (20.0%) | 35(36.5%) | 42 (37.2%) | ||
| ypN stage | 7.746 | 0.257 | |||
| ypN0 | 27 (33.8%) | 34 (35.4%) | 35 (31.0%) | ||
| ypN1 | 18 (22.5%) | 20 (20.8%) | 21 (18.6%) | ||
| ypN2 | 20 (25.0%) | 24 (25.0%) | 22 (19.5%) | ||
| ypN3 | 15 (18.8%) | 18 (18.8%) | 35 (31.0%) | ||
| Lauren typeb | 3.140 | 0.539 | |||
| Intestinal | 18 (22.5%) | 23 (23.9%) | 30 (26.5%) | ||
| Diffused | 42 (52.5%) | 50 (52.1%) | 59 (52.2%) | ||
| Mixed | 8 (1.0%) | 5 (5.2%) | 15 (13.3%) | ||
| Unknown | 12(15.0%) | 8(8.3%) | 9(8.0%) | ||
| Borrmann type | 6.923 | 0.141 | |||
| Infiltrating type | 22 (27.5%) | 20 (20.8%) | 30 (26.5%) | ||
| Ulcerative type | 46 (57.5%) | 50 (52.1%) | 67 (59.3%) | ||
| Protruding type | 12 (15.0%) | 26 (27.1%) | 16 (14.2%) | ||
| Differentiationb | 3.882 | 0.425 | |||
| Low | 36 (45.0%) | 42 (43.8%) | 58 (51.3%) | ||
| Moderate | 29 (36.3%) | 36 (37.5%) | 40 (35.4%) | ||
| High | 3 (3.8%) | 10 (10.4%) | 6 (5.3%) | ||
| Vascular invasion (yes) | 43 (53.8%) | 65 (67.7%) | 66 (58.4%) | 3.800 | 0.158 |
| Perineural invasion (yes) | 42 (52.5%) | 64 (66.7%) | 60 (53.1%) | 5.013 | 0.083 |
b 29 patients who achieved complete pathological remission were excluded
Postoperative complication
Of the 289 patients, 53 experienced postoperative complications, with a total complication rate of 18.3%. Among them, 16 patients (20.0%) in the total laparoscopic group developed complications, comprising five (6.3%) with Clavien-Dindo grade II, five (6.3%) with grade IIIa, and three (3.8%) with grade IV. Two patients (2.5%) were readmitted to the hospital due to the occurrence of complications on the 30th and 90th days after surgery. 15 patients (15.6%) in the laparoscopic-assisted group developed complications, comprising one (1.0%) with Clavien-Dindo grade I, four (4.2%) with grade II, three (3.1%) with grade IIIa, and one (1.0%) with grade IV. Three patients (3.1%) were readmitted to the hospital due to the occurrence of complications on the 30th and 90th days after surgery. In the open surgery group, 22 patients (19.5%) developed complications, comprising one (0.9%) with Clavien-Dindo grade I, eight (7.1%) with grade II, three (2.7%) with grade IIIa, and four (3.5%) with grade IV. The total postoperative complication rate, Clavien-Dindo classification, and readmission rates at 30 and 90 days after surgery did not differ significantly among the three surgical methods (all p > 0.05). Patients with complications received symptomatic treatment after the confirmation of complications and were discharged after improvement (Table 4).
Table 4.
Postoperative complications in patients treated with neoadjuvant therapy
| Complications | TLTG group (n = 80) |
LATG group (n = 96) |
OTG Group (n = 113) |
F/H/X2 value | P-value |
|---|---|---|---|---|---|
| Postoperative complications (yes) | 16 (20.0%) | 15 (15.6%) | 22 (19.5%) | 0.716 | 0.699 |
| Clavien-Dindo classification | |||||
| Grade I a | 0 (0%) | 1 (1.0%) | 1 (0.9%) | 0.789 | 0.674 |
| Lymphatic leakage a | 0 (0%) | 1 (1.0%) | 1 (0.9%) | 0.789 | 0.674 |
| Grade II | 5 (6.3%) | 4 (4.2%) | 8 (7.1%) | 0.823 | 0.663 |
| Pulmonary infection a | 3 (3.8%) | 0 (0%) | 5 (4.4%) | 4.172 | 0.124 |
| Anastomotic leakage a | 2 (2.5%) | 2 (2.1%) | 1 (0.9%) | 0.824 | 0.662 |
| Duodenal stump fistula a | 0 (0%) | 1 (1%) | 0 (0%) | 2.017 | 0.365 |
| Gastroplegia a | 0 (0%) | 1 (1%) | 0 (0%) | 2.017 | 0.365 |
| Anastomotic bleeding a | 0 (0%) | 0 (0%) | 2 (1.8%) | 3.137 | 0.208 |
| Grade IIIa a | 5 (6.3%) | 3 (3.1%) | 3 (2.7%) | 1.836 | 0.399 |
| Pleural effusion a | 4 (5%) | 3 (3.1%) | 1 (0.9%) | 3.015 | 0.221 |
| Pneumothorax a | 1 (1.3%) | 0 (0%) | 2 (1.8%) | 1.631 | 0.442 |
| Grade IV a | 3 (3.8%) | 1 (1%) | 4 (3.5%) | 1.600 | 0.449 |
| Septic shock a | 0 (0%) | 1 (1%) | 1 (0.9%) | 0.789 | 0.674 |
| Intraperitoneal bleeding a | 0 (0%) | 0 (0%) | 1 (0.9%) | 1.563 | 0.458 |
| Anastomotic leakage a | 3 (3.8%) | 0 (0%) | 2 (1.8%) | 3.611 | 0.164 |
| Other complications (yes) | |||||
| Duodenal obstruction a | 0 (0%) | 1 (1%) | 0 (0%) | 1.861 | 0.609 |
| Lower extremity venous thrombosis a | 1 (1.3%) | 0 (0%) | 0 (0%) | 2.622 | 0.270 |
| Abdominal pain a | 0 (0%) | 1 (1%) | 2 (1.8%) | 1.428 | 0.490 |
| Vomiting a | 0 (0%) | 2 (2.1%) | 1 (0.9%) | 1.886 | 0.389 |
| Diarrhea a | 1 (1.3%) | 0 (0%) | 2 (1.8%) | 1.631 | 0.442 |
| Constipation a | 0 (0%) | 1 (1%) | 1 (0.9%) | 0.789 | 0.674 |
| Acute cholangitis a | 0 (0%) | 1 (1%) | 0 (0%) | 1.861 | 0.609 |
| Anemiaa | 1 (1.3%) | 0 (0%) | 0 (0%) | 2.622 | 0.270 |
| 30-day unplanned readmission a | 2 (2.5%) | 3 (3.1%) | 3 (2.7%) | 0.072 | 0.965 |
| 90-day unplanned readmission a | 2 (2.5%) | 3 (3.1%) | 5 (4.4%) | 0.568 | 0.753 |
a Fisher’s exact test was used
Survival outcomes
The median follow-up time was 26.4 months (mean 31.2 months, range 1–72 months). The DFS (p = 0.17) and OS rates (p = 0.21) were no significantly difference between the three groups (Fig. 2).
Fig. 2.
Kaplan-meier analysis of disease-free survival (DFS) and overall survival (OS) in the three groups of patients with gastric cancer. A Kaplan-meier DFS estimates. B Kaplan-meier OS estimates
Discussion
Multiple studies [5, 6] have confirmed the safety and feasibility of total laparoscopic surgery in patients with GC, but its benefits in patients undergoing NAT still require further research and verification. Fujisaki et al. [15] showed that laparoscopic surgery requires a longer operation time than traditional open surgery. Our results also showed that the operative time was longer in the TLTG group than in the LATG and OTG groups. Domestic researchers have provided different perspectives. Cheng et al. [11] found that the average operation time in the TLTG group after NACT was significantly shorter than that in the OTG group (173 min vs. 215 min, p < 0.001), which is inconsistent with our findings, possibly due to the inconsistent patient inclusion in this study. We believe that although laparoscopy has a magnifying effect, allowing for more precise identification of vascular lymphoid tissue, it requires greater operational skills from surgeons, a longer preparation time, and more cumbersome steps than traditional surgery, thereby significantly extending the operation time. Furthermore, the increased exudation in the surgical field after NAT for advanced GC further increases the difficulty of surgery.
The number of retrieved lymph nodes is an important indicator of the quality of gastric surgery and is positively correlated with the prognosis of GC [16, 17]. In this study, there was no significant difference in the number of retrieved lymph nodes among the three groups of patients, but all were more than the number specified by the current guidelines. However, previous studies [18] have found that the number of retrieved lymph nodes is significantly higher during laparoscopic surgery than during open surgery. We speculate that after NAT, most lymph nodes retract, and neither center performs lymph node sorting, which did not result in significant differences among the three groups. However, this result also indicates that there is no significant difference in lymph node dissection ability between the TLTG, LATG, and OTG groups, indicating their comparability with traditional surgical methods in this indicator.
Typically, blood loss is less with laparoscopic surgery than with open surgery; however, there was no difference in our study. Indeed, the magnified visualization and enhanced precision of laparoscopic techniques facilitate anatomical dissection along surgical planes, which has been consistently shown in numerous studies to reduce intraoperative blood loss in standard gastric cancer surgeries [19]. However, our study specifically focused on patients with advanced gastric cancer receiving neoadjuvant therapy, which presents unique surgical challenges: (1)Tissue edema induced by neoadjuvant treatment often obscures anatomical planes; (2)The inflammatory changes significantly compromise the precision advantages of laparoscopic surgery; (3)This frequently results in more pronounced oozing and increased blood loss compared to non-treated cases.These findings align with existing literature reporting that both open and laparoscopic approaches demonstrate greater blood loss in neoadjuvant-treated patients compared to treatment-naïve cases [5, 8]. The diminished tissue clarity after neoadjuvant therapy appears to offset some of the conventional bleeding-reduction benefits typically associated with laparoscopic techniques.We have incorporated these valuable points into the revised discussion section to provide a more comprehensive interpretation of our results.
In terms of postoperative recovery, gastrointestinal tract reconstruction in the TLTG group was performed under total laparoscopy, which has the advantages of reducing the postoperative inflammatory response, promoting intestinal function recovery, small incisions, mild pain, and clear vision [1, 20, 21]. These advantages promote faster postoperative bowel movements and earlier feeding times, which is consistent with previous research results. In addition, patients with advanced GC often present with nutritional and psychological disorders, and NAT can aggravate this damage to a certain extent. Therefore, minimally invasive treatment is necessary to reduce this traumatic stress.Recently, Park et al., [22]reported that TLTG provided a better quality of life in terms of some functioning and STO 22 eating, compared to LATG (reference A), which means much less invasiveness might have better quality of life. Since Totally laparoscopic gastrectomy (TLTG or TLDG) does not expose the intestine to manual manipulation by surgeon’s hand, it might cause less adhesion-induced pain than laparoscopy-assisted gastrectomy (LATG or LADG), which inevitably requires bowel handling during GI tract reconstruction [19]. Similarly, the authors suggest that minimally invasive LTG may improve eating-related and certain functional aspects of quality of life, ultimately leading to faster recovery and shorter hospital stays. Specifically, the earlier return of bowel motility, resumption of oral intake, and defecation can be directly attributed to the absence of manual intestinal manipulation during the procedure.
In terms of postoperative complications, a domestic retrospective study reported that the complication rate was significantly lower in the TLTG group than in the LATG group (19.0% vs. 56.5%, p = 0.011) [7]. Another retrospective propensity-matching analysis showed that the complication rates in the TLTG and OTG groups were 32.3% and 42.9%, respectively, with no statistically significant difference (p > 0.05) [10]. Moreover, a previous randomized trial demonstrated that TLTG was comparable to OTG in terms of tumor quality control and postoperative complications after NACT [23]. Our results showed that the total complication rate in the TLTG group was 20.0%, which was comparable to that of the abovementioned studies, further indicating that NAT combined with TLTG is both safe and feasible. However, the total complication rates in the LATG and OTG groups were 15.5% and 19.5%, respectively, which were significantly different from those in the above mentioned studies. There was no statistical significance among the three groups. This may be because the above mentioned studies were single-center retrospective studies, and follow-up bias was inevitable. Additionally, our center has been performing enhanced recovery after surgery since 2018, which further reduced the overall complication rate in the LATG and OTG groups. However, the higher incidence of Grade IVanastomotic leakage (3.8% vs. 0% vs. 1.8%, p = 0.449) in the TLTG group in this study is worthy of attention, even there was no statistically significant difference. A previous study [24] reported that preoperative NACT did not increase the risk of anastomotic leakage and that a BMI > 25 kg/m2 and longer operation time were associated with an increased risk of postoperative anastomotic leakage. A domestic single-center retrospective study [25] also showed that an operation time exceeding 240 min was an independent risk factor for postoperative complications in TLTG (odds ratio = 3.021, 95% confidence interval: 1.160–7.868, p = 0.024). Additionally, the learning curve of total laparoscopic surgery may affect the operative time and complication rate [26]. In this study, the surgical operators at each center were experienced. Furthermore, there was no significant difference in BMI among the three groups, and the mean values were less than 25 kg/m2. Therefore, we believe that the high incidence of anastomotic leakage in the TLTG group is not due to the learning curve or BMI; instead, the longer operation time may be a causative factor [25, 27]. Both the technical requirements of full laparoscopic surgery and the difficulty of anastomosis are high, and prolonged excessive traction or damage to the esophagogastric anastomosis may lead to poor anastomosis or aggravate edema and bleeding at the anastomotic site, thereby increasing the risk of anastomotic leakage.
Therefore, future research should focus on further exploring ways to reduce operation time and continuously improve surgical techniques to improve postoperative prognoses.Our results indicate that there is no statistically significant difference in long-term outcomes among the three surgical approaches. Previous studies have confirmed that in gastric cancer populations without neoadjuvant therapy, the surgical approach does not affect long-term survival outcomes [5]. Furthermore, in patients who have undergone neoadjuvant therapy, there is no statistically significant difference in survival between laparoscopy-assisted and open distal gastrectomy [8, 28]. This further demonstrates that laparoscopic surgery does not significantly improve patient survival outcomes but instead plays a more prominent role in promoting postoperative recovery.
As a retrospective analysis, this study has certain limitations. First, the chemotherapy modes of the three groups of patients were different. Some patients received NAT with two drugs, whereas others received NAT with three or even four drugs. Different chemotherapy regimens may have research bias.Second, the sample size in the TLTG group was relatively small, preventing the use of propensity score matching (PSM) to minimize selection bias, and variations in surgical team experience and learning curves across centers could further affect outcomes.Third, due to the lack of follow-up data, it is impossible to evaluate the postoperative quality of life of patients. Additionally, the non-randomized assignment of surgical techniques introduces potential selection bias; although baseline characteristics were comparable, unmeasured confounders such as surgeon expertise or subtle patient differences may have influenced the results. Further research involving larger cohorts or randomized controlled trials is necessary to validate these findings and provide more evidence.
In summary, our research results indicate that although total laparoscopic gastrectomy has a longer operation time, it has the advantages of faster postoperative recovery and early feeding and is generally safe. However, further multicenter, large-sample prospective randomized controlled studies are needed to verify our results and further explore the optimal application strategy of NAT under different treatment plans to further guide clinical practice.
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Authors’ contributions
Jiangpeng Wei, Xiaopeng Gao, Jia Yuan, Ziyang Li, Yanyang Son contributed to the conception, design and assembled the data; Xiaopeng Gao, Jiangpeng Wei, Ziyang Li, XianghuangMei, Guoqiang Huang collected the data. Jiangpeng Wei, Gang Ji, XinGuo contributed to data analysis and interpretation.Xiaopeng Gao, Ziyang Li and Jiangpeng Wei wrote the manuscript. All authors finally approved the manuscript.
Funding
This work was supported by the Shanxi Province Key Medical Research Projects (Grant No. 2023XM054), with Yangyang Song as the fund holder.
Data availability
The corresponding author Jiangpeng Wei had full access to all the data in the study and took responsibility for the data’s integrity and the data analysis’s accuracy.All data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Yuncheng Central Hospital in Shanxi Province (YXLL2023068), the Medical Ethics Committee of the First Affiliated Hospital of Air Force Medical University (KY20232198-C-1), the Medical Ethics Committee of Qinghai Provincial People’s Hospital (2020-154),Air Force 986th Hospital(KYLL-2020-986-28) and the Medical Ethics Committee of Changzhi Medical College Affiliated Heji Hospital (202307).All patients have signed the informed consent form.All research procedures in our study were conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All the authors declare no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaopeng Gao, Ziyang Li and Jia Yuan share co-first authorship.
Contributor Information
Yanyang Song, Email: 13603598188@163.com.
Jiangpeng Wei, Email: weijiangpeng2015@163.com.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Yu W, Xie F, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su PF, Yu JC. Progress in neoadjuvant therapy for gastric cancer. Oncol Lett. 2022;23(6):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jongh C, Cianchi F, Kinoshita T, et al. Surgical techniques and related perioperative outcomes after robot-assisted minimally invasive gastrectomy (RAMIG): results from the prospective multicenter international Ugira gastric registry. Ann Surg. 2024;280(1):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321(20):1983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey MG, Temperley HC, O’Sullivan NJ, et al. Minimally invasive and open gastrectomy for gastric cancer: A systematic review and network Meta-Analysis of randomized clinical trials. Ann Surg Oncol. 2023;30(9):5544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing J, Wang Y, Shan F, et al. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol. 2021;47(8):2023–30. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Shan F, Ying X, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: A randomized clinical trial. JAMA Surg. 2019;154(12):1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Wang C, Li F, et al. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12(1):10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong H, Liu X, Tian Y, et al. Comparison of short- and long-term outcomes between laparoscopic and open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy: a propensity score matching analysis. Surg Endosc. 2023;37(8):5902–15. [DOI] [PubMed] [Google Scholar]
- 11.Zheng HL, Shen LL, Xu BB, et al. Oncological outcomes of laparoscopic versus open radical total gastrectomy for upper-middle gastric cancer after neoadjuvant chemotherapy: a study of real-world data. Surg Endosc. 2023;37(8):6288–97. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer. 2011;14(2):101–12. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisaki M, Mitsumori N, Shinohara T, et al. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc. 2021;35(4):1682–90. [DOI] [PubMed] [Google Scholar]
- 16.Abate M, Drebin H, Shimada S, et al. Feasibility and efficacy of sentinel lymph node mapping in gastric cancer. Ann Surg Oncol. 2024. 10.1245/s10434-024-15642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurokawa Y, Doki Y, Kitabayashi R, et al. Short-term outcomes of preoperative chemotherapy with docetaxel, oxaliplatin, and S-1 for gastric cancer with extensive lymph node metastasis (JCOG1704). Gastric Cancer. 2024;27(2):366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Peña M, Suarez L, Valbuena DE, Rey Chaves CE, Conde Monroy D, Guevara R. Laparoscopic and open gastrectomy for locally advanced gastric cancer: a retrospective analysis in Colombia. BMC Surg. 2023;23(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Lee CM, Hur H, et al. Totally laparoscopic versus laparoscopy-assisted distal gastrectomy: the KLASS-07: a randomized controlled trial. Int J Surg. 2024;110(8):4810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Xiong J, Wang P, et al. Analysis of the safety and efficacy of the self-pulling and latter transected technique in modified overlap anastomosis in total laparoscopic total gastrectomy. Front Oncol. 2024;14: 1334141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Liu DY, Leng J, Tao XM, Wu HQ, Zhu YP. Comparison efficacy and safety of total laparoscopic gastrectomy and laparoscopically assisted total gastrectomy in treatment of gastric cancer. World J Gastrointest Surg. 2024;16(6):1871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SH, Suh YS, Kim TH, et al. Postoperative morbidity and quality of life between totally laparoscopic total gastrectomy and laparoscopy-assisted total gastrectomy: a propensity-score matched analysis. BMC Cancer. 2021;21(1):1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Wielen N, Straatman J, Daams F, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24(1):258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskins IN, Kroh MD, Amdur RL, Ponksy JL, Rodriguez JH, Vaziri K. The effect of neoadjuvant chemoradiation on anastomotic leak and additional 30-Day morbidity and mortality in patients undergoing total gastrectomy for gastric cancer. J Gastrointest Surg. 2017;21(10):1577–83. [DOI] [PubMed] [Google Scholar]
- 25.Cui H, Cui JX, Wang YN, et al. Could neoadjuvant chemotherapy increase postoperative complication risk of laparoscopic total gastrectomy? A mono-institutional propensity score-matched study in China. World J Gastrointest Surg. 2021;13(5):429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan KS, Oo AM. Learning curve of laparoscopic and robotic total gastrectomy: A systematic review and meta-analysis. Surg Today. 2024;54(6):509–22. [DOI] [PubMed] [Google Scholar]
- 27.Barchi LC, Ramos M, Pereira MA, et al. Esophagojejunal anastomotic fistula: a major issue after radical total gastrectomy. Updates Surg. 2019;71(3):429–38. [DOI] [PubMed] [Google Scholar]
- 28.Etoh T, Ohyama T, Sakuramoto S, et al. Five-year survival outcomes of laparoscopy-assisted vs open distal gastrectomy for advanced gastric cancer: the JLSSG0901 randomized clinical trial. JAMA Surg. 2023;158(5):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author Jiangpeng Wei had full access to all the data in the study and took responsibility for the data’s integrity and the data analysis’s accuracy.All data is provided within the manuscript.




