Abstract
Transforming growth factor (TGF)-β signaling enhances cancer cell plasticity by inducing epithelial-to-mesenchymal transition (EMT). Here, we identified a TGF-β-induced long non-coding RNA, LIMD1 Antisense RNA 1 (LIMD1-AS1) that strengthens the SMAD-mediated transcriptional response to TGF-β. LIMD1-AS1 expression is upregulated in breast cancer tissues compared to normal breast tissues, and high LIMD1-AS1 expression is associated with poor prognosis in breast cancer patients. Depletion of LIMD1-AS1 hinders TGF-β-induced EMT, migration, and extravasation of breast cancer cells. Mechanistically, LIMD1-AS1 promotes the interaction between SMAD3 and its transcriptional coactivator p300, thereby enhancing SMAD3 transcriptional activity and TGF-β/SMAD signaling. We demonstrated that LIMD1-AS1 binds to the MAD homology 2 (MH2) domain of SMAD3 and the interferon-binding domain (IBiD) of p300. Displacing LIMD1-AS1 from p300 by its competitor interferon regulatory factor 3 (IRF3) suppressed the effects of LIMD1-AS1 on potentiating TGF-β/SMAD signaling. Furthermore, blockage of p300 acetyltransferase activity with a pharmacological inhibitor A-485 reduced the ability of LIMD1-AS1 to enhance SMAD3 transcriptional activity, TGF-β-induced EMT, and migration. This study identifies LIMD1-AS1 as a novel stimulator of TGF-β signaling by establishing a positive feedback loop and highlights its potential as a therapeutic target for breast cancer.
Graphical Abstract
Graphical Abstract.
Introduction
Epithelial cancer cells undergo a process called epithelial-to-mesenchymal transition (EMT), in which they lose cell–cell adhesion and transition into a mesenchymal state [1, 2]. This shift is marked by a reduction in the expression of epithelial markers, such as E-cadherin and EpCAM, alongside an increase in the expression of mesenchymal markers, including fibronectin, N-cadherin, and vimentin [3, 4]. EMT endows epithelial cancer cells with enhanced migratory and invasive abilities, allowing them to escape from primary tumors and form metastases in distant organs [2–4]. Moreover, a large proportion of cancer cells are in a hybrid/partial EMT state, characterized by high plasticity, which contributes to their aggressiveness and resistance to chemotherapy [5, 6].
Signaling initiated by the transforming growth factor (TGF)-β cytokine plays a key role in inducing EMT and promoting cancer progression and therapy resistance [7, 8]. Upon TGF-β ligand binding to the serine/threonine kinase type I and type II receptors (TβRI and TβRII), TβRI recruits and phosphorylates the receptor-regulated SMAD (R-SMAD) proteins SMAD2/3. The activated SMAD2 and SMAD3 form complexes with SMAD4, which translocate to the nucleus to regulate the transcription of target genes, such as PAI-1, CTGF, and SMAD7 [9]. Transcriptional coactivators, including p300/CREB-binding protein (CBP) and p300/CBP-associated factor (PCAF), are recruited to the SMAD complex to remodel chromatin status and stabilize the transcriptional machinery [10–12]. The RIG-I-like receptors (RLR) signaling mediator, interferon regulatory factor 3 (IRF3), suppresses TGF-β/SMAD signaling by interacting with SMAD3 to disrupt its association with TβRI, while also interfering with the formation of functional transcriptional complexes between SMAD3 and coactivators, including p300 [11].
Long non-coding RNAs (lncRNAs), defined as longer than 200 nucleotides in length, serve as critical regulatory molecules in modulating signal transduction [12, 13]. LncRNAs can act as scaffolds or decoys by directly interacting with DNA, RNA, and proteins, influencing their activity and stability, or altering their interactions [13]. Additionally, lncRNAs can function as sponges to compete with microRNAs for their messenger RNA (mRNA) binding to affect mRNA degradation and translation [14, 15]. Emerging evidence has revealed that a proportion of lncRNAs possess coding potential to encode small peptides to impact cancer progression [16, 17]. LIMD1 antisense RNA 1 (LIMD1-AS1) is a lncRNA implicated in promoting the progression of prostate cancer [18] and glioma [19], while suppressing non-small-cell lung cancer progression [20]. However, the role of LIMD1-AS1 in breast cancer remains unexplored.
LncRNAs regulate TGF-β signaling at multiple levels to influence EMT and cancer progression [21, 22]. In particular, lncRNAs interact with SMAD3 to modulate SMAD3-driven transcription [23, 24], inhibit its interaction with TβRI [25], retain SMAD3 in the cytoplasm [26], and enhance SMAD3 stability [27]. In this study, we performed a loss-of-function genetic screen and identified TGF-β-induced LIMD1-AS1 as a key lncRNA to potentiate TGF-β signaling. LIMD1-AS1 potentiated TGF-β-induced EMT, migration, and extravasation in breast cancer cells. Mechanistically, LIMD1-AS1 facilitated the interaction between SMAD3 and p300, and thereby promoted SMAD3 transcriptional activity. These findings uncover a lncRNA-directed positive feedback mechanism that regulates TGF-β/SMAD signaling. Targeting LIMD1-AS1 may present a promising therapeutic strategy to restrain TGF-β-induced EMT and breast cancer progression.
Materials and methods
Selection of lncRNA hits
The selection of 107 TGF-β-induced lncRNAs for the CRISPR interference (CRISPRi) screen was based on transcriptome analysis [28] (GEO accession: GSE203119), as detailed in our earlier study [29]. Briefly, RNA-seq was performed on MDA-MB-231 cells stimulated with TGF-β (5 ng/mL) at early (2 h), intermediate (8 h), and late (24 h) time points. A total of 107 lncRNAs that were consistently upregulated across all three time points (compared to 0 h) were selected for inclusion in the CRISPRi screen (Fig. 1A). The complete list of these lncRNAs is available in the referenced study [29].
Figure 1.
A CRISPRi screen identifies LIMD1-AS1 as a TGF-β signaling enhancer. (A) Schematic overview of the selection of 107 TGF-β-induced lncRNAs in MDA-MB-231 cells. (B) Schematic overview of the CRISPRi-mediated lncRNA screen in MDA-MB-231 cells using the (CAGA)12-green fluorescent protein (GFP) SMAD3/SMAD4-dependent transcriptional reporter. (C) Diagram of lncRNA screening results in MDA-MB-231 cells. LncRNAs promoting TGF-β-induced (CAGA)12-GFP reporter activity are indicated in purple, and SMAD3 is indicated in yellow. The x- and y-axes represent the −log10 transformation of the positive robust ranking aggregation (RRA) scores comparing the GFP-high population to the GFP-low population in two replicates. The dashed line indicates the cutoff of −log10 (RRA score) at 1.5. (D-F) Reverse transcription quantitative polymerase chain reaction (RT-qPCR) of LIMD1-AS1 expression in MDA-MB-231 (D), MCF10A-M1 (E), and MCF10A-M2 (F) cells upon TGF-β stimulation for the indicated durations. The results are expressed as the mean ± SEM values from three biological replicates. Significance was calculated using one-way ANOVA followed by Dunnett’s multiple comparisons test. (G) Subcellular localization analysis of LIMD1-AS1 in MDA‐MB‐231 cells by RT-qPCR. NEAT1 is a positive control for the nuclear fraction, whereas H19 and GAPDH are positive controls for the cytoplasmic fraction. The data are presented as the mean ± SEM from three biological replicates. (H) RNA fluorescence in situ hybridization was performed to evaluate LIMD1-AS1 expression and subcellular localization in MDA-MB-231 cells. Representative images are shown in the left panel, and signal quantification data are shown in the right panel. Scale bar = 4.64 μm. The results from 28 (−TGF-β) and 20 (+TGF-β) cells are quantified as a box plot with min-to-max whiskers using unpaired Student’s t-test. (I) Schematic working model. TGF-β induces the expression of LIMD1-AS1, which promotes the TGF-β-induced transcriptional response. *0.01 < P < 0.05; ***0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P < 0.0001.
Lentiviral CRISPRi screen
MDA-MB-231-CAGA12-GFP cells were created by lentiviral, single-copy integration of CAGA12-GFP and isolating a single cell clone that showed good induction in response to TGF-β. pLenti-dCas9-ZIM3-KRAB- blue fluorescent protein (BFP) (Addgene, 196712) was packaged into lentivirus in HEK293T cells using plasmids psPAX2 (Addgene, 12260, a gift from Didier Trono) and pCMV-VSV-G (Addgene, 8454, a gift from Robert A. Weinberg). The virus-containing supernatant was concentrated 40-fold with Lenti-X concentrator (Takara), aliquoted, and stored in liquid nitrogen. MDA-MB-231-CAGA12-GFP cells were then transduced with the virus, and a stable BFP-positive population was isolated by repeated cell sorting (Sony SH800). gRNA sequences were designed against the 694 cap analysis of gene expression (CAGE) peaks from the FANTOM5 (GRCh38 v9 DPI clustering) dataset [30, 31] that fell within 500 bp upstream and 200 bp downstream of the transcripts’ annotated start sites, using a −25 to 250 window relative to the maximum of the CAGE peak (Fig. 1B). CRISPick [32, 33] was used to design and rank gRNAs, and the top five ranked gRNAs were included in the library. Four hundred non-targeting control gRNAs and positive control gRNAs against known TGF-β pathway genes were also included. The gRNA library was synthesized (Genscript) and included an anchor sequence to enable the synthesis of multiple libraries on the same array [34], double-stranded using an ultramer containing random sequence labels [35], and amplified with primers CRISPRi_fw and CRISPRi_rev. The anchor sequence was removed by digestion with EcoRV, and the resulting final insert was cloned by Gibson assembly into pLentiGuide-Puro.3xBsmBI (Addgene, 196709) digested with BsmBI. The library plasmid was sequenced by next-generation sequencing (NGS) to confirm gRNA representation and packaged into lentivirus as described earlier. The functional titer of the library virus was estimated from the fraction of surviving cells after transduction with serial dilutions of the virus, followed by puromycin selection. dCas9-ZIM3-KRAB-BFP-expressing MDA-MB-231-CAGA12-GFP cells were then transduced with the library virus in duplicate at an approximate multiplicity of infection of 0.3 and a coverage of 1000 cells per gRNA in the presence of 2 μg/mL polybrene. Transduced cells were selected with 2 μg/ml puromycin. A control sample was harvested on day 5 post transduction (p.t., early time point). Cell numbers per replicate were kept at 1000× coverage throughout. On day 7 p.t., cells were treated with TGF-β (5 μg/ml) for 48 h. An unsorted control sample was harvested, and cells were sorted based on GFP expression, collecting the top and bottom 10% GFP. Genomic DNA was isolated using the QIAamp DNA Blood Mini (Qiagen), and gRNA and RSL sequences were amplified by PCR as described [35], but with modified primers PCR2_fw and PCR3_fw. The amplicon was sequenced (Illumina), reading 20 cycles of Read 1 with custom primer CRISPRSeq, 10 cycles of index read i7 to read the RSL (not used in data analysis), and six cycles of index read i5 for the sample barcode. Next-generation sequencing (NGS) data was analyzed with the MaGeCK software, v.0.5.6 [36]. A summary of lncRNA and gRNA enrichment, along with gRNA sequences, is provided in Supplementary Table S1. Primers used for library construction and NGS sequencing are listed in Supplementary Table S2.
Cell culture and reagents
HEK293T (CRL-1573), A549 (CRM-CCL-185), HepG2 (HB-8065), MDA-MB-231 (CRM-HTB-26), HeLa (CCL-2), and MCF7 (HTB-22) cells were purchased from the American Type Culture Collection. hTERT-immortalized human breast cancer-associated fibroblasts LACAF cells were obtained from Dr Thomas A. Hughes (University of Leeds, Leeds, UK). These cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, 41965062) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, 16000044) and 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific, 15140163). MCF10A-M1 and MCF10A-M2 cells were kindly provided by Dr Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, USA) and cultured in DMEM/F12 (GlutaMAX™ Supplement; Thermo Fisher Scientific, 31331028) containing 5% horse serum (Thermo Fisher Scientific, 26050088), 0.1 μg/mL cholera toxin (Sigma−Aldrich, C8052), 0.02 μg/mL epidermal growth factor (EGF; Sigma−Aldrich, 01-107), 0.5 μg/mL hydrocortisone (Sigma−Aldrich, H0135), 10 μg/mL insulin (Sigma−Aldrich, I6634), and 100 U/mL penicillin/streptomycin. All cell lines were maintained in a 5% CO2, 37°C humidified incubator, tested monthly for mycoplasma contamination, and checked for authenticity by short tandem repeat (STR) profiling. Recombinant TGF-β3 is a kind gift from Dr Andrew Hinck (University of Pittsburgh). A-485 (1 μM unless indicated otherwise; MedChemExpress, HY-107455) and PCAF-IN-2 (5 μM or 10 μM as indicated; MedChemExpress, HY-147895) were used in the cell culture experiments.
Plasmid construction
Full-length LIMD1-AS1 was amplified by PCR from MDA-MB-231 cell-derived complementary DNA (cDNA) and inserted into the lentiviral vector pCDH-EF1α-MCS-polyA-Blast. The LIMD1-AS1 ATG mutant was synthesized as a mini-gene (Integrated DNA Technologies) and ligated into pCDH-EF1α-MCS-polyA-Blast. CRISPR activation (CRISPRa), CRISPRi, and Cas13d gRNAs were inserted into the lentiviral vectors lenti_sgRNA(MS2)_puro optimized backbone (Addgene, 73797), pLKO.1-U6-PURO-AA19 (kindly provided by Dr M.A.F.V. Gonçalves, LUMC, Leiden, The Netherlands), and pHR_hU6-crScaffold_EF1a-PURO (modified from pSLQ5465_pHR_hU6-crScaffold_EF1a-PuroR-T2A-BFP; Addgene, 155307), respectively. LIMD1-AS1 open reading frames (ORFs) were amplified from pCDH-EF1α-LIMD1-AS1-polyA-Blast and cloned to pLV-FLAG-PURO and pLV-GFP-ΔATG-PURO vectors, respectively. PB_L30-3xFLAG-Halo-linker-p300-CTR-ΔTAZ2_PGK-Puro, PB_L30-3xFLAG-Halo-linker-p300-CTR-ΔIBiD_PGK-Puro, and PB_L30-3xFLAG-Halo-linker-p300-IBiD_PGK-Puro plasmids were constructed by PCR and re-cloning into the corresponding vectors. All plasmids were verified by Sanger sequencing, and the primers used for plasmid construction are listed in Supplementary Table S4.
siRNA transfection
For transfection of non-targeting siRNA (Horizon), siRNA ON-TARGETplus targeting SMAD2 (Horizon, L-003561-00-0005), SMAD3 (Horizon, L-020067-00-0005), or SMAD4 (Horizon, L-003902-00-0005), HEK293T cells were seeded at 80% confluence and incubated with complex formed by DharmaFECT transfection reagent 1 (Horizon, T-2001-02) and siRNA (10 nM at final concentration). Medium was changed at 6 h post siRNA transfection. Protein samples for western blot analysis were collected 2 days after transfection.
Lentiviral transduction
Production of lentivirus was performed as described elsewhere [28]. We used two shRNAs [Sigma–Aldrich; TRCN0000323060 (sh1) and TRCN0000322985 (sh2)] for LIMD1 knockdown. pLX-307-LIMD1 (Addgene, 98349) was used for LIMD1 ectopic expression.
RT-qPCR
To detect the expression of TGF-β target genes and LIMD1-AS1, cells were treated with TGF-β (1 ng/ml) or a vehicle control for indicated time points or 8 h, if the treatment duration is not specified. A NucleoSpin RNA kit (Macherey Nagel, 740955) was used to extract RNA from cells. Reverse transcription was performed using a RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific, K1691). The indicated genes were amplified using the synthesized cDNA with specific primer pairs (listed in Supplementary Table S5), and signals were visualized with a CFX Connect Real-Time PCR Detection System (Bio-Rad). GAPDH was used as the reference gene for normalization by the 2−ΔΔCt method. All experiments were performed at least three times, and representative results are shown.
Western blotting
To detect epithelial-to-mesenchymal transition (EMT) marker expression, MCF10A-M2 cells were treated with TGF-β (2.5 ng/ml) or a vehicle control for 3 days in DMEM medium. To check TGF-β-induced p-SMAD2, TGF-β (1 ng/ml) or a vehicle control was added for 1 h. Western blotting was performed as previously described [37]. The primary antibodies are listed in Supplementary Table S6. All experiments were performed at least three times, and representative results are shown.
Transcriptional reporter assays
Luciferase reporter assays were performed as previously described [28] to measure the indicated reporter activities in HEK293T or HepG2 cells. Cells transfected with indicated constructs were serum-starved for 6 h and stimulated with TGF-β (5 ng/mL), bone morphogenic protein (BMP)6 (50 ng/mL), Activin A (50 ng/mL), IL1β (10 ng/mL), Wnt3A conditional medium, or a vehicle control for 16 h. Luciferase activity was measured with the substrate D-luciferin (Promega) and a luminometer (PerkinElmer) and normalized to β-galactosidase activity. MDA-MB-231 cells with stable expression of the CAGA12-dynGFP SMAD3/SMAD4-dependent reporter [38] was used to monitor the TGF-β-induced transcriptional response in an IncuCyte live cell imaging system (Essen BioScience). Cells were serum starved for 16 h and stimulated with TGF-β (0.5 ng/mL) or a vehicle control. GFP intensity was quantified as total green integrated intensity normalized with cell confluence. All experiments were performed three times, and representative results are shown.
F-actin staining
A549 cells were treated with TGF-β (2.5 ng/mL) or a vehicle control for 48 h and incubated with phalloidin conjugated with Alexa Fluor 488 (1:500 dilution; Thermo Fisher Scientific, A12379) as described before [37]. Images were acquired with a Leica SP8 confocal microscope (Leica Microsystems). Quantification of F-actin fluorescence intensity was performed using the ImageJ software. Experiments were performed twice, and representative results are shown.
MTS cell proliferation assays
MTS assays were performed to evaluate the cell viability following the manufacturer’s instructions (Promega, G3581). Cells were seeded at a density of 1 × 103 cells in wells of 96-well plates (Corning). The absorbance of the samples was measured at 490 nm with a luminometer at the indicated days after seeding.
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) experiments were performed as previously described [37]. Briefly, HEK293T cells were transfected with the indicated plasmids using polyethyleneimine. Forty-eight hours post-transfection, cells were lysed using TNE lysis buffer, centrifuged, and incubated with anti-FLAG agarose beads (Sigma–Aldrich, A2220) for 30 min at 4°C with gentle rotation. Beads were washed 5 times with TNE buffer for 5 min with rotation. Afterward, samples were boiled with 1× sample buffer for 5 min and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis.
Sequential co-IP
HEK293T cells transfected with the indicated plasmids were lysed using TNE lysis buffer. After centrifugation, the clarified lysates were incubated with anti-FLAG agarose beads (Sigma–Aldrich, A2220) for 30 min at 4°C with gentle rotation. Beads were then washed 5 times with TNE buffer for 5 min with rotation. Ten percent of beads were collected for SDS–PAGE analysis. The remaining beads were incubated with FLAG® peptide (Sigma–Aldrich, F3290) at a final concentration of 100 μg/mL for 1 h at 4°C with rotation to elute FLAG-tagged proteins. The eluates were then divided equally into two Eppendorf tubes and incubated with either 1.5 μL normal IgG control (Cell Signaling Technology, 5415) or anti-MYC antibody (Sigma–Aldrich, M4439) for 16 h at 4°C with rotation. The immune complexes were subsequently captured by incubating with 40 μL of Protein G Sepharose (GE Healthcare, 17-0618-02) for 3 h at 4°C with rotation. Beads were washed 5 times with the TNE buffer for 5 min with rotation and then split into two portions. One portion was boiled in 1× sample buffer for 5 min and analyzed by SDS–PAGE, while the other portion was used for RNA extraction followed by RT-qPCR.
RNA pull-down
A MEGAscript Kit (Thermo Fisher Scientific, AM1334) was used for in vitro RNA synthesis as previously described [29]. Fifty picomoles of RNA were biotinylated with an RNA 3′ End Desthiobiotinylation Kit (Thermo Fisher Scientific, 20160). The tertiary structure of each lncRNA was recovered by 10 min of incubation at 70°C followed by gradual cooling to room temperature. HEK293T cell lysates expressing FLAG-p300 or 6xMYC-SMAD3 were incubated with biotinylated LIMD1-AS1-AS, LETS1, LIMD1-AS1, or its truncation mutants for 16 h at 4°C. Proteins were eluted from the beads and analyzed by western blotting. Experiments were performed three times, and representative images are shown.
Transwell migration assays
An IncuCyte Clearview 96-well plate (Essen BioScience, 4582) was used to seed the cells. An IncuCyte live cell imaging system (Essen BioScience) was used to monitor chemotactic cell migration as previously described [39]. Cells were treated with TGF-β (5 ng/mL) or a vehicle control during the assays. Cells in the top and bottom chambers were imaged and quantified using the IncuCyte system. Experiments were performed twice, and representative results are shown.
Subcellular fractionation
Cytoplasmic and nuclear fractions were collected from MDA-MB-231 cells as previously described [28]. Experiments were performed three times, and representative results are shown.
RNA immunoprecipitation
RNA immunoprecipitation (RIP) was performed with a Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Merck Millipore, 17-700) as previously described [28, 29]. Anti-FLAG antibody (Sigma−Aldrich, F3165), anti-MYC antibody (Sigma–Aldrich, M4439), or normal IgG control, was incubated with HEK293T cell lysates for 16 h at 4°C. RNA was extracted from the beads, and RT-qPCR was performed as mentioned earlier. Experiments were performed three times, and representative results are shown.
Proximity ligation analysis
Proximity ligation analysis (PLA) was performed to analyze the endogenous interaction between p300 and SMAD3 as previously described [28]. MDA-MB-231 cells were treated with TGF-β (5 ng/mL) or a vehicle control for 1 h. Cells were fixed and incubated with primary antibodies against SMAD3 (Abcam, ab208182) and p300 (Abcam, ab14984) at a 1:500 dilution for 16 h at 4°C. PLUS and MINUS PLA probes conjugated to secondary antibodies (Sigma−Aldrich, DUO92001 and DUO92005) were used to incubate cells for 1 h at 37°C. Ligase (Sigma−Aldrich, DUO92008) was added to the cells for a 30-min incubation before Duolink® Polymerase (Sigma−Aldrich, DUO82028) incubation for 90 min at 37°C. Images were acquired with a Leica SP8 confocal microscope (Leica Microsystems). Experiments were performed twice, and representative images are shown.
In situ hybridization staining
MDA‐MB‐231 cells were treated with TGF-β (5 ng/mL) or a vehicle control for 8 h. An RNAScope® Multiplex Fluorescent Kit (Advanced Cell Diagnostics, 323100) was utilized to evaluate the expression and localization of LIMD1-AS1 in cells, as described elsewhere [29]. A LIMD1-AS1-specific probe set (Advanced Cell Diagnostics, 1271931-C1), comprising 20 pairs of probes targeting the 885–2185 region of LIMD1-AS1, was employed for detection. Images were acquired with a Leica SP8 confocal microscope (Leica Microsystems). Representative results from two independent experiments are shown. To analyze LIMD1-AS1 expression in patient samples, in situ hybridization was performed in tissue microarrays using a 2.5 HD Detection Kit–BROWN (Advanced Cell Diagnostics, 322300). A tissue microarray with breast cancer and matched breast tissues (Biomax, BR804b) and a tissue microarray with invasive breast cancer tissues (Biomax, BC081116e) were used for LIMD1-AS1 in situ hybridization staining. Images were acquired with a digital slide scanner (Pannoramic 250 Flash III, 3DHISTECH). The staining index was quantified by the following formula: staining intensity (0, no staining; 1, light brown; 2, brown; 3, dark brown) × proportion of positive cells (0, no positive cells; 1, <10%; 2, 10%–50%; 3, >50%). The staining was independently evaluated by two researchers in a blinded manner, yielding similar staining index scores.
Embryonic zebrafish extravasation assay
The experiments were conducted in a licensed establishment for the breeding and use of experimental animals (LU) and subject to internal regulations and guidelines, stating that advice is taken from the animal welfare body to minimize suffering for all experimental animals housed at the facility. The zebrafish assays described are not classified as animal experiments under the Experiments on Animals Act (Wod, effective 2014), the applicable legislation in the Netherlands in accordance with the European guidelines (EU directive no. 2010/63/EU) regarding the protection of animals used for scientific purposes, because non-self-eating larvae were used. Therefore, no license specific to these assays on zebrafish larvae (<5 days post fertilization) was required. MDA-MB-231 cells labeled with mCherry were injected into the duct of Cuvier of embryos from transgenic zebrafish (fli; EGFP) as previously described [40]. An inverted SP5 STED confocal microscope (Leica Microsystems) was used to visualize the injected cancer cells and zebrafish embryos.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed as previously described [29, 41]. In brief, 1 × 107 MDA-MB-231 cells were collected, cross-linked with 1% formaldehyde, and sonicated into 200–700 bp fragments using sonication beads (Diagenode, C01020031). The collected supernatant was diluted 5 times and incubated with 5 μg of IgG (Cell Signaling Technology, 2729) or anti-SMAD3 antibody (Abcam, ab208182) for 16 h at 4°C with rotation. The next day, the antibody-chromatin complex was captured by 30 μL of Protein A Sepharose beads (GE Healthcare, 17-0963-03). After washing 5 times, ribonuclease A (Thermo Fisher Scientific, R1253) and proteinase K (Thermo Fisher Scientific, 25530049) treatment, DNA was extracted by isopropanol. RT-qPCR was performed to quantify protein enrichment at the regions of interest. Representative results from three independent experiments are shown.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 10.2.3. P < 0.05 was considered statistically significant. All measurements in this study were taken from distinct samples.
Results
A CRISPRi functional screen identifies LIMD1-AS1 as a TGF-β signaling potentiator
TGF-β-induced gene products, including lncRNAs, often function as feedback regulators of TGF-β signaling [21, 41]. To identify critical lncRNAs that potentiate TGF-β signaling in breast cancer cells, we conducted a CRISPRi-based genomic screen [42] targeting 107 lncRNAs potently induced by TGF-β in a triple-negative breast cancer cell line, MDA-MB-231 [28, 29] (Fig. 1A and B and “Materials and Methods” section). We designed five gRNAs for each lncRNA and transduced them as a pool into MDA-MB-231 cells, which stably express dCas9-ZIM3-KRAB [43] for transcriptional inhibition (Fig. 1B). A selective synthetic SMAD3/4-driven transcriptional reporter, CAGA12-dynGFP [38], was also expressed in the same MDA-MB-231 cells to track the TGF-β/SMAD-induced transcriptional activity (Fig. 1B). Upon TGF-β stimulation for 48 h, cells were sorted into the GFP-high and GFP-low groups (Fig. 1B and “Materials and Methods” section). LncRNA hits were ranked based on the −log10 transformation of positive robust rank aggregation (RRA) scores comparing GFP-high and GFP-low populations across two biological replicates (Fig. 1C). A RRA score threshold of 1.5 was applied to identify lncRNA candidates that function as positive regulators of TGF-β-induced GFP expression. Notably, seven candidate lncRNAs (marked in purple) were found to enhance the TGF-β-induced transcriptional response as potently as SMAD3 (Fig. 1C). After assessing the enrichment of the five gRNAs targeting each candidate lncRNA, we focused on one of the top hits, LIMD1-AS1, for further investigation.
The kinetic evaluation of LIMD1-AS1 expression following TGF-β treatment revealed that LIMD1-AS1 is an early induced target gene of TGF-β, with sustained induction observed for up to 72 h in MDA-MB-231 cells (Fig. 1D), MCF10A-M1 normal breast cells (Fig. 1E), and MCF10A-M2 pre-malignant breast cells (Fig. 1F). The localization of lncRNAs often hints at their mechanism of action [13, 22]. To this end, we performed subcellular fractionation followed by RT-qPCR to determine the distribution of LIMD1-AS1 in MDA-MB-231 cells. LIMD1-AS1 was mainly localized in the nucleus of MDA-MB-231 cells (Fig. 1G), which was further consolidated by fluorescence in situ hybridization (FISH) with a LIMD1-AS1-specific probe set, which targets the 885–2185 region of LIMD1-AS1 (Fig. 1H). The specificity of the probe set for detecting LIMD1-AS1 was validated in MDA-MB-231 cells following CRISPR/Cas13d [44]-mediated LIMD1-AS1 knockdown (Supplementary Fig. S1A). Next, we characterized the full-length LIMD1-AS1 sequence using cDNA derived from MDA-MB-231 cells as a template for amplification. We identified two exons in LIMD1-AS1, which is inconsistent with the National Center for Biotechnology Information (NCBI) database annotation indicating that LIMD1-AS1 comprises three exons (Supplementary Fig. S1B and C). This observation was confirmed in multiple cell lines, including HEK293T kidney epithelial cells, LACAF cancer-associated fibroblasts, HeLa cervical cancer cells, MCF10A-M2 cells, and MCF7 luminal breast cancer cells (Supplementary Fig. S1C and D). We have included the validated full-length LIMD1-AS1 sequence in Supplementary Table S3. Taken together, our findings identify LIMD1-AS1 as a TGF-β-induced lncRNA that promotes the TGF-β-induced transcriptional response in breast cancer cells (Fig. 1I).
LIMD1-AS1 promotes TGF-β signaling independent of its coding potential
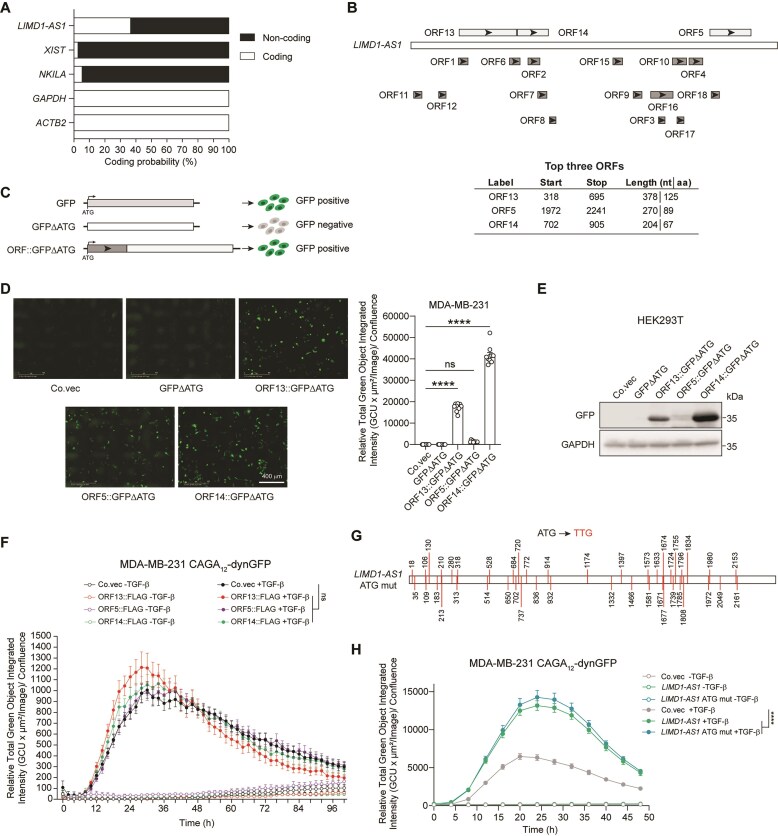
Emerging evidence suggests that lncRNAs are capable of encoding small functional peptides in cancer cells [16, 17]. Bioinformatic analysis with Coding Potential Assessment Tool (CPAT) [45] predicted that the coding potential of LIMD1-AS1 is higher than that of the well-characterized lncRNAs XIST and NKILA, suggesting that LIMD1-AS1 may encode small peptides (Fig. 2A). Although a 100-amino acid (aa) threshold has been conventionally used to predict functional open reading frames (ORFs), recent studies have demonstrated that small peptides with fewer than 100 aa can exert pivotal biological functions [46, 47]. To explore the coding potential of LIMD1-AS1, we used the NCBI ORF Finder tool (https://www.ncbi.nlm.nih.gov/orffinder/) and identified 18 putative ORFs (Fig. 2B and Supplementary Table S3). We selected the top three candidate ORFs longer than 65 aa for further coding potential analysis, while acknowledging that shorter ORFs may also have functional relevance (Fig. 2B and Supplementary Table S3). We generated reporter constructs by placing each LIMD1-AS1 ORF at the 5′ end of a GFP-expressing gene that lacks a start codon (GFP-ΔATG; Fig. 2C). All three ORFs could drive GFP expression in both MDA-MB-231 and HEK293T cells (Fig. 2D and E). However, we failed to observe an effect of FLAG-tagged LIMD1-AS1 ORFs on the TGF-β-induced CAGA12-dynGFP reporter activity in MDA-MB-231 cells (Fig. 2F). To further exclude the possibility that LIMD1-AS1-derived small peptides may play a role in the effect of LIMD1-AS1 on TGF-β signaling, we generated a LIMD1-AS1 ATG mutant in which all the putative start codons were mutated (ATG to TTG; Fig. 2G). Among the 44 ATG start codons identified in LIMD1-AS1, three correspond to the ORFs analyzed above (Fig. 2D and E), while the rest either fall within these three ORFs or generate putative ORFs shorter than 65 aa, which were not included for coding potential analysis (Fig. 2B and Supplementary Table S3). The LIMD1-AS1 ATG mutant enhanced the TGF-β/SMAD-induced transcriptional response in MDA-MB-231 cells to a level comparable to that of wild-type LIMD1-AS1(Fig. 2H and Supplementary Fig. S2). These results suggest that LIMD1-AS1 elicits its effect on promoting TGF-β/SMAD signaling by acting as a non-coding transcript.
Figure 2.
LIMD1-AS1 promotes TGF-β signaling independent of its coding potential. (A) Coding probability prediction of LIMD1-AS1 with the CPAT software. Protein‐coding mRNA (GAPDH and ACTB2) and well‐annotated lncRNAs (XIST and NKILA) serve as positive controls. (B) ORF analysis of LIMD1-AS1 sequence by using the ORF Finder tool from NCBI. Information on the top three ORFs is presented in the table, while details for the remaining ORFs are provided in Supplementary Table S3. (C) Schematic representation of the GFP reporter used to determine the coding potential of ORFs from LIMD1-AS1. (D) Representative images of MDA-MB-231 cells stably expressing LIMD1-AS1 ORFs fused to GFP without the start codon (ΔATG). Co.vec, control empty vector. Scale bar = 400 μm. Signal quantification data are shown in the right panel. Significance was assessed using one-way ANOVA followed by Dunnett’s multiple comparisons test. (E) Western blotting analysis of the expression of LIMD1-AS1 ORFs fused to GFPΔATG in HEK293T cells. GAPDH, loading control. Co.vec, control empty vector. (F) Effect of FLAG-tagged LIMD1-AS1 ORFs on GFP intensity in MDA-MB-231 cells expressing the CAGA12-dynGFP reporter. The results are expressed as mean ± SEM from four biological replicates. Significance was assessed using two-way ANOVA followed by Tukey’s multiple comparisons test. Co.vec, control empty vector. (G) Schematic representation of the LIMD1-AS1 mutant with all 44 ATGs mutated to TTGs (LIMD1-AS1 ATG mut). (H) Effect of ectopic expression of LIMD1-AS1 or LIMD1-AS1 ATG mut on GFP intensity in MDA-MB-231 cells expressing the CAGA12-dynGFP reporter. The results are expressed as mean ± SD from nine biological replicates. Significance was assessed using two-way ANOVA followed by Tukey’s multiple comparisons test. Co.vec, control empty vector. ****P < 0.0001; ns, not significant.
LIMD1-AS1 expression is correlated with poor prognosis in breast cancer patients
To explore the correlation between LIMD1-AS1 expression and breast cancer progression, and to motivate subsequent functional studies in breast cancer cell models, we analyzed several The Cancer Genome Atlas (TCGA) datasets [48] and observed that LIMD1-AS1 was expressed significantly more highly in tumor samples from breast cancer patients compared to normal breast tissue samples (Fig. 3A). A combined analysis of TCGA [48] and Genotype-Tissue Expression (GTEx) project [49] breast cancer datasets demonstrated that LIMD1-AS1 expression was significantly increased in breast cancer patient samples compared to normal breast tissue (Fig. 3B). In situ hybridization in a tissue microarray revealed that LIMD1-AS1 expression was elevated in breast cancer samples compared to matched adjacent normal samples, with a higher expression in 75% (30 of 40) of the tested samples (Fig. 3C). Moreover, LIMD1-AS1 expression was significantly upregulated in breast cancer patient samples with advanced N stage, indicative of lymph node invasion (Fig. 3D). Furthermore, Kaplan–Meier survival analysis demonstrated that high LIMD1-AS1 expression was associated with an unfavorable outcome in two breast cancer patient cohorts [50, 51] (Fig. 3E and F). Taken together, our results suggest that LIMD1-AS1 expression is correlated with poor clinical outcomes in patients with breast cancer.
Figure 3.
LIMD1-AS1 expression is correlated with poor prognosis in breast cancer patients. (A) Comparison of LIMD1-AS1 expression between normal breast tissues (Normal) and breast cancer samples (Tumor) from the TCGA dataset. The results are expressed as the mean ± SEM. Significance was assessed using unpaired Student’s t-test. (B) Comparison of LIMD1-AS1 expression between normal breast tissues (Normal) and breast cancer samples (Tumor) from the TCGA and GTEx datasets. The data were generated via Gene Expression Profiling Interactive Analysis (GEPIA)2 [85]. (C) Quantification of LIMD1-AS1 expression by in situ hybridization in a breast cancer tissue microarray. Representative images (bar = 200 μm) and zoomed images (bar = 50 μm) of in situ hybridization results in breast cancer and matched adjacent normal tissues are shown in the left panel. The comparison of the LIMD1-AS1 staining index between the paired tissues is shown in the right panel. Tissue pairs with higher LIMD1-AS1 expression in the normal tissue than that in the breast cancer tissue are highlighted in green, whereas tissue pairs with lower LIMD1-AS1 expression in the normal tissue than that in the tumor tissue are highlighted in red. Significance was assessed using paired t-test. (D) Quantification of LIMD1-AS1 expression by in situ hybridization in breast cancer tissue microarrays comprising breast cancer samples with different N stages. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (E, F) Kaplan–Meier survival curves in breast cancer patients stratified by LIMD1-AS1 expression. The data in panels (E) and (F) were generated via R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl) and Kaplan–Meier Plotter [86, 87] (https://kmplot.com/analysis/), respectively. *0.01 < P < 0.05; ****P < 0.0001.
LIMD1-AS1 promotes TGF-β/SMAD signaling
To validate the enhancing effect of LIMD1-AS1 on TGF-β/SMAD signaling, we employed the CRISPR activation (CRISPRa) [52] system to upregulate endogenous LIMD1-AS1 expression. As expected, CRISPRa-mediated activation led to a pronounced increase in LIMD1-AS1 expression, with 15- and 30-fold upregulation observed using two independent gRNAs, respectively, which substantially exceeded the induction achieved by TGF-β stimulation alone (∼3-fold; Fig. 4A). Consistent with the initial CRISPRi screen results, CRISPRa-mediated LIMD1-AS1 overexpression significantly enhanced TGF-β-induced CAGA12-dynGFP reporter activity in MDA-MB-231 cells (Fig. 4B). Ectopic LIMD1-AS1 expression also potentiated TGF-β/SMAD signaling in MDA-MB-231 cells (Supplementary Fig. S3A and B). On the contrary, Cas13d-mediated LIMD1-AS1 knockdown mitigated the TGF-β/SMAD-induced transcriptional response in MDA-MB-231 cells (Fig. 4C and D). In line with these results, CRISPRa-mediated LIMD1-AS1 overexpression promoted, whereas Cas13d-mediated LIMD1-AS1 knockdown attenuated, TGF-β-induced expression of target genes, including PAI-1, SMAD7, SNAI1, and PTHRP (Fig. 4E and F, and Supplementary Fig. S3C and D). In the absence of TGF-β stimulation, LIMD1-AS1 had no significant effect on the expression of these genes (Fig. 4E and F, Supplementary Fig. S3C and D). These results were further consolidated by LIMD1-AS1 ectopic expression (Supplementary Fig. S3E). Moreover, analyses of the TCGA breast cancer samples revealed a positive correlation between LIMD1-AS1 expression and the TGF-β response gene signature [53] (Fig. 4G). We further checked the impact of LIMD1-AS1 on TGF-β-induced SMAD2 and SMAD3 phosphorylation, which is a direct indicator of TGF-β receptor activity. However, LIMD1-AS1 did not affect TGF-β-induced SMAD2 and SMAD3 phosphorylation in MDA-MB-231 cells (Fig. 4H–J and Supplementary Fig. S3F), indicating that LIMD1-AS1 affects TGF-β/SMAD signaling downstream of the R-SMAD activation. In addition, we observed that the activities of selective transcriptional reporters for BMP/SMAD signaling, Activin/SMAD signaling, WNT/β-catenin signaling, and interleukin (IL)1β/NFκB signaling remained unchanged upon LIMD1-AS1 ectopic expression (Supplementary Fig. S4A–D), suggesting that LIMD1-AS1 selectively regulates TGF-β/SMAD signaling.
Figure 4.
LIMD1-AS1 promotes TGF-β/SMAD signaling. (A, C)The efficiency of CRISPRa-mediated LIMD1-AS1 overexpression (A) and Cas13d-mediated LIMD1-AS1 knockdown (C) with two independent gRNAs in MDA-MB-231 cells. gEV, empty vector for gRNA expression. The data are shown as mean ± SEM (A) and mean ± SD (C) from three biological replicates. Significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (B, D) Effect of CRISPRa-mediated LIMD1-AS1 overexpression (B) and Cas13d-mediated LIMD1-AS1 knockdown (D) with two independent gRNAs on GFP intensity in MDA-MB-231 cells expressing the CAGA12-dynGFP reporter. The results are expressed as mean ± SEM from nine (B) and four (D) biological replicates. Significance was assessed using two-way ANOVA followed by Dunnett’s multiple comparisons test. gEV, empty vector for gRNA expression. (E, F) Effect of CRISPRa-mediated LIMD1-AS1 overexpression (E) and Cas13d-mediated LIMD1-AS1 knockdown (F) with two independent gRNAs on TGF-β-induced PAI-1 and SMAD7 expression in MDA-MB-231 cells. RT-qPCR results are shown as mean ± SEM (E) and mean ± SD (F) from three biological replicates. Significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. gEV, empty vector for gRNA expression. (G) Correlation between LIMD1-AS1 expression and TGF-β response gene signature in TCGA breast cancer samples. The data were generated via R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl). The statistical analysis was performed using Pearson’s correlation (r) test. (H-J) Effect of CRISPRa-mediated LIMD1-AS1 overexpression (H), Cas13d-mediated LIMD1-AS1 knockdown (I), and LIMD1-AS1 ectopic expression (J) on TGF-β-induced SMAD2 phosphorylation (p-SMAD2) in MDA-MB-231 cells. The p-SMAD2 and total SMAD2 (t-SMAD2) levels were analyzed by western blotting. GAPDH, loading control. Co.vec and gEV, control empty vectors. *0.01 < P < 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P < 0.0001; ns, not significant.
LIMD1-AS1 enhances TGF-β-induced EMT, migration, and extravasation
To investigate the impact of LIMD1-AS1 on TGF-β-induced EMT, we ectopically expressed LIMD1-AS1 in epithelial MCF10A-M2 cells (Fig. 5A). LIMD1-AS1 overexpression, achieved either by ectopic expression or CRISPRa-mediated activation, enhanced TGF-β-induced suppression of the epithelial marker E-cadherin and upregulation of mesenchymal markers, including N-cadherin, fibronectin, and vimentin (Fig. 5B and Supplementary Fig. S5A and B). On the contrary, Cas13d-mediated LIMD1-AS1 depletion reduced the changes in TGF-β-induced EMT marker expression in MCF10A-M2 cells (Fig. 5C and D). In line with these findings, CRISPRa-directed LIMD1-AS1 overexpression promoted TGF-β-induced filamentous actin (F-actin) stress fiber formation in non-small-cell lung adenocarcinoma A549 cells, a commonly used model to investigate TGF-β-induced EMT (Fig. 5E). We observed a positive correlation between LIMD1-AS1 expression and the EMT gene signature [54] in TCGA breast cancer samples (Fig. 5F). Since bulk RNA-seq data include non-cancerous cells, particularly stromal cells like cancer-associated fibroblasts, their presence can elevate the mesenchymal score, potentially influencing the overall EMT score [55, 56]. Furthermore, we found that LIMD1-AS1 overexpression enhanced, whereas LIMD1-AS1 knockdown reduced, TGF-β-induced cell migration in MDA-MB-231 cells (Fig. 5G–I). However, the viability of MDA-MB-2231 cells remained unaffected upon LIMD1-AS1 ectopic expression (Supplementary Fig. S5C). We further expanded our findings by examining the role of LIMD1-AS1 in TGF-β-induced inhibition of cell proliferation in normal human keratinocyte HACAT cells. In this context, LIMD1-AS1 enhanced TGF-β-induced expression of the cell proliferation inhibitory genes p15 and p21, as well as the canonical TGF-β target gene PAI-1 (Supplementary Fig. S5D). Moreover, LIMD1-AS1 ectopic expression potentiated TGF-β-induced inhibition of cell proliferation in HACAT cells (Supplementary Fig. S5E). These findings suggest that LIMD1-AS1 enhances the cytostatic effect of TGF-β in normal epithelial cells, in addition to promoting TGF-β-induced EMT in cancer cells. Next, we applied an in vivo zebrafish embryo xenograft model [40] to evaluate the effect of LIMD1-AS1 on cancer cell extravasation (Fig. 5J). We found that Cas13d-mediated LIMD1-AS1 depletion suppressed MDA-MB-231 cell extravasation in zebrafish embryos (Fig. 5K). Collectively, our results demonstrate that TGF-β-induced LIMD1-AS1 promotes TGF-β-induced EMT, migration, and extravasation of breast cancer cells.
Figure 5.
LIMD1-AS1 enhances TGF-β-induced EMT, migration, and extravasation. (A, C) Efficiency of LIMD1-AS1 ectopic expression (A) and Cas13d-mediated LIMD1-AS1 knockdown (C) in MCF10A-M2 cells. Co.vec and gEV, control empty vectors. RT-qPCR results are shown as mean ± SD from three independent experiments (A) and three biological replicates (C). Significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (B, D) Effect of LIMD1-AS1 on TGF-β-induced EMT marker expression in MCF10A-M2 cells upon LIMD1-AS1 ectopic expression (B) and Cas13d-mediated LIMD1-AS1 knockdown (D). GAPDH, loading control. Co.vec and gEV, control empty vectors. (E) Immunofluorescence analysis of F-actin expression and localization in A549 cells upon CRISPRa-mediated LIMD1-AS1 overexpression with two independent gRNAs. Scale bar = 36.8 μm. gEV, empty vector for gRNA expression. Quantification results of the relative F-actin fluorescence intensity are shown as mean ± SD from three biological replicates. Significance was assessed using two-way ANOVA followed by Dunnett’s multiple comparisons test. (F) Correlation between LIMD1-AS1 expression and EMT gene signature in TCGA breast cancer samples. The data were generated via R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl). The statistical analysis was performed using Pearson’s correlation (r) test. (G) Schematic representation of the transwell migration assay. (H, I) Effect of LIMD1-AS1 on TGF-β-induced migration in MDA-MB-231 cells as analyzed by a transwell migration assay. LIMD1-AS1 overexpression was achieved by ectopic expression (H), and LIMD1-AS1 knockdown was achieved by Cas13d-mediated knockdown (I). Co.vec and gEV, control empty vectors. The results are expressed as mean ± SEM (H) and mean ± SD (I) from 16 (H) and 6 (I) biological replicates, respectively. Significance was calculated using two-way ANOVA followed by Tukey’s multiple comparisons test. (J) Schematic representation of the zebrafish embryo xenograft assay. (K) In vivo zebrafish xenograft experiments with mCherry-labelled MDA-MB-231 cells upon Cas13d-mediated LIMD1-AS1 knockdown. Representative zoomed images of the tail fin area are shown in the left panel. Extravasated breast cancer cell clusters are indicated with yellow arrows. gEV, empty vector for gRNA expression. Analysis of the extravasated cell cluster numbers is expressed as mean in the right panel. Significance was assessed using one-way ANOVA followed by Dunnett’s multiple comparisons test. Whole zebrafish image, bar = 618.8 μm; zoomed image, bar = 154.7 μm. *0.01 < P < 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P < 0.0001.
LIMD1-AS1 binds and facilitates SMAD3 transcriptional activity
Based on the localization of LIMD1-AS1, we hypothesized that LIMD1-AS1 may interact with SMAD proteins in the nucleus. We performed RNA immunoprecipitation (RIP) followed by RT-qPCR in HEK293T cells with ectopic expression of LIMD1-AS1 and FLAG-tagged SMAD2, SMAD3, or SMAD4. We found that LIMD1-AS1 coprecipitated with SMAD3, but not SMAD2 or SMAD4 (Fig. 6A), indicating that LIMD1-AS1 selectively binds SMAD3. To map the domains of SMAD3 for LIMD1-AS1 binding, we expressed MYC-tagged SMAD3 truncation mutants together with LIMD1-AS1 in HEK293T cells (Fig. 6B and C). RIP-qPCR assays demonstrated that LIMD1-AS1 coprecipitated with the SMAD3 Mad Homology 2 (MH2) domain, but not with the Mad Homology 1 (MH1) or linker domains (Fig. 6D). However, LIMD1-AS1 did not bind to the isolated MH2 domain of SMAD2 or SMAD4 (Supplementary Fig. S6A and B). The MH2 domain of SMAD3 mediates protein–protein interactions to regulate SMAD3 oligomerization, transcriptional complex formation, and degradation [57]. Notably, co-IP experiments demonstrated that LIMD1-AS1 did not influence the formation of complexes between SMAD3 and SMAD4 or between SMAD2 and SMAD4 (Supplementary Fig. S6C). Moreover, ectopic expression of LIMD1-AS1 in MDA-MB-231 cells did not alter SMAD3 mRNA or protein expression (Supplementary Fig. S6D and E). Afterward, we applied a luciferase reporter driven by six galactose-responsive transcription factor GAL4 upstream activating sequences (UASs) to evaluate the effect of LIMD1-AS1 on SMAD3 transcriptional activity (Fig. 6E). We uncoupled SMAD3 transcriptional activation from its DNA binding ability by fusing the GAL4 DNA binding domain (DBD) to SMAD3 (Fig. 6E). LIMD1-AS1 significantly potentiated both basal and TGF-β-induced SMAD3 transcriptional activation in HEK293T cells (Fig. 6F). As a negative control, LIMD1-AS1 failed to enhance the transcriptional activity of GAL4-DBD alone (Fig. 6F). To further rule out the involvement of the MH1 domain in this effect, a SMAD3 truncation mutant GAL4-DBD-SMAD3-ΔMH1 was utilized for transcriptional activity analysis. Although this truncation mutant lost the responsiveness to TGF-β stimulation, its transcriptional activity was still promoted by LIMD1-AS1 in HEK293T cells (Fig. 6F). Of note, the basal transcriptional activity of GAL4-DBD-SMAD3-ΔMH1 was higher than that of GAL4-DBD-SMAD3 (Fig. 6F), consistent with previous reports that SMAD proteins are intrinsically autoinhibited through intramolecular interactions between their MH1 and MH2 domains [58, 59]. Moreover, LIMD1-AS1 did not change the transcriptional activity of reporters driven by SMAD2-ΔMH1 or SMAD4-ΔMH1, suggesting that LIMD1-AS1 specifically promotes SMAD3 transcriptional activity (Fig. 6G). Next, we used siRNA to knock down SMAD2, SMAD3, or SMAD4 to assess their roles in LIMD1-AS1-mediated promotion of TGF-β signaling (Supplementary Fig. S6F). We observed that the effect of LIMD1-AS1 on promoting the TGF-β-induced transcriptional response was reduced upon knockdown of SMAD3 and SMAD4, but not SMAD2, in HEK293T cells (Supplementary Fig. S6G). Although SMAD4 does not directly interact with LIMD1-AS1, this result suggests that it is essential for LIMD1-AS1-mediated regulation of TGF-β/SMAD signaling. Finally, ChIP-qPCR analysis showed that the DNA binding ability of SMAD3 to the promoter of its target genes (PAI-1, SMAD7, and SNAI1) was not affected by LIMD1-AS1 in MDA-MB-231 cells (Fig. 6H). These results suggest that LIMD1-AS1 binds SMAD3 to enhance its transcriptional activity without altering its DNA binding capacity.
Figure 6.
LIMD1-AS1 binds and enhances SMAD3 transcriptional activity. (A) The interactions between LIMD1-AS1 and SMAD proteins in HEK293T cells were analyzed by RIP. RT-qPCR was performed to detect LIMD1-AS1 expression in immunoprecipitates and input. The results are expressed as the mean ± SEM from three biological replicates, and significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (B) Schematic representation of full-length (FL) SMAD3 and the truncation mutants tested. (C) Western blotting was used to analyze the expression of SMAD3 and its truncation mutants in HEK293T cells. Vinculin, loading control. (D) The interactions between LIMD1-AS1 and SMAD3 and its truncation mutants in HEK293T cells were analyzed by RIP. RT-qPCR was performed to detect LIMD1-AS1 expression in immunoprecipitates and input. The results are expressed as the mean ± SEM from three biological replicates, and significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (E) Schematic representation of the luciferase reporter to examine the transcriptional activity of SMAD3. UAS, upstream activating sequence; DBD, DNA binding domain. (F, G) Effect of LIMD1-AS1 on the transcriptional activity of GAL4-DBD-SMAD3 and GAL4-DBD-SMAD3-ΔMH1 (F), as well as GAL4-DBD-SMAD2/3/4-ΔMH1 (G), as determined by luciferase reporter assays. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (H) ChIP-qPCR analysis of the effect of LIMD1-AS1 on SMAD3 binding to the promoter of PAI-1, SMAD7, and SNAI1 in MDA-MB-231 cells. The results are expressed as the mean ± SD from three biological replicates. Significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. **0.001 < P < 0.01; ****P < 0.0001; ns, not significant.
It has been reported that LIMD1-AS1 binds and stabilizes LIM domain-containing protein 1 (LIMD1) mRNA in lung adenocarcinoma cells [20]. LIMD1-AS1 transcribes in the opposite direction to the LIMD1 gene in the human genome (Supplementary Fig. S7A). We found no changes in LIMD1 expression upon LIMD1-AS1 misexpression in MDA-MB-231 cells (Supplementary Fig. S7B–D). To further rule out the influence of LIMD1 on TGF-β/SMAD signaling, LIMD1 was ectopically expressed or depleted by two independent shRNA constructs in MDA-MB-231 cells (Supplementary Fig. S7E and F). We observed that neither LIMD1 overexpression nor knockdown affected the TGF-β/SMAD-induced transcriptional response (Supplementary Fig. S7G and H). Nuclear lncRNAs can affect neighboring gene expression through cis-regulation [12]. LIMD1-AS1 is located in a head-to-head orientation to SAC1 Like Phosphatidylinositide Phosphatase (SACM1L) (Supplementary Fig. S7A). However, we found that LIMD1-AS1 had no effect on the expression of SACM1L (Supplementary Fig. S7B–D). These results suggest that LIMD1-AS1 is unlikely to promote TGF-β/SMAD signaling by acting as an antisense or cis-regulatory lncRNA.
LIMD1-AS1 binds p300 and promotes p300–SMAD3 complex formation
Given that LIMD1-AS1 binds and enhances SMAD3 transcriptional activity, we hypothesized that LIMD1-AS1 may facilitate the formation of the SMAD3/p300 transcriptional complex [60, 61]. First, we performed sequential co-IP followed by RT-qPCR and found that LIMD1-AS1 could be co-precipitated with SMAD3 and p300 in a complex in HEK293T cells, supporting the formation of a ternary complex among these three molecules (Fig. 7A and B). Moreover, LIMD1-AS1 ectopic expression potentiated the interaction between SMAD3 and p300, but had no effect on the interactions between SMAD2 and p300 or SMAD4 and p300 in HEK293T cells (Fig. 7C and Supplementary Fig. S8A). Proximity ligation assay (PLA) consolidated that both basal and TGF-β-induced endogenous SMAD3-p300 interactions were potentiated upon LIMD1-AS1 ectopic expression in MDA-MB-231 cells (Fig. 7D). RIP-qPCR experiments in HEK293T cells confirmed that LIMD1-AS1 interacts with p300 (Fig. 7E). However, p300-induced histone H3 lysine 27 acetylation (H3K27ac), which reflects its intrinsic acetyltransferase activity that can be inhibited by the small-molecule inhibitor A-485 [62, 63], was not affected upon LIMD1-AS1 ectopic expression in MDA-MB-231 cells (Supplementary Fig. S8B). We next performed RNA pull-down assays using LIMD1-AS1 truncation mutants to map the binding regions for SMAD3 and p300. Full-length LIMD1-AS1, but not the negative control LIMD1-AS1 antisense RNA (LIMD1-AS1-AS) or the previously reported nuclear lncRNA LETS1 [41], successfully pulled down both FLAG-tagged p300 and MYC-tagged SMAD3 from HEK293T cell lysates (Fig. 7F). Notably, the LIMD1-AS1 truncation mutants T3 (1807–1702) and T4 (1681–2417) specifically interacted with p300 and SMAD3, respectively (Fig. 7F). We then mapped the binding domains of LIMD1-AS1 on p300 by analyzing p300 truncation mutants (Fig. 7G and Supplementary Fig. S8C). RIP-qPCR analysis showed that the IRF3-Binding Domain (IBiD) within the C-terminal region (CTR) of p300 was responsible for its interaction with LIMD1-AS1 (Fig. 7H and Supplementary Fig. S8D). As IRF3 also binds to the IBiD domain of p300 [11, 64], we tested whether IRF3 can displace LIMD1-AS1 from p300. RIP-qPCR results demonstrated that IRF3-5SD, a constitutively active form of IRF3, disrupted the interaction between LIMD1-AS1 and p300 or its IBiD domain (Fig. 7I and Supplementary Fig. S8E). Consistently, both LIMD1-AS1-induced enhancement of SMAD3 transcriptional activity and the TGF-β-induced transcriptional response were dampened upon IRF3-5SD ectopic expression (Fig. 7J and K). Taken together, our results suggest that LIMD1-AS1 functions as a scaffold to potentiate p300–SMAD3 interaction.
Figure 7.
LIMD1-AS1 promotes p300–SMAD3 interaction. (A, B) Sequential co-IP experiments to confirm the formation of SMAD3/p300/LIMD1-AS1 ternary complex in HEK293T cells. Western blotting analysis (A) was performed to detect MYC and FLAG expression in whole-cell lysates (Input) and immunoprecipitates (IP). GAPDH, loading control. RT-qPCR was subsequently performed to assess LIMD1-AS1 expression in the IP samples (B). The results are shown as mean ± SEM from three biological replicates. Significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (C) The interaction between p300 and SMAD3 upon LIMD1-AS1 ectopic expression was analyzed by co-IP assays in HEK293T cells. Western blotting analysis was performed to detect MYC and FLAG expression in the input and IP samples. Vinculin, loading control. (D) The endogenous interaction between p300 and SMAD3 was evaluated by PLA in MDA-MB-231 cells. The red and blue dots indicate the p300–SMAD3 interaction and the staining of nuclei by DAPI, respectively. Scale bar = 9.2 μm. The quantification of the PLA signal is shown in the right panel. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (E) The interaction between LIMD1-AS1 and MYC-tagged p300 in HEK293T cells, which was analyzed by RIP. RT-qPCR was performed to detect LIMD1-AS1 expression in input and IP samples. The results are expressed as the mean ± SD from three biological replicates, and significance was assessed using unpaired Student’s t-test. (F) The interaction between LIMD1-AS1 and its truncation mutants and FLAG-p300 or 6xMYC-SMAD3 in MDA-MB-231 cells was analyzed by RNA pull-down. LIMD1-AS1 antisense RNA (LIMD1-AS1-AS) and LETS1 lncRNA were used as negative controls. Western blotting analysis was performed to detect FLAG and MYC expression in the input and IP samples. The RNA amounts used for pull-down were evaluated by agarose gel electrophoresis. (G) Schematic representation of the p300 truncation mutants. (H) The interactions between LIMD1-AS1 and the p300 truncation mutants in HEK293T cells were analyzed by RIP. RT-qPCR was performed to detect LIMD1-AS1 expression in immunoprecipitates and input. The results are expressed as the mean ± SEM from three biological replicates, and significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (I) The effect of IRF3-5SD on the interaction between LIMD1-AS1 and FLAG-p300-IBiD in HEK293T cells were analyzed by RIP. RT-qPCR was performed to detect LIMD1-AS1 expression in immunoprecipitates and input. The results are expressed as the mean ± SEM from three independent experiments, and significance was assessed using two-way ANOVA followed by Šídák’s multiple comparisons test. (J) Effect of IRF3-5SD on LIMD1-AS1-induced GAL4-DBD-SMAD3 transcriptional activity in HEK293T cells, as determined by luciferase reporter assays. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (K) Effect of IRF3-5SD on TGF-β/SMAD-induced transcriptional activity, which was enhanced by LIMD1-AS1, in HEK293T cells with a CAGA12 luciferase transcriptional reporter. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. *0.01 < P < 0.05; **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P < 0.0001.
p300 inhibition suppresses LIMD1-AS1-mediated enhancement of SMAD3 transcriptional activity, TGF-β/SMAD signaling, and TGF-β-induced EMT and migration
To further investigate the role of p300 in LIMD1-AS1-mediated enhancement of SMAD3 transcriptional activity, we utilized A-485 [62] to selectively suppress p300 histone acetyltransferase activity. The promoting effect of LIMD1-AS1 on TGF-β-induced SMAD3 transcriptional activity was suppressed by A-485 in a dose-dependent manner, but not by the negative control inhibitor PCAF-IN-2 [65], which blocks PCAF activity (Fig. 8A). Meanwhile, we used the adenovirus early region 1A (E1A) protein to disrupt p300 activity and its interaction with SMAD3 [66, 67]. As expected, E1A ectopic expression reduced the LIMD1-AS1-triggered increase of TGF-β-induced SMAD3 transcriptional activity in HEK293T cells (Fig. 8B). Moreover, A-485 significantly suppressed the enhancing effect of LIMD1-AS1 on the transcriptional activity of GAL4-DBD-SMAD3-ΔMH1 (Fig. 8C) and the TGF-β-induced transcriptional response (Fig. 8D). To further consolidate these results at the transcriptional level under physiological conditions, we tested the engagement of p300 in the role of LIMD1-AS1 in MDA-MB-231 cells. LIMD1-AS1-triggered increase of the expression of TGF-β target genes (PAI-1, CTGF, and IL11) was significantly inhibited by A-485 treatment (Fig. 8E). Additionally, the promotion of TGF-β-induced EMT and migration triggered by LIMD1-AS1 was attenuated by A-485 in MCF10A-M2 cells (Fig. 8F) and MDA-MB-231 cells (Fig. 8G), respectively. Collectively, our results suggest that pharmacological inhibition of p300 acetyltransferase activity suppresses LIMD1-AS1-induced enhancement of SMAD3 transcriptional activity, TGF-β/SMAD signaling, and TGF-β-induced EMT and migration.
Figure 8.
p300 inhibition suppresses LIMD1-AS1-induced promotion of SMAD3 transcriptional activity and TGF-β/SMAD signaling. (A) Effect of A-485 (p300 inhibitor) or PCAF-IN-2 (PCAF inhibitor) on LIMD1-AS1-induced GAL4-DBD-SMAD3 transcriptional activity in HEK293T cells, as determined by luciferase reporter assays. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (B) Effect of E1A on LIMD1-AS1-induced GAL4-DBD-SMAD3 transcriptional activity in HEK293T cells, as determined by luciferase reporter assays. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (C) Effect of A-485 on LIMD1-AS1-induced GAL4-DBD-SMAD3-ΔMH1 transcriptional activity in HEK293T cells, as determined by luciferase reporter assays. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (D) Effect of A-485 on the TGF-β/SMAD-induced transcriptional activity, which was enhanced by LIMD1-AS1, in HEK293T cells with a CAGA12 luciferase reporter. The data are presented as the mean ± SD from three biological replicates. Significance was assessed using one-way ANOVA followed by Tukey’s multiple comparisons test. (E) Effect of A-485 on TGF-β-induced PAI-1, CTGF, and IL-11 expression, which was increased upon LIMD1-AS1 ectopic expression, in MDA-MB-231 cells. RT-qPCR results are shown as mean ± SD from three biological replicates. Significance was assessed by using two-way ANOVA followed by Tukey’s multiple comparisons test. (F) Effect of A-485 on changes in TGF-β-induced EMT marker expression, which was increased upon LIMD1-AS1 ectopic expression, in MCF10A-M2 cells. GAPDH, loading control. (G) Effect of A-485 on TGF-β-induced migration, which was increased upon LIMD1-AS1 ectopic expression, in MDA-MB-231 cells as analyzed by a transwell migration assay. The results are expressed as mean ± SD from four biological replicates. Significance was calculated using two-way ANOVA followed by Tukey’s multiple comparisons test. (H) Schematic of the working model. TGF-β-induced LIMD1-AS1 acts as a scaffold to promote p300–SMAD3 interaction and thereby enhances TGF-β target gene expression to reinforce TGF-β-induced cellular responses and to promote EMT, migration, and invasion in cancer cells. **0.001 < P < 0.01; ***0.0001 < P < 0.001; ****P < 0.0001.
Discussion
In this study, we propose that TGF-β-induced nuclear lncRNA LIMD1-AS1 acts as a scaffold to reinforce SMAD3/p300 transcriptional complex formation, thereby potentiating TGF-β/SMAD signaling in a positive feedback loop. Consequently, TGF-β-induced EMT, migration, and extravasation are enhanced by LIMD1-AS1 in breast cancer cells (Fig. 8H). LIMD1-AS1 has been reported to promote cancer cell migration and invasion by sponging a tumor-suppressive microRNA miR-29c-3p in prostate cancer cells [18]. Moreover, super enhancer-driven LIMD1-AS1 interacts with heat shock protein family A member 5 (HSPA5) to enhance glioma cell migration and invasion [19]. Given the pivotal role of TGF-β signaling in triggering migration and invasion in cancer cells [8, 9], it is likely that the tumor-promoting effects of LIMD1-AS1 observed in these studies may, at least in part, be attributed to its role in potentiating TGF-β signaling.
In our efforts to elucidate the mechanism by which LIMD1-AS1 regulates TGF-β/SMAD signaling, we have ruled out several potential mechanisms. Some lncRNAs can encode small peptides to elicit their functions [16, 17]. We experimentally validated the production of three small peptides from LIMD1-AS1. However, none of them was functionally involved in the regulation of TGF-β/SMAD signaling in breast cancer cells. Furthermore, mutation of all the ATGs within LIMD1-AS1 did not alter its promoting effect on the TGF-β/SMAD-induced transcriptional response, consolidating the notion that LIMD1-AS1 potentiates TGF-β signaling by serving as a lncRNA itself. We observed no change in LIMD1 mRNA expression following either ectopic expression or depletion of LIMD1-AS1, excluding its function as an antisense lncRNA, as reported in lung adenocarcinoma cells [20]. Furthermore, the possibility of cis-regulation by LIMD1-AS1 was eliminated, based on the observation that the expression of its neighboring gene SACM1L remained unaffected upon LIMD1-AS1 misexpression.
Our results show that LIMD1-AS1 interacts with the MH2 domain of SMAD3 to stimulate its transcriptional activity. The MH1 domain of SMAD3 is responsible for DNA binding, while the MH2 domain interacts with TβRI to enable phosphorylation and engages with other proteins to regulate SMAD3 activity and stability [68, 69]. In addition to LIMD1-AS1, LINC01977 and lnc-TSI have also been shown to interact with the MH2 domain of SMAD3 [25, 70], suggesting that the SMAD3 MH2 domain may have the ability to bind a range of lncRNA partners to modulate its activity. Interestingly, although the MH2 domains of SMAD2 and SMAD3 share a 98% primary sequence similarity [71], RIP experiments demonstrated that LIMD1-AS1 selectively binds SMAD3 but not SMAD2. Previous studies also revealed that some lncRNAs selectively interact with SMAD3, but not SMAD2 [23, 25]. Further investigation is needed to determine how the subtle amino acid differences between the MH2 domains of SMAD2 and SMAD3 contribute to their distinct binding abilities to lncRNAs, including LIMD1-AS1.
We revealed that LIMD1-AS1 failed to promote the transcriptional activity of SMAD3 and TGF-β/SMAD signaling upon inhibition of p300 histone acetyltransferase activity by A-485 and E1A. p300 regulates gene transcription by functioning as a histone acetyltransferase to remodel chromatin status [61, 66, 72–73] and acts as a scaffold to facilitate chromatin looping [74, 75]. Besides CBP/p300, SMAD3 can interact with other transcriptional co-activators, such as PCAF [10], Activator Protein 1 (AP-1) [76], and Forkhead box H1 (FoxH1) [77], through its MH2 domain. PCAF does not appear to play a role in LIMD1-AS1-induced SMAD transcriptional activation as the pharmacological PCAF inhibitor has no effect on this response. However, the possibility that LIMD1-AS1 promotes the interaction between SMAD3 and the other co-activators, besides p300, to strengthen TGF-β/SMAD signaling cannot be excluded.
We unraveled that LIMD1-AS1 recruits p300 to SMAD3 by interacting with the IBiD domain of p300. p300 interacts with SMAD3 through its C-terminal transcriptional adapter zinc-binding domain 2 (TAZ2) [78, 79]. Analyses of p300 deletion mutants showed that LIMD1-AS1 could still interact with p300 in the absence of its TAZ2 domain, suggesting that LIMD1-AS1 binds p300 independent of its interaction with SMAD3. Moreover, our results demonstrated that IRF3-5SD, previously shown to interact with IBiD of p300 [11, 64], displaced LIMD1-AS1 from p300 binding. IRF3 can interact with the IBiD domain of p300 to interfere with the formation of SMAD3/p300 transcriptional complex, thereby suppressing TGF-β signaling and TGF-β-induced EMT [11, 64]. Our findings revealed that IRF3-5SD ectopic expression blocked LIMD1-AS1-directed promotion of SMAD3 transcriptional activity and TGF-β/SMAD signaling, indicating that IRF3 may elicit its suppressive effect on TGF-β/SMAD signaling, at least partially, by disrupting the interaction between LIMD1-AS1 and p300. Since IRF3 binds to SMAD3 and inhibits TGF-β signaling through mechanisms beyond disrupting the SMAD3-p300 interaction [11], we cannot exclude the possibility that IRF3 suppresses SMAD3 transcriptional activity independently of its competition with LIMD1-AS1 for p300 binding.
p300-associated lncRNAs have been shown to recruit p300 to targeted genomic loci for chromatin remodeling [80–84]. For example, LncSmad7 guides p300 to enhancers to facilitate histone acetylation and target gene activation in an intrans manner [81]. We cannot eliminate the possibility that LIMD1-AS1 may direct p300, independently of its interaction with SMAD3, to specific chromatin loci for transcriptional regulation. Besides LIMD1-AS1, another TGF-β-induced lncRNA, LINC01977, has been reported to interact with both SMAD3 and p300 to facilitate the establishment of the SMAD3/CBP/p300 complex and the promotion of TGF-β/SMAD signaling in lung adenocarcinoma cells [70]. Whether LIMD1-AS1 and LINC01977 exist in the same SMAD3/p300 transcriptional complex requires further investigation. Moreover, it remains unclear whether these two lncRNAs cooperate with each other or function in a redundant manner to potentiate TGF-β/SMAD signaling in cancer cells.
Our analyses of clinical data and samples demonstrated that LIMD1-AS1 expression was significantly higher in breast cancer samples compared to normal breast tissue. Moreover, high LIMD1-AS1 expression was associated with advanced clinical stages and poor prognosis in breast cancer patients, suggesting that LIMD1-AS1 may serve as a potential biomarker for breast cancer patients. LIMD1-AS1 depletion diminished TGF-β/SMAD signaling, impairing TGF-β-induced EMT and migration in breast cancer cells. These results indicate that targeting LIMD1-AS1 may serve as a promising strategy to inhibit the overactive TGF-β signaling in highly aggressive mesenchymal breast cancer cells. The positive correlations between LIMD1-AS1 expression and both the TGF-β response gene signature and the EMT gene signature in breast cancer patients suggest that LIMD1-AS1 could serve as a clinical marker for identifying patients who may benefit from TGF-β targeted therapies.
Supplementary Material
Acknowledgements
The authors thank Gerard van der Zon for his excellent technical assistance with molecular cloning and Chao Li for providing cDNA samples for the PCR amplification of the LIMD1-AS1 fragment shown in Supplementary Fig. S1C. The authors thank Manuel A.F.V. Gonçalves (Leiden University Medical Centre), Longping Xu (Zhejiang University), and Xinhua Feng (Zhejiang University) for providing plasmids pLKO.1-U6-PURO-AA19, pRK5-MYC-IRF3-5SD, and GAL4-DBD-SMAD4-ΔMH1, respectively. The authors thank Philip H. Howe (Medical University of South Carolina) for providing anti-p-SMAD3 antibody. The authors are also thankful to Xavier Darzacq (University of California) for providing constructs PB_L30-3xFLAG-Halo-linker-p300-NTR_PGK-Puro, PB_L30-3xFLAG-Halo-linker-NLS-p300-Core_PGK-Puro, and PB_L30-3xFLAG-Halo-linker-p300-CTR_PGK-Puro. Part of this work was carried out at the CRISPR Functional Genomics unit funded by SciLifeLab. The results here are in part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga). We also acknowledge support from the Swedish National Genomics Infrastructure (NGI) and the National Academic Infrastructure for Supercomputing in Sweden (NAISS).
Author contributions: C.F. generated and characterized reagents, designed and performed experiments, analyzed the results, and wrote the paper. D.C. and H.M. analyzed the clinical data. M.S., O.K., S.D. and B.S. performed CRISPRi screen and analyzed the results. P.T.D. designed and supervised the research, analyzed the data, and wrote the paper. Q.W. designed and performed experiments, analyzed the results, supervised the research, and wrote the paper. All authors reviewed the manuscript.
Contributor Information
Chuannan Fan, Oncode Institute and Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, 2300 RC, the Netherlands.
Davy Cats, Department of Biomedical Data Sciences, Sequencing Analysis Support Core, Leiden University Medical Center, Leiden, 2300 RC, the Netherlands.
Miriam Selle, Department of Medical Biochemistry and Biophysics, SciLifeLab and Karolinska Institute, Solna, 171 65, Sweden.
Olga Khorosjutina, Department of Medical Biochemistry and Biophysics, SciLifeLab and Karolinska Institute, Solna, 171 65, Sweden.
Soniya Dhanjal, Department of Medical Biochemistry and Biophysics, SciLifeLab and Karolinska Institute, Solna, 171 65, Sweden.
Bernhard Schmierer, Department of Medical Biochemistry and Biophysics, SciLifeLab and Karolinska Institute, Solna, 171 65, Sweden.
Hailiang Mei, Department of Biomedical Data Sciences, Sequencing Analysis Support Core, Leiden University Medical Center, Leiden, 2300 RC, the Netherlands.
Peter ten Dijke, Oncode Institute and Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, 2300 RC, the Netherlands.
Qian Wang, Oncode Institute and Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, 2300 RC, the Netherlands.
Supplementary data
Supplementary data is available at NAR online.
Conflict of interest
None declared.
Funding
This work was supported by the ZonMW grant (09120012010061).
Data availability
The data supporting this study are included in the article and accompanying supplementary files. The raw data of CRISPRi screen from this publication have been deposited to the GEO database and assigned the identifier GSE288909.
References
- 1. Pastushenko I, Blanpain C EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019; 29:212–26. 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D Hallmarks of cancer: new dimensions. Cancer Discov. 2022; 12:31–46. 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 3. Bakir B, Chiarella AM, Pitarresi JR et al. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020; 30:764–76. 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brabletz T, Kalluri R, Nieto MA et al. EMT in cancer. Nat Rev Cancer. 2018; 18:128–34. 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 5. Yang J, Antin P, Berx G et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020; 21:341–52. 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sha YT, Haensel D, Gutierrez G et al. Intermediate cell states in epithelial-to-mesenchymal transition. Phys Biol. 2019; 16:021001. 10.1088/1478-3975/aaf928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan C, Zhang J, Hua W et al.. Boffetta P, Hainaut P Biphasic role of TGF-β in cancer progression: from tumor suppressor to tumor promotor. Encyclopedia of Cancer (Third Edition). 2018; Cambridge, MA; London: Academic Press (Elsevier) 10.1016/B978-0-12-801238-3.64983-8. [DOI] [Google Scholar]
- 8. ten Dijke P, Miyazono K, Heldin CH et al. Special issue: TGF-β and epithelial-mesenchymal transition in cancer. Semin Cancer Biol. 2024; 102-103:1–3. 10.1016/j.semcancer.2024.06.002. [DOI] [PubMed] [Google Scholar]
- 9. Liu S, Ren J, ten Dijke P Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021; 6:8. 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itoh S, Ericsson J, Nishikawa J et al. The transcriptional co-activator P/CAF potentiates TGF-β/Smad signaling. Nucleic Acids Res. 2000; 28:4291–8. 10.1093/nar/28.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu P, Bailey-Bucktrout S, Xi Y et al. Innate antiviral host defense attenuates TGF-β function through IRF3-mediated suppression of Smad signaling. Mol Cell. 2014; 56:723–37. 10.1016/j.molcel.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gil N, Ulitsky I Regulation of gene expression by cis-acting long non-coding RNAs. Nat Rev Genet. 2020; 21:102–17. 10.1038/s41576-019-0184-5. [DOI] [PubMed] [Google Scholar]
- 13. Lin C, Yang L Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018; 28:287–301. 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tay Y, Rinn J, Pandolfi PP The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014; 505:344–52. 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomson DW, Dinger ME Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016; 17:272–83. 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 16. Rion N, Rüegg MA LncRNA-encoded peptides: more than translational noise?. Cell Res. 2017; 27:604–5. 10.1038/cr.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian H, Tang L, Yang ZH et al. Current understanding of functional peptides encoded by lncRNA in cancer. Cancer Cell Int. 2024; 24:252. 10.1186/s12935-024-03446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu Y, He N, Song Z et al. LIMD1-AS1 promotes the progression of prostate cancer and affects the function of prostate cancer cells by down-regulating miR-29c-3p. J Cancer Res Clin Oncol. 2024; 151:5. 10.1007/s00432-024-06046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen ZG, Tian DS, Chen XR et al. Super-enhancer-driven lncRNA LIMD1-AS1 activated by CDK7 promotes glioma progression. Cell Death Dis. 2023; 14:383. 10.1038/s41419-023-05892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan J, Tang Y, Liu S et al. LIMD1-AS1 suppressed non-small cell lung cancer progression through stabilizing LIMD1 mRNA via hnRNP U. Cancer Med. 2020; 9:3829–39. 10.1002/cam4.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues-Junior DM, Moustakas A Unboxing the network among long non-coding RNAs and TGF-β signaling in cancer. Ups J Med Sci. 2024; 129:e10614. 10.48101/ujms.v129.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papoutsoglou P, Moustakas A Long non-coding RNAs and TGF-β signaling in cancer. Cancer Sci. 2020; 111:2672–81. 10.1111/cas.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakai S, Ohhata T, Kitagawa K et al. Long noncoding RNA ELIT-1 acts as a Smad3 cofactor to facilitate TGFβ/Smad signaling and promote epithelial-mesenchymal transition. Cancer Res. 2019; 79:2821–38. 10.1158/0008-5472.CAN-18-3210. [DOI] [PubMed] [Google Scholar]
- 24. Xu L, Liu W, Li T et al. Long non-coding RNA SMASR inhibits the EMT by negatively regulating TGF-β/Smad signaling pathway in lung cancer. Oncogene. 2021; 40:3578–92. 10.1038/s41388-021-01760-2. [DOI] [PubMed] [Google Scholar]
- 25. Wang P, Luo ML, Song E et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway. Sci Transl Med. 2018; 10:eaat2039. 10.1126/scitranslmed.aat2039. [DOI] [PubMed] [Google Scholar]
- 26. Gelabert C, Papoutsoglou P, Golan I et al. The long non-coding RNA LINC00707 interacts with Smad proteins to regulate TGFβ signaling and cancer cell invasion. Cell Commun Signal. 2023; 21:271. 10.1186/s12964-023-01273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ni H, Tang S, Lu G et al. Linc00673-V3 positively regulates autophagy by promoting Smad3-mediated LC3B transcription in NSCLC. Life Sci Alliance. 2024; 7:e202302408. 10.26508/lsa.202302408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan C, Wang Q, Kuipers TB et al. LncRNA LITATS1 suppresses TGF-β-induced EMT and cancer cell plasticity by potentiating TβRI degradation. EMBO J. 2023; 42:e112806. 10.15252/embj.2022112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fan C, Wang Q, Krijger PHL et al. Identification of a SNAI1 enhancer RNA that drives cancer cell plasticity. Nat Commun. 2025; 16:2890. 10.1038/s41467-025-58032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abugessaisa I, Noguchi S, Hasegawa A et al. FANTOM5 CAGE profiles of human and mouse reprocessed for GRCh38 and GRCm38 genome assemblies. Sci Data. 2017; 4:170107. 10.1038/sdata.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lizio M, Harshbarger J, Shimoji H et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015; 16:22. 10.1186/s13059-014-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doench JG, Fusi N, Sullender M et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat Biotechnol. 2016; 34:184–91. 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanson KR, Hanna RE, Hegde M et al. Optimized libraries for CRISPR–Cas9 genetic screens with multiple modalities. Nat Commun. 2018; 9:5416. 10.1038/s41467-018-07901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeWeirdt PC, Sanson KR, Sangree AK et al. Optimization of AsCas12a for combinatorial genetic screens in human cells. Nat Biotechnol. 2021; 39:94–104. 10.1038/s41587-020-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmierer B, Botla SK, Zhang J et al. CRISPR/Cas9 screening using unique molecular identifiers. Mol Syst Biol. 2017; 13:945. 10.15252/msb.20177834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li W, Xu H, Xiao T et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014; 15:554. 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan C, Wang Q, van der Zon G et al. OVOL1 inhibits breast cancer cell invasion by enhancing the degradation of TGF-β type I receptor. Signal Transduct Target Ther. 2022; 7:126. 10.1038/s41392-022-00944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marvin DL, You L, Bornes L et al. Dynamic visualization of TGF-β/SMAD3 transcriptional responses in single living cells. Cancers. 2022; 14:2508. 10.3390/cancers14102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sinha A, Mehta P, Fan C et al. Visualizing dynamic changes during TGF-β-induced epithelial to mesenchymal transition. Methods Mol Biol. 2022; 2488:47–65. [DOI] [PubMed] [Google Scholar]
- 40. Ren J, Liu S, Cui C et al. Invasive behavior of human breast cancer cells in embryonic zebrafish. J Vis Exp. 2017; 122:55459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fan C, González-Prieto R, Kuipers TB et al. The lncRNA LETS1 promotes TGF-β-induced EMT and cancer cell migration by transcriptionally activating a TβR1-stabilizing mechanism. Sci Signal. 2023; 16:eadf1947. 10.1126/scisignal.adf1947. [DOI] [PubMed] [Google Scholar]
- 42. Gilbert LA, Horlbeck MA, Adamson B et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014; 159:647–61. 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alerasool N, Segal D, Lee H et al. An efficient KRAB domain for CRISPRi applications in human cells. Nat Methods. 2020; 17:1093–6. 10.1038/s41592-020-0966-x. [DOI] [PubMed] [Google Scholar]
- 44. Konermann S, Lotfy P, Brideau NJ et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018; 173:665–76. 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, Park HJ, Dasari S et al. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013; 41:e74. 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Su M, Ling Y, Yu J et al. Small proteins: untapped area of potential biological importance. Front Genet. 2013; 4:286. 10.3389/fgene.2013.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leong AZX, Lee PY, Mohtar MA et al. Short open reading frames (sORFs) and microproteins: an update on their identification and validation measures. J Biomed Sci. 2022; 29:19. 10.1186/s12929-022-00802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70. 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Consortium GT The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013; 45:580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Györffy B, Surowiak P, Budczies J et al. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013; 8:e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gyorffy B Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J. 2021; 19:4101–9. 10.1016/j.csbj.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Konermann S, Brigham MD, Trevino AE et al. Genome-scale transcriptional activation by an engineered CRISPR–Cas9 complex. Nature. 2015; 517:583–8. 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Padua D, Zhang XH, Wang Q et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008; 133:66–77. 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Irshad S, Bansal M, Guarnieri P et al. Bone morphogenetic protein and Notch signalling crosstalk in poor-prognosis, mesenchymal-subtype colorectal cancer. J Pathol. 2017; 242:178–92. 10.1002/path.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshihara K, Shahmoradgoli M, Martínez E et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013; 4:2612. 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tyler M, Tirosh I Decoupling epithelial-mesenchymal transitions from stromal profiles by integrative expression analysis. Nat Commun. 2021; 12:2592. 10.1038/s41467-021-22800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi YG, Massagué J Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003; 113:685–700. 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 58. Hata A, Lo RS, Wotton D et al. Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature. 1997; 388:82–7. 10.1038/40424. [DOI] [PubMed] [Google Scholar]
- 59. Heldin CH, Miyazono K, ten Dijke P TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997; 390:465–71. 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 60. Topper JN, DiChiara MR, Brown JD et al. CREB binding protein is a required coactivator for Smad-dependent, transforming growth factor β transcriptional responses in endothelial cells. Proc Natl Acad Sci USA. 1998; 95:9506–11. 10.1073/pnas.95.16.9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng XH, Zhang Y, Wu RY et al. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998; 12:2153–63. 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lasko LM, Jakob CG, Edalji RP et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017; 550:128–32. 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Raisner R, Kharbanda S, Jin LY et al. Enhancer activity requires CBP/P300 bromodomain-dependent histone H3K27 acetylation. Cell Rep. 2018; 24:1722–9. 10.1016/j.celrep.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 64. Qin BY, Liu C, Srinath H et al. Crystal structure of IRF-3 in complex with CBP. Structure. 2005; 13:1269–77. 10.1016/j.str.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 65. Turky A, Bayoumi AH, Ghiaty A et al. Design, synthesis, and antitumor activity of novel compounds based on 1,2,4-triazolophthalazine scaffold: apoptosis-inductive and PCAF-inhibitory effects. Bioorg Chem. 2020; 101:104019. 10.1016/j.bioorg.2020.104019. [DOI] [PubMed] [Google Scholar]
- 66. Pouponnot C, Jayaraman L, Massagué J Physical and functional interaction of SMADs and p300/CBP. J Biol Chem. 1998; 273:22865–8. 10.1074/jbc.273.36.22865. [DOI] [PubMed] [Google Scholar]
- 67. Eckner R, Ewen ME, Newsome D et al. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994; 8:869–84. 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 68. Macias MJ, Martin-Malpartida P, Massague J Structural determinants of Smad function in TGF-β signaling. Trends Biochem Sci. 2015; 40:296–308. 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. ten Dijke P, Hill CS New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004; 29:265–73. 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 70. Zhang T, Xia W, Song X et al. Super-enhancer hijacking LINC01977 promotes malignancy of early-stage lung adenocarcinoma addicted to the canonical TGF-β/SMAD3 pathway. J Hematol Oncol. 2022; 15:114. 10.1186/s13045-022-01331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu JW, Hu M, Chai J et al. Crystal structure of a phosphorylated Smad2. Recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-β signaling. Mol Cell. 2001; 8:1277–89. 10.1016/S1097-2765(01)00421-X. [DOI] [PubMed] [Google Scholar]
- 72. Dai P, Akimaru H, Tanaka Y et al. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996; 10:528–40. 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 73. Lee JS, See RH, Galvin KM et al. Functional interactions between YY1 and Adenovirus E1A. Nucl Acids Res. 1995; 23:925–31. 10.1093/nar/23.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fang F, Xu YF, Chew KK et al. Coactivators p300 and CBP maintain the identity of mouse embryonic stem cells by mediating long-range chromatin structure. Stem Cells. 2014; 32:1805–16. 10.1002/stem.1705. [DOI] [PubMed] [Google Scholar]
- 75. Kagey MH, Newman JJ, Bilodeau S et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010; 467:430–5. 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liberati NT, Datto MB, Frederick JP et al. Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA. 1999; 96:4844–9. 10.1073/pnas.96.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aragon E, Wang Q, Zou Y et al. Structural basis for distinct roles of SMAD2 and SMAD3 in FOXH1 pioneer-directed TGF-β signaling. Genes Dev. 2019; 33:1506–24. 10.1101/gad.330837.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Waltzer L, Bienz M A function of CBP as a transcriptional co-activator during Dpp signalling. EMBO J. 1999; 18:1630–41. 10.1093/emboj/18.6.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nishihara A, Hanai J, Imamura T et al. E1A inhibits transforming growth factor-β signaling through binding to Smad proteins. J Biol Chem. 1999; 274:28716–23. 10.1074/jbc.274.40.28716. [DOI] [PubMed] [Google Scholar]
- 80. Wei L, Li JW, Han ZJ et al. Silencing of lncRNA MALAT1 prevents inflammatory injury after lung transplant ischemia-reperfusion by downregulation of IL-8 via p300. Mol Ther Nucleic Acids. 2019; 18:285–97. 10.1016/j.omtn.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maldotti M, Lauria A, Anselmi F et al. The acetyltransferase p300 is recruited to multiple enhancer sites by lncSmad7. Nucleic Acids Res. 2022; 50:2587–602. 10.1093/nar/gkac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen H, Xiao LS, Xie GH et al. LINC00355 promotes gastric carcinogenesis by scaffolding p300 to activate CDC42 transcription and enhancing HNRNPA2B1 to stabilize CDC42 mRNA dependent on m6A. Mol Carcinog. 2024; 63:430–47. 10.1002/mc.23662. [DOI] [PubMed] [Google Scholar]
- 83. Liu Y, Wang X, Zhu Y et al. The CTCF/LncRNA-PACERR complex recruits E1A binding protein p300 to induce pro-tumour macrophages in pancreatic ductal adenocarcinoma via directly regulating PTGS2 expression. Clin Transl Med. 2022; 12:e654. 10.1002/ctm2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Navarro-Corcuera A, Sehrawat TS, Jalan-Sakrikar N et al. Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex. J Hepatol. 2022; 76:921–33. 10.1016/j.jhep.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang Z, Kang B, Li C et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019; 47:W556–W560. 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gyorffy B Transcriptome-level discovery of survival-associated biomarkers and therapy targets in non-small-cell lung cancer. British J Pharmacology. 2024; 181:362–74. 10.1111/bph.16257. [DOI] [PubMed] [Google Scholar]
- 87. Gyorffy B Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation (Camb). 2024; 5:100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study are included in the article and accompanying supplementary files. The raw data of CRISPRi screen from this publication have been deposited to the GEO database and assigned the identifier GSE288909.