Abstract
Background
Angular kyphosis, often resulting from congenital anomalies, trauma, infections, or tumors, can cause severe spinal cord compression, ischemia, and neurological dysfunction. Due to its sharp curvature and complexity, angular kyphosis remains challenging to treat surgically. This study aimed to establish a rabbit model to mimic the progression of angular kyphosis and its neurological consequences.
Methods
Fifty-six New Zealand white rabbits were divided into four groups: Group A (sham), and Groups B–D (2, 4, and 8 weeks postoperative, respectively). Angular kyphosis was induced via a V-shaped osteotomy between the L2 and L3 vertebrae. Motor function was assessed using Basso-Beattie-Bresnahan Locomotor Rating Scale(BBB scores). Radiological evaluations included Cobb angle and spinal canal occupancy. Histological and apoptosis analyses were conducted to evaluate spinal cord damage.
Results
The induced model reliably produced progressive kyphosis with worsening neurological function. BBB scores declined over time, while Cobb angles and canal occupancy rates increased significantly. Histological examination revealed spinal cord ischemia and increased neuronal apoptosis, aligning with observed motor deficits.
Conclusion
This rabbit model effectively replicates the clinical features of angular kyphosis, including progressive spinal cord compression and neurological impairment. It provides a reliable platform for investigating the pathophysiology of spinal deformities and evaluating therapeutic interventions.
Keywords: Spinal kyphosis with spinal cord ischemia, Animal model, Behavioral studies, Imaging
Introduction
Spinal kyphosis is a common structural disorder of the spine caused by congenital conditions, trauma, infections, or tumors [1–4]. Clinical symptoms vary, and in severe cases, it can lead to spinal cord compression and ischemia, resulting in motor and sensory dysfunction, or even life-threatening complications [5–10]. Angular kyphosis is one of the most frequent types, often associated with a high disability rate and treatment challenges [11]. Surgical intervention alone often fails to significantly improve neurological outcomes [12, 13]. Therefore, establishing an ideal animal model to simulate the progression of angular kyphosis is crucial for advancing its diagnosis and treatment.
Several animal models have been used to study angular kyphosis, including rats, chickens, and cats, with modeling methods such as gene knockout, protease injection, ovariectomy, activity restriction, and laminectomy [14–19]. Although some of these models have successfully induced spinal deformities, they often face limitations such as high cost, technical complexity, long modeling time, and poor reproducibility, making them difficult to apply widely and limiting their ability to reflect clinical progression. In addition, most models rely mainly on imaging to confirm spinal deformity and lack comprehensive evaluation of spinal cord ischemia and injury, which are common in clinical cases. Rabbits offer several advantages for establishing such models: they are widely available, cost-effective to maintain, and easy to handle; their moderate body size is suitable for spinal surgery and functional assessments; they have a short developmental cycle, with skeletal maturity achieved soon after sexual maturity; and they possess strong intracortical bone remodeling ability, with bone metabolism characteristics closer to those of humans [20]. These features make rabbits an ideal species for modeling spinal deformity and spinal cord injury.
An ideal animal model of angular kyphosis should reflect the underlying cause, disease progression, and associated complications. In this study, we induced angular kyphosis in rabbits by surgically damaging the anterior and middle spinal columns without internal fixation, allowing the spine to stabilize and deform naturally. The model was evaluated using behavioral tests, imaging, histological analysis, ischemic lesion assessment, and apoptosis detection, providing a practical platform for studies related to angular kyphosis.
Methods
Experimental object
Fifty-six healthy New Zealand white rabbits, regardless of gender, aged 4–5 months, weighing between 2.5 and 3.5 kg, were purchased from the Department of Experimental Animals at Kunming Medical University. They were housed under identical conditions in individual cages (temperature: 21 ± 3 °C, humidity: 51 ± 19%), and provided with complete nutritional rabbit feed.
Experimental methods
Experimental groups
The rabbits were randomly divided into four groups, each comprising 14 individuals: Group A (sham surgery group), Group B (2 weeks postoperative group), Group C (4 weeks postoperative group), and Group D (8 weeks postoperative group). Before surgery, all experimental animals underwent preoperative spinal anteroposterior and lateral X-rays and spinal MRI. The experiment was conducted strictly in accordance with the guidelines of Kunming Medical University for animal research.
Model establishment
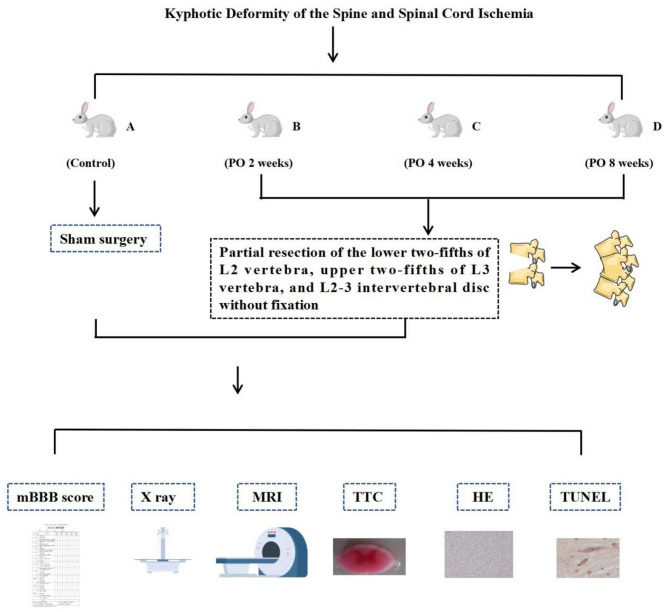
All experimental animals were anesthetized with 3% pentobarbital sodium (1 ml/kg) via the marginal ear vein, positioned in the left lateral decubitus position. The L5-6 intervertebral disc was used as an anatomical landmark, and the L2 and L3 spinous processes and transverse processes were localized. After routine iodine disinfection, a longitudinal incision was made from the lower rib margin to the pelvic margin. The skin, subcutaneous tissue, and bluntly dissected retroperitoneal fat and muscle tissue were sequentially opened to expose the L2 and L3 vertebrae. In Group A, no treatment was performed on the vertebrae. In Groups B, C, and D, a V-shaped osteotomy was performed between the L2 and L3 vertebrae, removing the lower two-fifths of the L2 vertebra, the L2-3 intervertebral disc, and the upper two-fifths of the L3 vertebra. The apex of the V-shaped osteotomy pointed toward the spinal canal and was close to the dura mater, with no internal fixation performed in the osteotomy area. The incision was closed layer by layer, and the animals were housed individually postoperatively.(Fig. 1.)
Fig. 1.
Research framework
Postoperative care
Postoperatively, close attention was paid to the animals’ eating and activity. If bilateral hind limb neurological and motor dysfunction occurred in the awake experimental animals after surgery, acute spinal cord injury caused by modeling during surgery was considered, and these cases were excluded from the statistical analysis.
Evaluation indicators
Behavioral assessment
At 2, 4, and 8 weeks postoperatively, two non-surgical personnel evaluated the hind limb motor function of the animals in each group using the modified Basso-Beattie-Bresnahan Locomotor Rating Scale(BBB scores) rating scale in a blinded manner.
Radiological evaluation
At 2, 4, and 8 weeks postoperatively, spinal X-rays and Magnetic Resonance Imaging(MRI) were performed to evaluate the degree of spinal kyphosis and spinal cord compression in each group. The Cobb angle was defined as the average angle of kyphosis in each group, and the vertebral canal occupation rate was calculated as the ratio of the narrowest anterior-posterior diameter of the compressed segment after surgery to the anterior-posterior diameter before surgery.
Observation of ischemic lesions
The spinal cords of the animals in each group were removed, including the compression zone, pre-compression zone (2 cm from the anterior end of the compression zone), and post-compression zone (2 cm from the posterior end of the compression zone). 2,3,5 - Triphenyl Tetrazolium Chloride Staining(TTC staining) was performed on the spinal cord specimens to observe the size of the ischemic lesions.
Hematoxylin-eosin staining(HE Staining)
HE staining was performed on the spinal cords of the animals in each group from the compression zone, pre-compression zone, and post-compression zone (2 cm from both ends of the compression zone) to observe the condition of the spinal cord, neurons, and glial cells.
Terminal deoxynucleotidyl transferase - mediated DUTP nick end labeling staining (TUNEL Staining)
TUNEL staining was performed on the spinal cords of the animals in each group from the compression zone, pre-compression zone, and post-compression zone (2 cm from both ends of the compression zone) to observe the distribution of TUNEL-positive cells. The ImageJ software was used to count the number of TUNEL-positive cells to determine the distribution and proportion of apoptotic cells.
Blinding
Throughout the entire experimental process, the personnel responsible for anesthesia, model establishment, group allocation, postoperative care, and various outcome assessments operated independently and in a blinded manner to ensure objectivity and reduce bias.
Statistical analysis
Using Statistical Product and Service Solutions(SPSS) 27.0 for statistical analysis, quantitative data are expressed as (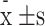 ). One-way analysis of variance (ANOVA) was used for comparisons of means among multiple groups, with the Least Significant Difference (LSD) method applied for pairwise comparisons. Comparisons between two samples were performed using independent-sample t-tests. A P-value of < 0.05 was considered statistically significant.
). One-way analysis of variance (ANOVA) was used for comparisons of means among multiple groups, with the Least Significant Difference (LSD) method applied for pairwise comparisons. Comparisons between two samples were performed using independent-sample t-tests. A P-value of < 0.05 was considered statistically significant.
In this study, rabbits were randomly divided into Groups A, B, C, and D. Group A (sham) served as the control group without surgical intervention, while Groups B, C, and D(2,4,and 8 weeks postoperative) underwent surgical resection of the lower two-fifths of the L2 vertebra, the L2-3 intervertebral disc, and the upper two-fifths of the L3 vertebra. The effectiveness of the model was evaluated using modified Basso-Beattie-Bresnahan Locomotor Rating Scale(BBB scoring), X-ray, Magnetic Resonance Imaging(MRI), 2,3,5 - Triphenyl Tetrazolium Chloride Staining(TTC staining), Hematoxylin-eosin staining(HE staining), and Terminal Deoxynucleotidyl Transferase - Mediated DUTP Nick End Labeling Staining(TUNEL staining).
Result
During the modeling process, one rabbit died due to anesthesia, and three rabbits died from acute spinal cord injury. The deceased animals were promptly replaced.
Behavioral assessment
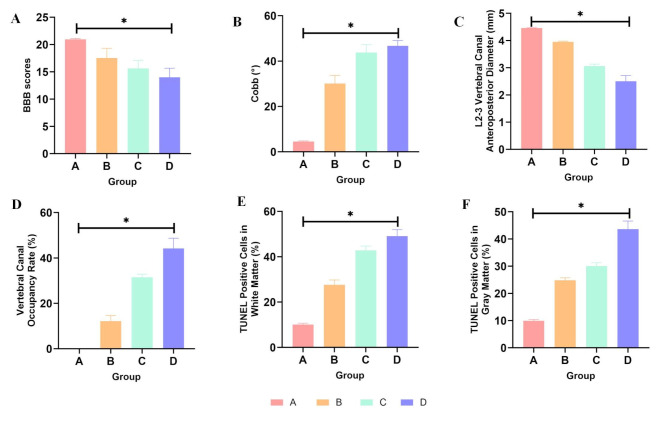
During the observation period, the hind limb motor function of animals in Group A(sham) is normal. Animals in Group B(2 weeks postoperative) exhibit mild impairment in hind limb motor function, characterized by swaying of the hips and uncoordinated walking. Animals in Group C(4 weeks postoperative) show significant coordination deficits in hind limb walking and difficulty bearing weight. In Group D(8 weeks postoperative), animals show obvious instability in walking, and some exhibit urinary and fecal retention. The modified BBB scores are as follows: Group A (20.93 ± 0.18), Group B (17.51 ± 1.80), Group C (15.61 ± 1.49), and Group D (14.00 ± 1.66). There are significant differences between each group (P < 0.05).(Table 1; Fig. 2A).
Table 1.
Modified BBB behavior scores for each group
| Group | Number (individuals) | Modified BBB Behavior Score (points) |
|---|---|---|
| A | 14 | 20.93 ± 0.18 |
| B | 14 | 17.51 ± 1.80 |
| C | 14 | 15.61 ± 1.49 |
| D | 14 | 14.00 ± 1.66 |
Note: ANOVA analysis showed that pairwise comparisons between groups revealed statistically significant differences (P < 0.05)
Fig. 2.
A-F: Statistical Analysis of Evaluation Metrics(ANOVA analysis). A: BBB scores for each group show statistically significant differences between the groups (P<0.05). B: Cobb angle(°) data for each group show statistically significant differences between the groups (P<0.05). C: Data on the L2-3 vertebral canal anteroposterior diameter(mm) for each group show statistically significant differences between the groups (P<0.05). D: Data on vertebral canal occupancy rate(%) for each group show statistically significant differences between the groups (P<0.05). E: Data on TUNEL positive cells in white matter(%) for each group show statistically significant differences between the groups (P<0.05). F: Data on TUNEL positive(%) cells in gray matter for each group show statistically significant differences between the groups (P<0.05)
Radiological evaluation
Spinal X-ray: During the observation period, Group A(sham) does not exhibit spinal kyphosis deformity, while Groups B, C, and D(2,4,and 8 weeks postoperative) show varying degrees of spinal kyphosis deformity. The deformity worsens over time, reaching its maximum at 8 weeks (46.73 ± 2.38)°. There are significant differences in Cobb angle between groups (P < 0.05).(Table 2; Figs. 2B and 3).
Table 2.
Imaging evaluation indicators for each group
| Group | Number (individuals) | Cobb (°) | L2-3 Vertebral Canal Anteroposterior Diameter (mm) | Vertebral Canal Occupancy Rate (%) |
|---|---|---|---|---|
| A | 14 | 4.54 ± 0.26 | 4.46 ± 0.01 | 0 |
| B | 14 | 30.08 ± 3.66 | 3.95 ± 0.03 | 12.14 ± 2.49 |
| C | 14 | 43.77 ± 3.51 | 3.06 ± 0.07 | 31.43 ± 1.44 |
| D | 14 | 46.73 ± 2.38 | 2.50 ± 0.21 | 44.13 ± 4.59 |
Note: ANOVA analysis showed that pairwise comparisons between groups revealed statistically significant differences (P < 0.05)
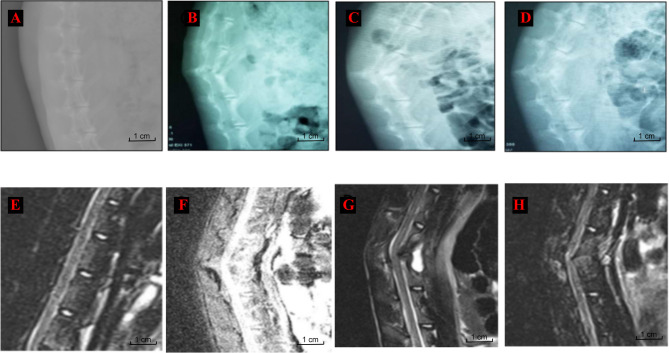
Fig. 3.
Rabbit Spinal Imaging Examination. A and E: Group A (sham) showed no obvious anterior kyphosis deformity(AKD); B and F: Group B (2 weeks postoperative) showed AKD formation with spinal cord compression; C and G: Group C (4 weeks postoperative) showed AKD formation with evident spinal cord compression; D and H: Group C (8 weeks postoperative) showed AKD formation with bony fusion and marked spinal cord compression
Spinal MRI: In Group A (sham), no significant spinal cord compression is observed during the observation period. However, in Groups B, C, and D(2,4,and 8 weeks postoperative), spinal cord compression gradually worsens, with an increase in vertebral canal occupation rate. There are significant differences in spinal cord compression between groups (P < 0.05).(Table 2; Figs. 2C-D and 3).
Observation of ischemic lesions
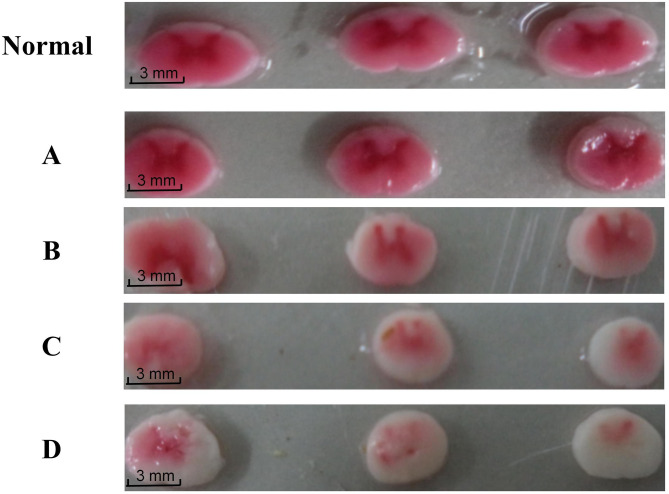
The spinal cords of the animals in each group are removed for observation. In Group A(sham), no obvious abnormalities are observed in the lumbar spinal cord. In Group B(2 weeks postoperative), a concave indentation is observed in the compression zone. In Group C(4 weeks postoperative), the indentation in the compression zone deepens, accompanied by swelling and loss of normal curvature of the spinal cord. In Group D(8 weeks postoperative), the spinal cord segment in the compression zone becomes significantly flattened and extremely irregular. TTC staining results show the following: In Group A(sham), no ischemic phenomena are observed in the spinal cord, similar to normal rabbit spinal cords. In Groups B, C, and D(2,4,and 8 weeks postoperative), different degrees of ischemia are observed in the pre-compression zone, compression zone, and post-compression zone, with the compression zone and post-compression zone being the most affected areas. With the increasing severity of deformity and prolonged compression time, the gray matter ischemic changes gradually worsen, with Groups B and C(2 and 4 weeks postoperative) showing small areas of pallor and Group D(8 weeks postoperative) exhibiting large areas of pallor and ischemic necrosis with cavitation in the post-compression zone.(Fig. 4).
Fig. 4.
Spinal Cord TTC Staining. Normal and A: Normal rabbits and Group A (sham) showed no signs of tissue ischemia; B and C: Group B (2 weeks postoperative) and Group C (4 weeks postoperative) showed small areas of ischemia; D: Group D (8 weeks postoperative) showed large pale ischemic regions
HE staining
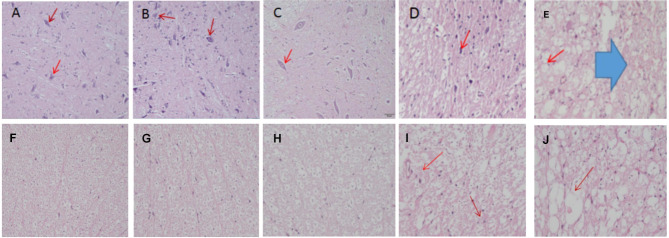
HE staining is performed on the spinal cords of the animals in each group to observe changes in neuronal, myelin sheath, and nerve fiber structures. In Group A(sham) and normal rabbit spinal cords, no abnormal pathological structural changes are observed in the gray matter. Numerous normal neurons are visible, with intact myelin sheaths and orderly arranged nerve fibers in the white matter, surrounded by small glial cells. In Group B(2 weeks postoperative), slight deformation of neurons in the gray matter is observed after mild compression, and occasional axonal swelling is observed in the white matter. In Group C(4 weeks postoperative), neurons in the gray matter become flattened, with a decrease in quantity and nuclear dissolution. Disorganization of nerve fibers in the white matter, swollen and thinned myelin sheaths, demyelination, and vacuoles are observed. In Group D(8 weeks postoperative), severe reduction in neuron volume, neuronal loss, vacuole formation, disorganization and reduction of nerve fibers in the white matter, widened myelin sheath gaps, extensive vacuole formation, and severe demyelination with massive glial cell proliferation are observed.(Fig. 5).
Fig. 5.
Spinal cord HE staining sections (×20). A, B: Spinal cord gray matter(GM) sections from normal rabbits and Group A (sham), showing normal neurons (→); C: Group B (2 weeks postoperative), neurons under slight compression (→); D: Group C (4 weeks postoperative), neurons appear flattened with signs of nuclear fragmentation (→); E: Group D (8 weeks postoperative), neurons appear shrunken with extensive vacuole formation (→) and vascular hemorrhage ( ). F, G: Spinal cord white matter(WM) sections from normal rabbits and Group A (sham), showing neatly arranged nerve fibers; H: Group B (2 weeks postoperative), occasional mild axonal swelling observed; I: Group C (4 weeks postoperative), axons appear thinner, nerve fibers are disorganized, and demyelination is present (→); J: Group D (8 weeks postoperative), vacuole formation (→), myelin sheath destruction, and demyelination changes are evident
). F, G: Spinal cord white matter(WM) sections from normal rabbits and Group A (sham), showing neatly arranged nerve fibers; H: Group B (2 weeks postoperative), occasional mild axonal swelling observed; I: Group C (4 weeks postoperative), axons appear thinner, nerve fibers are disorganized, and demyelination is present (→); J: Group D (8 weeks postoperative), vacuole formation (→), myelin sheath destruction, and demyelination changes are evident
TUNEL staining
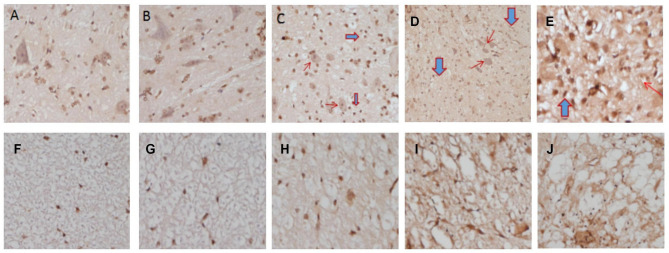
TUNEL staining is performed on spinal cord sections to determine the distribution and proportion of apoptotic cells. In Group A(sham) and normal rabbit spinal cords, a small number of scattered TUNEL-positive cells are present. In Group B(2 weeks postoperative), an increase in TUNEL-positive cells(%) is observed within a single field of view, with no changes in cell nuclei. In Group C(4 weeks postoperative), a significant increase in TUNEL-positive cells(%) is observed in the gray and white matter of the spinal cord, accompanied by nuclear dissolution, condensation, and staining, predominantly located on the ventral side of the spinal cord. In Group D(8 weeks postoperative), a significant increase in TUNEL-positive cells(%) is observed in the gray and white matter of the spinal cord, with nuclear fragmentation and vacuole formation, accumulating on both the ventral and dorsal sides of the spinal cord. Counting of TUNEL-positive cells show: There are significant differences in the proportion of TUNEL-positive cells(%) in the gray and white matter of the rabbit spinal cord between each group (P < 0.05). The comparison of the proportion of TUNEL-positive cells(%) in the gray and white matter within each group also show significant differences (P < 0.05), with the proportion of TUNEL-positive cells(%) in the white matter being significantly higher than that in the gray matter.(Table 3; Figs. 2E-F and 6).
Table 3.
Proportion of TUNEL positive Cells(%) in each group
| Group | Number (individuals) | WM(%) | GM(%) | P-value(WM and GM) |
|---|---|---|---|---|
| A | 6 | 10.03 ± 0.60 | 9.93 ± 0.47 | P<0.05 |
| B | 6 | 27.64 ± 2.12 | 24.86 ± 0.87 | P<0.05 |
| C | 6 | 42.81 ± 1.89 | 30.05 ± 1.19 | P<0.05 |
| D | 6 | 49.07 ± 2.88 | 43.64 ± 2.95 | P<0.05 |
Note: ANOVA analysis showed that pairwise comparisons between groups revealed statistically significant differences (P < 0.05)
Fig. 6.
Spinal cord TUNEL staining (×40). A, B: TUNEL staining of spinal cord gray matter in normal rabbits and Group A (sham); C: Group B (2 weeks postoperative), increased apoptotic cells and glial cell proliferation; D: Group C (4 weeks postoperative), increased apoptotic cells with nuclear fragmentation (→) and glial cell proliferation ( ); E: Group D (8 weeks postoperative), marked increase in apoptotic cells with vacuole formation (→) and extensive glial cell proliferation (
); E: Group D (8 weeks postoperative), marked increase in apoptotic cells with vacuole formation (→) and extensive glial cell proliferation ( ). F, G: TUNEL staining of spinal cord white matter in normal rabbits and Group A (sham); H: Group B (2 weeks postoperative), increased apoptotic cells and myelin swelling; I: Group C (4 weeks postoperative), disorganized nerve fiber arrangement with demyelination; J: Group D (8 weeks postoperative), severe demyelination and reticular changes
). F, G: TUNEL staining of spinal cord white matter in normal rabbits and Group A (sham); H: Group B (2 weeks postoperative), increased apoptotic cells and myelin swelling; I: Group C (4 weeks postoperative), disorganized nerve fiber arrangement with demyelination; J: Group D (8 weeks postoperative), severe demyelination and reticular changes
Discussion
Appropriate animal models are essential for studying the pathogenesis and treatment of spinal kyphosis. In this study, we successfully established an angular kyphosis model in rabbits by surgically removing portions of the L2-3 vertebral bodies. The model’s validity was confirmed through behavioral assessments, imaging studies, and histological analyses.
Although various animal models of spinal kyphosis have been developed, they often have limitations that reduce their translational value. For example, the TSC1 gene knockout mouse model created by Hwee Weng Dennis Hey et al. [17]. induces sarcopenia, leading to intervertebral disc and vertebral degeneration, eventually resulting in kyphosis. However, this model primarily reflects muscle atrophy-induced kyphosis, which is rare in clinical settings, limiting application value. Similarly, Yusuke Matsuhashi’s [14] LBX1 knockout mouse model highlights the role of muscle metabolism in spinal deformities, but lacks observations of severe complications, restricting its research scope. Other gene intervention models, such as Jiao Liang’s [15] Gdx knockout model, successfully induced kyphosis, but also caused systemic skeletal dysplasia due to gene defects, reducing its clinical applicability. K Iba’s [18] tetracycline-induced gene deficiency model is effective but faces challenges due to high costs and technical complexity, limiting its broader use. Laminectomy-based models, such as those developed by Kentaro Shimizu [19] and Dechao Kong [21], cause kyphosis by damaging the stability of the posterior spinal column. This damage leads to compression of the spinal cord. While these models are able to mimic spinal instability, they do not completely replicate the progression of the disease as seen in real clinical cases. Only a few studies have reported spinal cord ischemia as a complication in these models. When it comes to choosing animals for research, mouse models are often genetically modified. However, they are difficult to work with because of their small size. The procedures are also technically challenging, and the cost is high [14–16]. Rat models have their own limitations. They do not have a Haversian system, which is important for bone remodeling, and their bone healing process is very different from that of humans [22, 23]. This makes them less suitable for studying kyphosis. On the other hand, rabbits are more easily available and less expensive. Their bone remodeling system is more like that of humans, which makes rabbits better suited for developing kyphosis models [24, 25].
An ideal animal model should be easy to care for, simple to operate, with small individual differences, easy to repeat, and should try to simulate the clinical process and common complications of the disease. The evaluation of the model should be combined with multiple approaches and metrics to increase the credibility and clinical application value of the study.
In this study, we established an angular kyphosis model in rabbits and validated the model’s effectiveness by behavior, imaging, ischemic foci, HE staining and TUNEL staining. Rabbits of similar weight and appropriate age were selected for model construction, and the right anterolateral abdominal approach was used to avoid injury to major blood vessels and organs, with a low surgical mortality rate, with only 3 deaths. Postoperatively, the rabbits were housed in a single cage with controlled temperature and humidity. Behavioral assessment showed that the BBB score decreased from 20.93 ± 0.18 to 14.00 ± 1.66, accompanied by bilateral hind limb instability and dysfunction; imaging showed that the Cobb angle increased from 4.54 ± 0.26 to 46.73 ± 2.38, with a spinal canal occupancy rate of 44.13 ± 4.59% which, in combination with the BBB score, confirmed that the spinal cord was compressed. TTC staining showed ischemia in the ventral gray matter of the spinal cord, which worsened over time, consistent with disease progression. HE staining showed that the neurons were shrunken, and the white matter fibers were disorganized and demyelinated, which proved that the spinal cord was chronically damaged; TUNEL staining confirmed that the number of apoptotic cells increased, which provided a basis for the subsequent study on the mechanism of spinal cord ischemia associated with spinal lordosis. The results showed that with the aggravation of the degree of spinal deformity, the compression of the spinal cord in rabbits increased significantly, as evidenced by a significant decrease in behavioral scores, spinal canal stenosis (the rate of spinal canal encroachment increased to 44.13 ± 4.59%), and was accompanied by obvious spinal cord ischemia and apoptotic cell death. These findings were highly consistent with the progression of spinal cord injury in patients with clinical kyphosis, further validating the effectiveness of the model in simulating spinal cord compression and neurological dysfunction studies.Through behavioral and imaging assessments, the model in this study was able to accurately simulate the damage of spinal cord compression on neurological function, which provides a tool for understanding the pathophysiology of spinal cord injury due to compression. Histological and TUNEL staining further revealed degenerative and apoptotic processes in spinal cord neurons. The neuronal shrinkage, demyelination, and nerve fiber disorders seen in the gray and white matter of the spinal cord suggest chronic degenerative lesions of the spinal cord during compression. This finding provides a solid theoretical basis for exploring therapeutic strategies to mitigate spinal cord injury and slow the progression of lesions. With the prolongation of compression, spinal cord ischemia and apoptosis were further aggravated, suggesting that early intervention may have a critical role in preventing the deterioration of spinal cord injury. The correlation between spinal cord injury and compression time and degree of compression observed in this study provides a guiding direction for the development of time window-based interventions. Early intervention is not only expected to reduce the severity of spinal cord injury, but may also improve the rate of neurologic recovery and enhance treatment outcomes. These results provide an important reference for clinically optimizing the timing of treatment for patients with kyphotic spinal deformity and lay the foundation for exploring more effective neuroprotective strategies.
This model provides a important experimental basis for the study of the pathophysiology of spinal kyphosis and its related complications, provides strong support for the exploration of future treatment options. The model surgically simulates the natural progression of spinal kyphosis deformity, especially the formation of spinal cord compression and ischemic injury, which is highly consistent with the pathological process of clinical patients. As a result, it can aid clinicians in better understanding the disease’s course and explore more effective treatment options. Future research can utilize this model to assess the effects of different treatments (e.g. surgical decompression or pharmacological interventions) on the recovery of spinal cord function, and to explore strategies to alleviate spinal cord compression and reduce neuronal apoptosis. In addition, the model can also be used to test the potential of novel treatments in alleviating spinal cord ischemia and dysfunction, thus providing a direct reference for clinical practice.
In conclusion, the establishment of this model not only lays a solid foundation for studying the mechanisms of spinal kyphosis and developing intervention strategies, but also points out future clinical treatment research. By extending observation time and incorporating therapeutic interventions, future studies can further reveal the complex mechanisms of spinal kyphosis and its associated spinal cord injury, providing more detailed insights for clinical management.
Although this study successfully established a rabbit model of angular kyphosis and verified its feasibility and stability through behavioral, imaging, histological, and cellular-level assessments, several limitations remain and should be addressed in future research. First, manual osteotomy was used during model construction. Despite efforts to standardize preoperative planning and surgical procedures, slight variations in osteotomy depth, angle, or position were unavoidable and may affect inter-model consistency. Second, the follow-up period was relatively short, which limits the ability to observe the long-term pathological progression of chronic spinal cord compression. Future studies should extend the observation period to better investigate tissue adaptation and functional remodeling under sustained compression, thereby improving the model’s clinical relevance. In terms of histological and ischemic injury assessment, this study primarily relied on qualitative observations. The lack of quantitative metrics may restrict accurate evaluation of the severity of spinal cord compression, lesion extent, and dynamic pathological changes. It is recommended that future work incorporate quantitative imaging analysis, standardized histological scoring systems, or molecular markers such as apoptotic cells, inflammatory cytokines, and ischemia-related proteins to enhance the precision and objectivity of tissue injury evaluation. In addition, although the BBB score reflects basic motor function, it does not fully capture changes related to sensory deficits, coordination, or fine motor control, indicating a limitation in functional assessment. Given the complexity of functional impairment after spinal cord injury, future studies should consider combining broader neurobehavioral assessments with electrophysiological monitoring to improve the sensitivity and comprehensiveness of functional evaluation. Due to experimental limitations, real-time monitoring of animal posture and movement was not performed in this study. In future work, behavioral tracking systems could be introduced to provide supplementary dynamic functional data. Finally, although the sample size met the basic requirements for statistical analysis, its relatively small scale may affect statistical power. Increasing the sample size in future studies would help improve the reliability and generalizability of the findings.
Conclusion
This study established a rabbit model of angular kyphosis to investigate the progression of spinal cord compression and ischemic injury, along with associated neuropathological changes. Through behavioral assessment, imaging, histological examination, and apoptosis analysis, the model reproduced the gradual decline in neurological function, worsening spinal deformity, and progressive spinal cord damage. As the severity of kyphosis increased and compression time was prolonged, animals developed motor deficits, spinal canal narrowing, spinal cord ischemia, and neuronal apoptosis. These findings support the model’s feasibility in simulating clinical spinal cord compression and related neurological impairment. This model may be further applied in long-term studies and in evaluating surgical or pharmacological interventions to support translational research on spinal deformity-related spinal cord injury.
Acknowledgements
We would like to express our sincere gratitude to Kunming Medical University for providing the laboratory resources necessary for the completion of this research.
Abbreviations
- AKD
Anterior kyphosis deformity
- ANOVA
One-way analysis of variance
- BBB scores
Basso-Beattie-Bresnahan Locomotor Rating Scale
- GM
Gray Matter
- HE staining
Hematoxylin-eosin staining
- MRI
Magnetic Resonance Imaging
- SPSS
Statistical Product and Service Solutions
- TTC staining
2,3,5 - Triphenyl Tetrazolium Chloride Staining
- TUNEL staining
Terminal Deoxynucleotidyl Transferase - Mediated DUTP Nick End Labeling Staining
- WM
White Matter
Author contributions
Contributions to this work were made by Xueneng Yang; Huaiquan Gu; Ruijuan Li; Bo Li; Limin Guo; Jun Shu. Conceptualization: L.G. & J.S. Methodology: X.Y. & H.G. Data Curation: H.G. & X.Y. Formal Analysis: H.G. Investigation: X.Y. & R.L. Writing – Original Draft: X.Y. Writing – Review & Editing: X.Y. & L.G. & J.S. Visualization: H.G. & X.Y. & B.L. & R.L. All authors have thoroughly read and approved the final manuscript.
Funding
This research received no external funding.
Data availability
The relevant data from the literature is stored in the attachments. For further inquiries, please feel free to contact the author.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kunming Medical University (Approval No. KMMU20241612). All experimental procedures followed the ethical guidelines for the care and use of laboratory animals. To minimize pain and stress during model establishment, a combination of anesthetic and sedative agents was used during surgery. After modeling, animals were gradually rewarmed to reduce postoperative mortality. During the recovery period, daily assessments of food intake, activity, excretion, and neurological function were conducted. At the end of the study, all animals were humanely euthanized in accordance with ethical guidelines.
Consent for publication
All authors reviewed the final manuscript and provided their approval for submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xueneng Yang and Huaiquan Gu contributed equally to this work.
Contributor Information
Liming Guo, Email: guoliming2025@126.com.
Jun Shu, Email: yxn00yxn@163.com.
References
- 1.Garg B, Bansal T, Mehta N. Clinical, radiological, and functional outcomes of posterior-only three-column osteotomy in congenital kyphosis : a minimum of two years’ follow-up. Bone Joint J. 2021;103–b(7):1309–16. [DOI] [PubMed] [Google Scholar]
- 2.Kado DM, Miller-Martinez D, Lui LY, Cawthon P, Katzman WB, Hillier TA, Fink HA, Ensrud KE. Hyperkyphosis, kyphosis progression, and risk of non-spine fractures in older community dwelling women: the study of osteoporotic fractures (SOF). J Bone Miner Res. 2014;29(10):2210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods GN, Huang MH, Lee JH, Cawthon PM, Fink HA, Schousboe JT, Kado DM. Factors associated with kyphosis and kyphosis progression in older men: the MrOS study. J Bone Miner Res. 2020;35(11):2193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho W, Shepard N, Arlet V. The etiology of congenital scoliosis: genetic vs. environmental-a report of three monozygotic twin cases. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2018;27(Suppl 3):533-7. [DOI] [PubMed]
- 5.Blechacz B, Gajic O. Images in clinical medicine. Severe kyphosis. N Engl J Med. 2008;358(24):e28. [DOI] [PubMed] [Google Scholar]
- 6.Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468(3):687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z, Zhang Z, Yang X, Zhao Z, Li T, Bi N, Xie J, Wang Y. Posterior vertebral column resection for severe spinal deformity correction: comparison of pediatric, adolescent, and adult groups. Comput Intell Neurosci. 2022;2022:5730856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsirikos AI, Carter TH. The surgical treatment of severe scheuermann’s kyphosis. Bone Joint J. 2021;103–b(1):148–56. [DOI] [PubMed]
- 9.Terai H, Takahashi S, Yasuda H, Konishi S, Maeno T, Kono H, Matsumura A, Namikawa T, Kato M, Hoshino M, Tamai K, Toyoda H, Suzuki A, Nakamura H. Direct lateral corpectomy and reconstruction using an expandable cage improves local kyphosis but not global sagittal alignment. J Clin Med. 2021;10(17). [DOI] [PMC free article] [PubMed]
- 10.Gandhi SV, Januszewski J, Bach K, Graham R, Vivas AC, Paluzzi J, Kanter A, Okonkwo D, Tempel ZJ, Agarwal N, Uribe JS. Development of proximal junctional kyphosis after minimally invasive lateral anterior column realignment for adult spinal deformity. Neurosurgery. 2019;84(2):442–50. [DOI] [PubMed] [Google Scholar]
- 11.Pruszczynski B, Mackenzie WG, Rogers K, White KK. Spinal cord injury after extremity surgery in children with thoracic kyphosis. Clin Orthop Relat Res. 2015;473(10):3315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida G, Ushirozako H, Hasegawa T, Yamato Y, Kobayashi S, Yasuda T, Banno T, Arima H, Oe S, Mihara Y, Ide K, Watanabe Y, Yamada T, Togawa D, Matsuyama Y. Preoperative and postoperative sitting radiographs for adult spinal deformity surgery: upper instrumented vertebra selection using sitting C2 plumb line distance to prevent proximal junctional kyphosis. Spine. 2020;45(15):E950–8. [DOI] [PubMed] [Google Scholar]
- 13.Ye J, Rider SM, Lafage R, Gupta S, Farooqi AS, Protopsaltis TS, Passias PG, Smith JS, Lafage V, Kim HJ, Klineberg EO, Kebaish KM, Scheer JK, Mundis GM, Soroceanu A, Bess S, Ames CP, Shaffrey CI, Gupta MC. Distal junctional kyphosis in adult cervical deformity patients: where does it occur? European spine journal : official publication of the European spine society, the European spinal deformity society, and the European section of the cervical. Spine Res Soc. 2023;32(5):1598–606. [DOI] [PubMed] [Google Scholar]
- 14.Matsuhashi Y, Horiuchi K, Nakagawa T, Takahashi Y, Imabayashi H, Hosogane N, Watanabe K, Matsumoto M, Chiba K. Abrogation of LBX1 in skeletal muscle results in hypoplastic limbs and progressive kyphosis in mice. J Orthop Res. 2023;41(4):884–90. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, Li J, Fu Y, Ren F, Xu J, Zhou M, Li P, Feng H, Wang Y. GdX/UBL4A null mice exhibit mild kyphosis and scoliosis accompanied by dysregulation of osteoblastogenesis and chondrogenesis. Cell Biochem Funct. 2018;36(3):129–36. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Aruwajoye O, Sucato D, Richards BS, Feng GS, Chen D, King PD, Kamiya N. Induction of SHP2 deficiency in chondrocytes causes severe scoliosis and kyphosis in mice. Spine. 2013;38(21):E1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hey HWD, Lam WMR, Chan CX, Zhuo WH, Crombie EM, Tan TC, Chen WC, Cool S, Tsai SY. Paraspinal myopathy-induced intervertebral disc degeneration and thoracolumbar kyphosis in TSC1mKO mice model-a preliminary study. Spine J. 2022;22(3):483–94. [DOI] [PubMed] [Google Scholar]
- 18.Iba K, Durkin ME, Johnsen L, Hunziker E, Damgaard-Pedersen K, Zhang H, Engvall E, Albrechtsen R, Wewer UM. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol Cell Biol. 2001;21(22):7817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu K, Nakamura M, Nishikawa Y, Hijikata S, Chiba K, Toyama Y. Spinal kyphosis causes demyelination and neuronal loss in the spinal cord: a new model of kyphotic deformity using juvenile Japanese small game fowls. Spine. 2005;30(21):2388–92. [DOI] [PubMed] [Google Scholar]
- 20.Aimone JB, Leasure JL, Perreau VM, Thallmair M. Spatial and Temporal gene expression profiling of the contused rat spinal cord. Exp Neurol. 2004;189(2):204–21. [DOI] [PubMed] [Google Scholar]
- 21.Kong D, Zheng T, Fang J, Li X. Apoptosis of endplate chondrocytes in post-laminectomy cervical kyphotic deformity. An in vivo animal model in sheep. European spine journal : official publication of the European spine society, the European spinal deformity society, and the European section of the cervical. Spine Res Soc. 2013;22(7):1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalu DN, Hardin RH, Cockerham R, Yu BP. Aging and dietary modulation of rat skeleton and parathyroid hormone. Endocrinology. 1984;115(4):1239–47. [DOI] [PubMed] [Google Scholar]
- 23.Kalu DN, Liu CC, Hardin RR, Hollis BW. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology. 1989;124(1):7–16. [DOI] [PubMed] [Google Scholar]
- 24.Southard TE, Southard KA, Krizan KE, Hillis SL, Haller JW, Keller J, Vannier MW. Mandibular bone density and fractal dimension in rabbits with induced osteoporosis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2000;89(2):244–9. [DOI] [PubMed]
- 25.Seki T, Hida K, Tada M, Koyanagi I, Iwasaki Y. Graded contusion model of the mouse spinal cord using a pneumatic impact device. Neurosurgery. 2002;50(5):1075–81. discussion 81 – 2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant data from the literature is stored in the attachments. For further inquiries, please feel free to contact the author.