Abstract
Furfural (FF), an intermediate aldehyde compound, can serve as an indicator of the extent of the Maillard reaction on heat-induced food processing and storage, as well as for the aging assessment of the solid insulation of oil-immersed transformers because it is formed by the degradation of cellulosic insulation. By considering the concepts of green analytical chemistry, a new furfural–bis(4-aminophenyl) disulfide (APDS) colorimetric chemosensory assay, based on the Stenhouse reaction between furfural and APDS, was developed for the quantification of furfural by utilizing UV spectroscopy and colorimetric analyses. A strong correlation between UV–vis absorbance and furfural concentrations was observed, which confirmed the high sensitivity (0.00024 mM furfural) of the reaction system. The color change at 0.005 mM of furfural was noted by the naked eye. This was a unique and highly selective phenomenon because only furfural shows UV absorbance and color change within 450–600 nm of UV radiation, unlike other aromatic and aliphatic aldehydes. The highly sensitive method was applied for the qualification of 0–0.01 mM (0–1 ppm) of furfural in the power transformer insulating fluid. An APDS strip formulated with polyethylene glycol was used as the chromatography paper. This study demonstrates the successful surface Stenhouse coupling between furfural and APDS, as confirmed by X-ray photoelectron spectroscopy analysis. Therefore, this liquid- and solid-based assay is a novel, green analytical method as it uses safer solid APDS instead of toxic liquid aniline that is generally used in conventional methods. In addition, the method is simpler, which makes the on-site analysis possible.


Introduction
The principles of green analytical chemistry have resulted from the idea of sustainable development, which aims to reduce risks to humans and the environment. , Aside from the development in instrumentation and methodologies, which are necessary for improving the quality of chemical analyses, efforts are also being made to reduce the negative impact of chemical analyses on the environment and to enable the implementation of the principles of sustainable development to analytical laboratories.
Furfurals such as hydroxymethylfurfural (5-HMF) are intermediate aldehyde compounds. They are involved in the formation of various pigments (such as melanoidins) and are also closely linked to processes, such as caramelization and the Maillard reaction. − Furfurals and related compounds are produced through heat-induced food processing and storage. They are also present in trace levels in processed foods, although the final amount depends on the processing condition and storage duration and can serve as an indicator of the extent of the Maillard reaction. − Beyond a certain point of absorption in the body, these compounds begin to adversely impact the human health, especially the central nervous system, kidneys, the liver, and other organs. Therefore, it is important to develop a simple, fast, and sensitive method to determine their concentration in foods, such as bakery products, milk, and beer, and to investigate the relationship between the degree of heat treatment and the total amount of furfurals generated. − In addition, because furanic compounds found in transformers are solely formed by the degradation of cellulosic insulation, furfural analysis is a useful tool for assessing the aging of the solid insulation of oil-immersed transformers. ,
Currently, furan aldehydes are generally determined through traditional chromatographic methods, such as gas chromatography (GC), high-performance liquid chromatography (HPLC), and spectrophotometric methods. However, these methods are ineffective owing to matrix effects and insufficient selectivity. In addition, they often require several analytical steps, including extraction and derivatization, before analysis. The detection limits of these methods could be improved by employing precolumn derivatization reactions with various compounds. Dinitrophenylhydrazine (DNPH) is one such commonly used derivatization reagent for the HPLC–UV analysis of aldehydes. ,, Although these methods tend to produce accurate and reproducible results, they have several drawbacks, such as time-consuming sample preparation protocols and expensive instrumentation, which limit their application.
To overcome the drawbacks of traditional chromatographic methods, several sensitive, rapid, and inexpensive colorimetric methods for in situ determination of aldehydes have been developed with many important applications, including detection of chemical toxins, security screening, food inspection, and disease monitoring. Colorimetric techniques have gained high visibility with great research and commercial interest in the last two decades because of their simple operation, use of portable devices, low cost, and convenient readout with the naked eye. , These chemosensors allow simple and fast determination of a wide range of compounds in complex matrices and can measure compounds of interest by the naked eye or using portable spectrophotometers. As a result, they are being used as alternatives to traditional analytical methods.
Colorimetric techniques have attracted considerable interest as simple, low-cost, and rapid methods for on-site analysis, particularly in resource-limited environments. In recent years, digital image-based (DIB) methodsoften utilizing smartphone-assisted platformshave emerged as valuable tools for visual quantification, offering advantages such as minimal waste generation and low energy consumption. While such methods hold promise for future applications, the present study focuses on an UV–vis spectrophotometric approach to ensure reliable and precise quantitative analysis. ,
Colorimetric methods are based on chromogenic reactions, which convert a “colorless (or weakly colored)” compound into a “colored” compound through chemical and/or biological reactions. Generally, the colorimetric detection of an aldehyde involves the nucleophilic addition of an amine, such as anilines, 2,4-dinitrophenylhydrazine (DNPH), and 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald), to the carbonyl group of the aldehyde, resulting in the formation of an imine with a different UV–vis absorption band from that of the analyte. , Although DNPH can intensify the UV adsorption by aldehydes for UV-based detectors and spectrometers, the colorimetric response and detection are typically not useful when the color change is not drastic. Purpald is one of the most commonly used amine for aldehyde determination. Therefore, it is stable at room temperature and more sensitive than other methods such as those using acetyl-acetone or chromotropic acid. , Purpald-based commercially available formaldehyde test kits are extremely sensitive. Interestingly, however, Purpald-based colorimetric assay for furfural does not lead to any color development. In addition, this assay yields results that are completely different from those obtained through formaldehyde analysis, which is an extremely sensitive test and affords purple bands.
Various methods have been developed for the detection of furan-based compounds, including chromatographic techniques, electrochemical sensors, and optical detection systems. Among these, colorimetric approaches offer a simple and effective strategy for detecting furan derivatives. For instance, Meldrum’s acid furfural conjugate has been explored for amine sensing through donor–acceptor Stenhouse adduct (DASA) formation, highlighting the potential of furan-based conjugates in optical sensing applications.
Aniline acetate is used as a chromogenic agent in UV–vis spectrometry (519 nm)-based furfural analysis to eliminate the interference factors and improve the detection limit. A previous study proposed an analytical procedure for in situ determination of furfurals in sugar cane spirit samples using a method comprising a colorimetric spot test and a DIB method with aniline acetate, prepared by adding pure aniline to glacial acetic acid (AA) in a 1:9 (v/v) ratio, as a reagent. A colorimetric sensor array was successfully designed for identifying and differentiating aldehydes from ketones in the vapor phase using 4,4′-azodianiline and pararosaniline. This mechanism is based on the Stenhouse reaction in which both aniline and furfural are reacted in an acidic medium, resulting in the generation of a deep red cyanine derivative that shows absorption at 537 nm. ,,
Donor–acceptor Stenhouse adducts are attracting increasing interest because of their application as visible-light-responsive photoswitches with multiple emerging applications as photoresponsive materials. , However, liquid aniline and its derivatives are highly toxic and corrosive. They pose a serious health risk and are hazardous to the environment. In addition, their fabrication uses a large amount of the analyte. Aniline is highly toxic to aquatic life and is identified as a chemical capable of causing genetic defects and cancer by the European Union.
Moreno-Bondi et al. developed disposable color-changing polymeric films through radical polymerization of 4-vinylaniline, 2-hydroxymethyl methacrylate, and ethylene dimethyl methacrylate for quantifying furfural in beer. These polymer films could be further improved by removing interference, which will reduce the analysis time (to 30–60 min) and improve their selectivity to other aldehydes. This is evident from the baseline slope at 530 nm in the UV spectrum. Therefore, a novel facile, selective, and environmentally friendly analytical method for furfural needs to be developed by replacing toxic aniline with a greener alternative. Bis(4-aminophenyl) disulfide (APDS) is a solid aniline derivative coupled by sulfide bonds (S–S). Although it has been classified as a hazardous substance with health risks, it is much less harmful than aniline.
Aniline is known for its acute toxicity, potential carcinogenicity, and severe environmental impact. In contrast, APDS is not classified as a hazardous substance under GHS regulations, suggesting a relatively safer alternative. , Aniline can be replaced by APDS in the Stenhouse reaction-based colorimetric analysis of furfural. It can also be used to form aromatic amine-terminated oligomers and polymers through sulfide-bond rearrangement. In addition, it can be mixed with polyethylene glycol (PEG) to form a solid coating on the surface of a paper or plastic film, which can then be employed as a solid and stable colorimetric sensing strip.
This work proposes a furfural–APDS colorimetric method based on the coupling reaction of furfural and APDS, followed by color development. The response was evaluated to determine the optimum condition at 550 nm through UV spectrometry. The intensity and selectivity of APDS were compared to those of other aldehydes. Furthermore, a PEG–APDS-coated colorimetric sensing strip was prepared and employed for the diffuse reflectance analysis of furfural in the power transformer insulating fluid.
Results and Discussion
Using APDS for Analyzing Furfural under Different Acidic Conditions
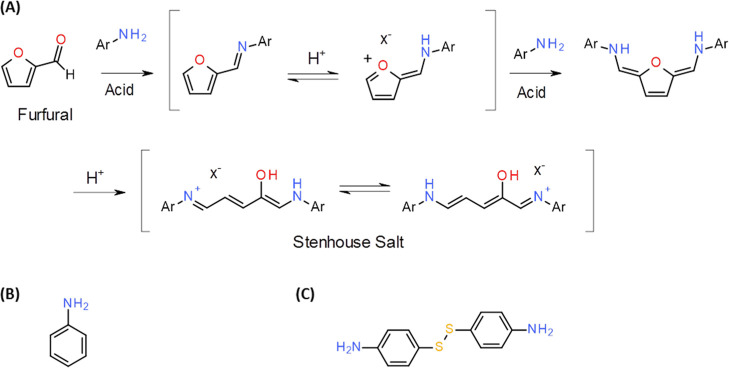
First isolated in 1850, the highly colored Stenhouse salts result from the furan ring-opening reaction through the condensation of anilines with furfuraldehyde at a molar ratio of 2:1 in the presence of protic acids (Scheme ). , Stenhouse salts are attracting increasing attention as the intermediates for the synthesis of cyclo-penten-2-enones. The photophysical properties of donor–acceptor Stenhouse salts are employed for the colorimetric detection of amines, functionalization of polymers for the detection of heat and nerve agents, photolithography, and orthogonal photoswitching systems.
1. (A) Formation of Stenhouse Salts from Aniline and Furfural (Ar = Ph from Aniline, X = Counter Ion (−) from Acid); , (B) Aniline; (C) Bis(4-Aminophenyl) Disulfide (APDS).
Similar to amines, the Stenhouse reaction can also be used for the colorimetric detection of furfural based on the formation of intensely colored conjugated triene structures. The overall transformation comprises ABB′-type coupling, involving the initial ring-opening of the furan ring, presumably resulting in the formation of an iminium ion, which could then be activated for a nucleophilic attack at the 5-position of furfural (Scheme A). Although the exact nature of this furan ring-opening step is yet to be established, it is possible that the addition reaction affords compound 7, followed by its ring-opening to form intermediate 8 (i.e., the deprotonated Stenhouse salt). The ring closure of intermediate 8, catalyzed by Sc(OTf), leads to the formation of compound 4, as described in ref .
APDS is a solid aniline derivative involving a disulfide bond (S–S). It can be used as a greener alternative to aniline in the Stenhouse reaction (Scheme S1). As the reaction is performed under acidic conditions, it is necessary to determine suitable acidic conditions for obtaining a high colorimetric response. The spectral shift and colorimetric response of furfural and APDS in AA, propionic acid, valeric acid, and 0.031 M HCl were compared (Figure ). Both of them afforded similar pH values (AA: 2.5; propionic acid: 2.5; valeric acid: 2.92; and 0.031 M HCl: 2.5). To investigate the necessary assay factors, the effects of reaction time, solvent, and water were evaluated on the reaction rate of furfural and APDS in AA by conducting UV–vis spectral analysis.
1.
UV–vis spectrum. (A) AA, 10 ppm furfural (FF), and 10 and 50 mM APDS in AA. (B) 50 mM APDS in AA, 20 ppm FF, and 50 mM APDS in AA, propionic acid, valeric acid, and 0.031 M HCl. (C) UV spectrum and (D) absorption at 550 nm on the reaction of 150 μL of 10 ppm FF and 150 μL of 10 mM APDS solution in AA in a 10 × 10 mm quartz cell in the reaction time course. (C,D) Share the same experimental conditions, with (C) displaying the full UV spectrum and (D) highlighting the specific absorption at 550 nm.
As expected, AA and furfural produced adsorption bands below 300 nm, while APDS gave rise to a strong absorption band below 450 nm in the UV spectrum (Figure A). However, significant shifts were noted in the absorption band and intensity of the reaction solution comprising APDS and furfural in AA (Figure B). The λmax value at 550 nm was not influenced by band hindrance or overlap with the absorption bands of the substrate (APDS and furfural) and AA, which were within the baseline region (475–600 nm). The absence of band hindrance or overlap with other absorption bands can lead to a higher sensitivity, which may help lower the detection limit. Because the Stenhouse reaction is performed under acidic conditions, other acids such as propionic (C3) acid and valeric (C5) acid can also be used as they too react with furfural, although weakly. A comparison of the reaction using 0.031 M HCl with that using AA showed that APDS and furfural could not properly react with each other. Hence, no significant adsorption in the UV spectrum was observed under the aforementioned conditions. Therefore, AA was selected as both the solvent and the catalyst for further investigation of the Stenhouse reaction.
The DNPH–furfural reaction can increase the UV absorption in the detection and analysis of aldehydes. In addition, this reaction can be coupled with HPLC and GC. The condensation reaction between the carbonyl group of aldehydes (furfural and formaldehyde) and 2,4-DNPH results in the formation of an aldehyde–DNP–hydrazone adduct, the spectrum band of which gradually broadens within the range of 375–450 nm during the reaction course without the formation of any new absorption band or wavelength (λmax). As shown in Scheme S1, the APDS–furfural adduct exhibits a unique adsorption band at 550 nm, allowing its use for the selective detection and identification of furfural in mixed samples via HPLC–UV–vis analysis. The reaction rate of furfural (10 ppm) and APDS (10 mM) in AA was monitored for detecting the emergence of any new band in the UV–vis spectrum (400–600 nm) by using an UV–vis spectrometer (Figure C,D). The band was compared with that of a blank (10 mM of APDS in AA), which afforded a relatively lower adsorption band (450–600 nm). The band intensity rapidly increased during the initial 10 min, followed by a gradual increase (Figure D).
Effects of Solvents and Furfural Concentration on the Colorimetric Reaction with APDS in AA
The effect of solvent was investigated using solvents such as acetonitrile (ACN), methanol (MeOH), and ethanol (EtOH), which produce a homogeneous solution with AA. Furfural (10 ppm) was reacted with APDS (10 mM), prepared using a solution of AA with solvents such as acetonitrile, methanol, and ethanol. High initial reaction rates with high UV adsorption at 550 nm were obtained in 100% AA within the first 5 min of the reaction. However, the final responses were even higher, especially in ethanol, after the first 5 min of the reaction had passed (Figure A). The effect of the ethanol concentration (0–100%) in AA was evaluated. Less than 60% of ethanol afforded higher adsorption responses at 550 nm of UV–vis radiation. However, the responses weakened as the ethanol concentration was increased (and the AA concentration was decreased) (Figure B).
2.

Effect of (A) solvent, (B) ethanol concentration, and (C) water addition on the reaction of 100 μL of 10 ppm furfural and 100 μL of 10 mM APDS solution in a 96-well plate in the reaction time course. Water was added at 1, 5, and 10 min and compared the reaction solution without water. UV–vis absorption at 550 nm.
Therefore, for optimum results, AA and ethanol concentration of more than 60% should be employed. This phenomenon might be a result of the equilibrium status of the reaction, which shifted more toward the product when a high concentration of ethanol was used. AA is necessary to maintain the acidic condition of the reaction system. However, AA is corrosive and irritates the eyes and the nose. Thus, AA should be partially replaced with ethanol for a better handling condition.
The water content could be another important factor influencing the reaction rate. Very low absorption intensity was observed in 0.031 M of HCl (Figure B). To understand the role of water in the reaction, we compared the result obtained from the reaction of furfural (10 ppm) and APDS (10 mM) in a reaction mixture comprising AA and ethanol under the same reaction conditions but with the addition of water at 1, 5, and 10 min of the reaction (Figure C). The addition of water led to the quenching effect, which greatly reduced the reaction rate.
The correlation between the analytes, furfural concentration, and absorbance at a given wavelength or λmax is related to the limit and range of valid detection, and thus, it is one of the critical factors in spectral analysis. The adsorption band of pure APDS, furfural, and solvent did not cause any hindrance or interference. In addition, the baseline change in the UV–vis spectrum was confirmed (Figure A). Therefore, we hypothesize that the new band is solely a result of the reaction adduct.
In this study, the maximum concentration of furfural employed was 0.5 mM (200 μL), which is 4 times more than that of 50 mM of APDS solution (50 μL). The reaction system with 0 mM of furfural was designed as control, while that with 0.0005–0.5 mM of furfural was employed for further studies. The concentrations and volumes of both furfural and APDS were employed for comparing absorbance linearity at 550 nm and any visual change in color under each condition. All combinations of furfural and APDS ((a) 100 μL of furfural and 50 μL of APDS, (b) 100 μL of furfural and 100 μL of APDS, (c) 200 μL of furfural and 10 μL of APDS) afforded a high linear correlation and significant color changes, depending on the concentration of furfural employed. No phase separation and precipitation were observed. Thus, the concentration range of furfural was appropriate for the assay condition and protocol. Systematic and consistent linear profiles were obtained for each furfural concentration under the optimal fixed conditions (Figure A). A strong correlation was obtained even at low furfural concentrations (0.0005–0.01 mM) (Figure A).
3.

(A) Linear correlation between UV absorbance at 550 nm and furfural concentrations and (B) colorimetric responses obtained from the reaction of furfural (0–0.5 mM) and APDS (50 mM) prepared in a mixture of 70% AA and 30% ethanol. (a) Reaction of furfural (100 μL) and APDS (50 μL). (b) Reaction of furfural (100 μL) and APDS (100 μL). (c) Reaction of furfural (200 μL) and APDS (100 μL). The insert plate indicates the linear correlation in low furfural concentration (0–0.01 mM).
Therefore, both the reaction profile and trend should be consistent and afford highly sensitive, stable, and correlated results, irrespective of the ratios of the two reactants (furfural and APDS). Furthermore, the color change and visual responses were observed and compared by the naked eye at three different ratios of furfural and APDS (Figure B). The color (pink) change was apparent for 0.01–0.5 mM of furfural. However, higher volumes of solutions were used for cases with 0.005 to 0.5 mM of furfural (Figure B). A gradual change from light pink to deep pink was observed based on the range of the furfural concentration employed. This color change can be analyzed to determine a lower limit of detection using a colorimetric instrument. Therefore, this assay protocol could be a suitable quantitative analysis method utilizing UV–vis spectroscopy and colorimetric assay for the reaction between furfural and APDS. The LOD and LOQ were calculated according to the ICH Q2(R1) guideline, using the standard error of the intercept (σ = 0.00072) and the slope (S = 10.061) from a regression based on five replicates per concentration point (N = 5). The resulting values were 0.00024 mM and 0.00071 mM, respectively. These results demonstrate the high sensitivity and reliability of the proposed method. ,
The LOD and LOQ described in the present study were sufficient for the detection of furfural in foods, including in fruits and vegetables. The following are some typical concentrations of furfural that have been reported in various food items: wheat bread: 0.8–14 ppm; cognac: 0.6–33 ppm; rum: 22 ppm; malt whisky: 10–37 ppm; port wine: 2–34 ppm; coffee: 55–255 ppm; and juices: 0.01–4.93 ppm. The color change for 0.01 mM (=0.961 ppm) of furfural from that of 0 mM of furfural can be easily identified through the naked eye (Figure ). This phenomenon can be used for a simple equipment-free on-site detection method.
To verify the reliability of the method, a comparative analysis was performed between the UV–vis–NIR spectrophotometer and a commercial microplate reader (Thermo Multiskan GO) at 0.01 mM furfural (n = 3). Welch’s t-test revealed a significant difference in absorbance due to instrumental configuration, whereas the F-test confirmed comparable precision. Detailed results are provided in the Supporting Information (Table S1).
While the UV–vis spectrophotometric method used in this study does not allow for in situ or online measurements, it is generally considered an energy-efficient and low-waste approach. Although we did not formally calculate an AGREE score, the method reflects several core principles of green analytical chemistrysuch as minimal reagent use, low generation of hazardous waste, and operational simplicitywhich together support its classification as an environmentally conscious laboratory method. ,
As shown in Figure S12, the absorbance of 50 mM of APDS at 550 nm of UV–vis radiation for each concentration of furfural was higher than that observed for 50 mM of aniline at its λmax (520 nm). Therefore, when the same molar concentrations of APDS and aniline are employed, the APDS protocol is a more green, reasonable, and sensitive method for detecting furfural than that involving aniline, a toxic compound. Comparison of the toxicity profiles of 4-aminophenyl disulfide (APDS) and aniline was carried out based on their respective Material Safety Data Sheets (MSDS). APDS is not classified as a hazardous substance under the GHS system, whereas aniline is categorized as an acutely toxic (category 3), mutagenic (category 2), and carcinogenic (category 2) substance. The oral LD50 of APDS (2501 mg/kg, rat) is significantly higher than that of aniline (250 mg/kg, rat), indicating a much lower acute toxicity. Additionally, aniline is associated with serious eye damage, respiratory toxicity, and systemic toxicity upon prolonged exposure, whereas such classifications are not reported for APDS. Furthermore, aniline poses significant environmental risks (aquatic acute 1, chronic 1), whereas APDS has no reported classification regarding environmental toxicity (Table S2). ,
Selectivity and Absorbance Responses of Aromatic and Aliphatic Aldehydes in Reactions with APDS by UV–Vis Spectroscopy
In the Stenhouse reaction, furfural is initially fused with the amine group of APDS to form an iminium species/Schiff base, followed by ring opening with a nucleophile to finally yield a Stenhouse adduct (Scheme ).
Because the Stenhouse reaction is based on the ring-opening reaction of furfural, an aromatic aldehyde, it is valuable to compare the reaction and colorimetric responses of other aromatic and aliphatic aldehydes, which may have a different reaction mechanism from that of the amine group. To compare the absorption and colorimetric responses, the same concentration (0.2 mM) and the same assay protocol were employed for both the aliphatic and aromatic aldehyde. The spectrum profiles of aliphatic aldehydes (e.g., formaldehyde, butanal, and pentanal) and APDS were similar, without any absorbance in the 500–600 nm region (Figure A). Similarly, aromatic aldehydes also did not demonstrate any absorption band within the 500–600 nm region, which was completely different from that of furfural, which showed strong absorbance, under the same condition. This phenomenon could be confirmed based on visual changes in color as observed by the naked eye. The only reaction of furfural showed significant color changes to pink (Figure B).
4.

UV–vis spectrum of (A) aldehyde–APDS reaction solutions obtained from the reaction of aliphatic aldehydes (0.2 mM of formaldehyde (FA), butanal (BA), and pentanal (PA)) and aromatic aldehydes (0.02 mM of furfural), 5-hydroxymethylfurfural (5-HMF), benzaldehyde (BzA), 4-hydroxy-benzaldehyde (4-H-benzaldehyde, 4HBzA), and vanillin (VA) with 50 mM of APDS. (B) Color development and comparison of the resection solution of 0.2 mM of aldehydes and 50 mM of APDS at 10 min in a 96-well plate. Aldehydes and APDS solution were prepared in a mixture of 70% AA and 30% ethanol.
In addition, the adducts of the respective aldehydes are presented in the mass spectra provided in the Supporting Information (Figures S4–S11). Also, the furfural–APDS adducts were characterized by 1H NMR, as shown in Figure S3. As a result, the expected mass peaks demonstrating ABB′-type coupling were not observed, except for furfural. Hence, other aldehydes did not form Stenhouse derivatives. Therefore, this APDS protocol can be employed as a highly selective and sensitive method for detecting furfurals without any interference from substrates and other aldehydes. As shown in Scheme S1, the resulting Stenhouse adduct from furfural is fully conjugated over the ring-opened furan with APDS by pi-electron delocalization, resulting in a colorimetric chemosensory response and shift of the absorption band (λmax = 550 nm). Although the carbonyl group of other aldehydes can react with the amine group of APDS, there may be no ring-opening reaction and pi-electron delocalization over the product without any color change. Based on the absorbance profile shown in Figures and , we can suggest that pi-electron delocalization in the adduct should be stable during the entire analysis time. Therefore, the assay protocol may be sufficiently valid for high-throughput screening and analysis to selectively detect furfural in the solution, including other aliphatic and aromatic aldehydes, within short analysis time course, especially for analysis at single wavelengths.
Furfural–APDS Assay for Determining Furfural Concentration in Power Transformer Insulating Fluid
Because furan compounds, although present in trace levels, are a result of heat-induced food processing and storage conditions, they are often used as the markers of heating processes in many products. − Recently, disposable color-changing polymeric films were prepared through the radical polymerization of 4-vinylaniline, 2-hydroxymethyl methacrylate, and ethylene dimethyl methacrylate and were employed for the quantification of furfural in beer by conducting the Stenhouse reaction at 537 nm, which is visible to the naked eye. The films were incubated for 1 h at room temperature in 10 mL of beer samples spiked with 200 μL of 1.5 M HCl, followed by air drying and measuring using a portable fiber-optic spectrometer.
Furfural analysis is a useful tool for the aging assessment of solid insulation of oil-immersed transformers because furanic compounds found in transformers are solely formed by the degradation of cellulosic insulation. , Currently, furan aldehydes are generally determined by using various traditional chromatographic methods, such as GC, HPLC, and spectrophotometric methods. − However, these methods are often hindered by matrix effects and poor selectivity. In addition, they typically require several analytical steps, including extraction and derivatization, before analysis. A Stenhouse reaction-based analysis method comprising solvent extraction, mixing with aniline and AA, colorimetric reaction, and absorbance measurement using a potable UV spectrometer was developed to determine the furfural concentration in the insulation oil of transformers. However, this method needs to be further developed because it uses a large amount of aniline and AA. In addition, the use of toxic aniline causes major environmental and human safety issues.
We employed the optimized conditions discussed in the previous section (Figure ) for the colorimetric and spectral analyses of the standard solutions of furfural (0.0–0.01 mM) in the insulation oil of transformers (Figure ). Because oil and 50 mM of APDS solution prepared in 70% AA and 30% ethanol were immiscible, the furfural was simply extracted from the oil to the solution. To improve the detection limit, the extraction ratio of oil and solution was maintained at 4:1 (v/v), and the phase separation was completed within 3 min after shaking. Although the centrifugation was not necessary, it can be used to obtain rapid and clearer phase separation (e.g., within 10 s). The procedure comprised the mixing of oil (2800 μL) and APDS (700 μL), extraction, phase separation, reaction, and analysis in a polystyrene cuvette, which is generally used in UV spectroscopy. Therefore, this was a very simple method without involving any additional pretreatment steps and solution transfer and could be used for direct and rapid analysis (within 10 min) using a potable UV spectrometer. No hindrance and overlap with other bands was observed within the UV absorbance range of 450–600 nm. A high correlation between the furfural concentration and UV absorbance at 550 nm was noted, although some fluctuations in the band also occurred because of the coextracted oil (Figure ). The baseline obtained from 0 ppm of furfural (oil) with 50 mM of APDS solution was a little higher than that obtained using 50 mM of APDS solution because of the use of a polystyrene cuvette in the former (Figure ). However, the detection efficiency was not affected because the intensity was very low at 550 nm, and the difference between 0 and 0.001 mM was significant enough (Figure A). The color change with the furfural concentration was also significant. The differences can be seen by the naked eye (Figure B). Approximately 76% of native furfural was recovered from the oil matrix through a single-step ethanol-acetic acid based extraction, without the need for concentration or enrichment. The recovery was calculated by comparing the amount detected in the aqueous layer (via HPLC) to the theoretical amount expected from complete transfer, based on a solution-phase calibration curve.
5.
(A) UV–vis spectrum. (B) Color development of furfural–APDS reaction solutions through an in situ extraction of 0–0.01 mM of model furfural in a power transformer insulating fluid with APDS (50 mM). In situ extraction and reaction were performed at a ratio of 4/1 (v/v, fluid/APDS solution). (C) Linear correlation of absorbance at 550 nm and furfural concentration in furfural standard solution and model insulating fluid. (D) Recovery of furfural extracted from the model insulating fluid.
Formulation of APDS in PEG for Furfural–APDS Assay
To realize a highly efficient and convenient colorimetric detection of furfural in the insulation oil of transformers, we obtained APDS using PEG-3350 on a chromatography paper, which can be used as a colorimetric sensing strip. Because solid APDS and PEG were used, leaking of chemicals from the strip could be avoided after drying the solvents.
Furfural was simply extracted from the oil using a solution comprising 70% AA and 30% ethanol. This solution can be directly applied for the furfural–APDS reaction in the strip. The extraction can be performed at the high oil-to-extraction solution ratio (10:1) to improve the detection range since clear phase separation was obtained.
The visual color change with each condition of the APDS strip corresponds to the concentration of the furfural solution (up to 0.2 and 0.01 mM, respectively, as shown in Figure A1,B1). The concentration of the furfural solution and relative reflectance (R %, UV–vis–NIR, PerkinElmer, Lambda 1050) of the APDS strip demonstrated a high linearity at 554 nm (Figure S7). The colorimetric responses were highly systematic, and a strong correlation and high linearity were observed among the furfural concentrations of R %. Because the extraction was performed at 10 times high ratio, 0.002 mM of furfural solution could be observed by the naked eye (Figure B1). Notably, the color range and R % value of 0.01 mM of the sample were similar to those of 0.05 and 0.1 mM of standard furfural solutions (Figure A2,B2). R % was the average value obtained from five APDS strips for each concentration of furfural solution. The relative standard deviation (RSD) of the response of the APDS strip for the samples with the same concentration (N = 5) was less than 8% (Figure ). Detailed data on the RSD are given in Figure S16 and Tables S3 and S4. The results obtained were reasonable because 76% recovery in extraction from oil was confirmed. Therefore, an APDS strip can be prepared on the paper by applying PEG. The resulting paper can be used for colorimetric sensing as a safe and simple method.
6.
Colorimetric responses on (A1) APDS strip and (A2) correlation between the diffuse reflectance value and furfural concentration prepared at 0, 0.01, 0.05, 0.1, and 0.2 mM in solution of 70% AA and 30% ethanol. Colorimetric responses on (B1) APDS strip and (B2) correlation between the diffuse reflectance value and furfural concentration extracted from the furfural model solution at 0, 0.001, 0.0025, 0.005, and 0.01 mM in the power transformer insulating fluid. The extraction was performed at the 10:1 ratio (oil: solution of 70% AA and 30% ethanol).
7.
Relative standard deviation (RSD %) of diffuse reflectance analysis for (A) furfural concentration prepared at 0, 0.01, 0.05, 0.1, and 0.2 mM in a solution comprising 70% AA and 30% ethanol and (B) furfural concentration extracted from a furfural model solution at 0, 0.001, 0.0025, 0.005, and 0.01 mM in a power transformer insulating fluid calculated from the standard deviation.
Characterization of APDS Strip Surface Reactions by XPS
The new peaks of Stenhouse adducts were determined using X-ray photoelectron spectroscopy (XPS). Before the reaction, the XPS spectra showed the following bands: (1) C 1s: C–C (phenyl of APDS) at 284.74 eV, C–N and C–S at 285.79 eV, C–O (PEG) at 286.61 eV, and a π–π* shakeup peak at 291.00 eV. (2) S 2p: S–C at 163.69 and 164.84 eV. (3) N 1s: N–C at 399.43 eV (Figure ).
8.
XPS spectra of APDS: (A) C 1s spectrum; (B) enlarged view of panel (A); (C) S 2p region; (D) N 1s spectrum; (E) enlarged view of panel (D).
The APDS strips were immersed in a 10 mM furfural solution prepared in 30 mM HCl for 5 min. To overcome the detection limit of XPS and the surface dryness caused by vacuum conditions prior to the measurement, an excess amount of furfural was used to ensure sufficient surface reaction. Furthermore, weak HCl was employed instead of AA as the O–CO peak in the C 1s region overlapped with the CN signal, complicating spectral deconvolution.
After the Stenhouse reaction, new peaks appeared at 289.18 eV (CN peak) in the C 1s region and 406.23 eV (Schiff base of π–π* satellite), 401.69 eV (Schiff base, NC peak), which are attributed to the Stenhouse adducts formation of Schiff base of CN (Figure ). In addition, the S–C peak showed no noticeable change, indicating that the sulfide bonding does not participate in the Stenhouse reaction and remains chemically stable. These surface chemical changes indicate the successful Stenhouse coupling between furfural and the APDS strip at the surface level during the reaction process.
9.
XPS spectra of APDS strips after the Stenhouse reaction. APDS strips were immersed in a solution of 10 mM furfural in 30 mM HCl. (A) C 1s spectrum; (B) enlarged view of panel (A); (C) S 2p region; (D) N 1s spectrum; (E) enlarged view of panel (D).
Discussion of Limitations and Future Directions
A summary comparison with previously reported methods is provided in Table . While some approaches, such as the polymer-based sensor by Rico-Yuste and co-workers, demonstrated lower LODs, they required lengthy incubation times and additional fabrication steps. In contrast, our method offers a favorable balance of sensitivity (LOD = 0.00024 mM), short reaction time (5–10 min), and operational simplicity under ambient conditions. Furthermore, it avoids toxic reagents or complex sample pretreatment, aligning with green chemistry principles and making the method suitable for rapid and eco-conscious furfural detection. Compared to our group’s prior studies, , the current method also expands the application scope to oil matrices and test strips, while improving analytical performance and environmental compatibility. The proposed method demonstrates high sensitivity and selectivity for detecting low levels of furfural in an environmentally friendly manner and is applicable as a polymer-based test strip. Compared to conventional HPLC-based detection techniques, our approach offers notable advantages in terms of cost-effectiveness, simplicity, and environmental sustainability.
1. Summary of Key Parameters for Furfural Detection in Recent Studies.
| method | detection method | application scope | reaction time (min) | LOD (mM) | green method | refs |
|---|---|---|---|---|---|---|
| this work | colorimetric assay, UV–vis | solution, oil (via aqueous extraction), solid strip | 5–10 | 0.00024 | yes | |
| Park et al., 2023 | UV–vis | solution, oil (via aqueous extraction) | 5–10 | 0.001 | yes | |
| Park et al., 2024 | colorimetric assay, UV–vis | solution, oil (via aqueous extraction) | 5–10 | 0.00176 | no | |
| Rico-Yuste et al., 2016 | colorimetric assay, optic sensor | polymer film | 60 | 0.00012 | partially |
Unlike HPLC, which requires expensive instrumentation and significant amounts of organic solvents for mobile phases, our method enables rapid, reagent-based visual detection, reducing both analysis time and solvent consumption.
However, this method also has several limitations. First, as it relies on visual interpretation, the quantitative accuracy is lower than that of HPLC, which provides precise concentration measurements. The sensitivity of the test strip could also be limited in detecting ultralow concentrations of furfural. Furthermore, reproducibility may be affected due to slight variations in color perception and environmental conditions. In addition, compared to aniline, APDS offers a significantly less toxic and more environmentally friendly alternative, making it a safer choice for sustainable applications. This highlights the potential of APDS as a sustainable substitute in applications where minimizing health and ecological risks is of paramount importance.
To address these limitations, future research will focus on improving the chemical stability of the polymer matrix by incorporating chemically synthesized polymers that directly participate in the Stenhouse reaction. This approach is expected to enhance resistance to acid catalysts and organic solvents, improving durability and reliability. Additionally, optimizing the formulation and detection conditions could enhance sensitivity and reproducibility, making the method more applicable in diverse real-world scenarios.
Conclusions
A new furfural–APDS colorimetric chemosensory assay was developed for the quantification of furfural, based on the Stenhouse reaction between furfural and APDS. The method could be employed for both UV spectroscopy and colorimetric analyses. A high correlation between UV–vis absorbance and furfural concentrations was obtained, which confirmed the high sensitivity (LOD, 0.00024 mM furfural), while recognizing the color change at 0.005 mM furfural by the naked eye. This was unique and highly selective because only furfural demonstrates UV absorbance within 450–600 nm and color change, in contrast to other aromatic and aliphatic aldehydes. Therefore, we used this method for the quantification of 0–0.01 mM of furfural in the power transformer insulating fluid. We further developed this method by using an APDS strip formulated with PEG as the chromatography paper. In addition, XPS analysis revealed distinct surface chemical changes that indicate successful Stenhouse coupling between furfural and the APDS-modified surface. The preservation of the S–C bonding and the appearance of CN related peaks support that the reaction occurred selectively at the surface without disrupting the sulfide structure. These findings demonstrate the feasibility of controlled surface functionalization via Stenhouse-type reactions under mild acidic conditions. While APDS’s toxicological profile is not as well-studied as aniline’s, its significantly higher LD50 value, nonhazardous classification, and lack of reported carcinogenicity suggest that it may serve as a safer alternative in chemical applications. We were able to replace the toxic liquid aniline, used in conventional methods, by solid APDS. The resulting procedure was straightforward and safer for handling and can be used for on-site analysis. Therefore, this liquid- and solid-based assay offers a novel green analytical concept to quantitatively determine the concentration of furfural in various fields, such as food, beverages, biological, clinical, and environmental sciences.
Methods
Materials
Ultrapure water was obtained from a Milli-Q water purification system (Millipore, USA). Furfural (99%), bis(4-aminophenyl) disulfide (APDS, 98%), aniline(99%), 5-hydroxymethylfurfural (99%), benzaldehyde (99%), 4-hydroxy-benzaldehyde (99%), vanillin (99%), acetonitrile (≥99%), butyraldehyde (98%), pentaldehyde (98%), methanol (99%), ethanol (99%), AA (99%), valeric acid (99%), propionic acid (99%), hydrochloric acid (HCl, 35 wt % in H2O purity), formaldehyde (35 wt % in H2O purity), polyethylene glycol-3350 (PEG), deuterium oxide (D2O, 99.9 atom %), dimethyl sulfoxide-d6 (99.8 atom %), and chloroform-d (99.8 atom %) were used. All chemicals and Whatman cellulose chromatography papers were provided by Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used without further treatment.
Colorimetric and Spectroscopic Analyses on the Reaction of Furfural with APDS by UV–Vis Spectroscopy
The furfural–APDS assay was performed by using a modified method based on the Stenhouse reaction. , During the APDS-colorimetric assay, the color development and absorbance shift for the reaction of furfural and APDS in acid were monitored at the absorbance range of 250–600 nm by using an UV–vis spectrometer (UV–vis–NIR, Agilent, Cary5000). Then, 50 mM of APDS solutions in AA, propionic acid, valeric acid, and 0.031 M HCl (1/1) were freshly prepared. Next, 20 ppm (0.21 mM) of furfural solutions in AA, propionic acid, valeric acid, and 0.031 M HCl were also freshly prepared. Then, 150 μL of furfural solutions and 150 μL of the APDS solution were mixed in an UV quartz cuvette (path length 10 × 10 mm). After performing the reaction for 10 min, the absorbance spectra for the furfural and APDS reaction at different reaction times were obtained within the absorbance range of 250–600 nm by using an UV–vis spectrometer. For reference, the absorbance spectra of AA, 0.1 mM furfural, and 10 and 50 mM of APDS in AA were obtained and compared within the absorbance range of 250–600 nm by using an UV–vis spectrometer. All the data were obtained from two independent experiments and are provided as the average of the replicates.
Optimization of the Colorimetric Reaction of APDS and Furfural in AA
The necessary assay factors were investigated for the reaction of furfural and APDS in AA by UV–vis spectral analysis. The reaction rate of 0.1 mM of furfural (150 μL) and 10 mM of APDS (150 μL) in AA was monitored in a quartz cell (5 × 5 mm) within the 400–600 nm range of UV–vis radiation in the reaction time course using an UV–vis spectrometer. To evaluate the effect of the solvent, 0.1 mM of furfural was prepared in AA, and 10 mM of APDS was prepared using a mixture of 10% AA and 90% solvent, such as acetonitrile, methanol, and ethanol. Next, 100 μL of furfural and 100 μL of APDS solution were placed in a 96-well plate and reacted in the reaction time course. Detail experiments were performed for ethanol solutions prepared at the ratios of 100/0 (AA/ethanol), 80/20, 70/30, 60/40, 40/60, 20/80, and 0/100. The UV adsorption at 550 nm for the reaction of furfural with each APDS solution was measured at 1, 3, 5, 10, 15, 20, 25, and 30 min using an UV–vis microplate spectrophotometer (Multiskan GO, Thermo Scientific, USA).
The effect of water was evaluated on the reaction of 100 μL of 0.1 mM furfural in AA and 100 μL of 10 mM APDS in a mixture of 10% AA and 90% ethanol in a 96-well plate. Four experiments were performed with and without the addition of water at 0, 5, and 10 min. The UV adsorption at 550 nm was measured for experiments at 1, 3, 5, 10, 15, 20, 25, and 30 min by using an UV–vis microplate spectrophotometer.
For evaluating the absorbance responses by using the concentration of furfural, furfural solution at 0, 0.05 (0.0005 mM), 0.1 (0.001 mM), 0.5 (0.005 mM), 1 (0.01 mM), 5 (0.005 mM), 10 (0.1 mM), 20 (0.2 mM), and 50 ppm (0.5 mM), and 50 mM APDS were freshly prepared in a mixture of 70% AA and 30% ethanol. Three different ratios of solution ((a) 100 μL of furfural + 50 μL of APDS, (b) 100 μL of furfural + 100 μL of APDS, (c) 200 μL of furfural + 100 μL of APDS) were placed and mixed in the wells of 96-well microplates at room temperature. The reactions and color development were analyzed at 550 nm and apparently monitored at 10 min by using an UV–vis microplate spectrophotometer and by the naked eye after taking pictures.
For comparing the Stenhouse reactivities of APDS and aniline, 0, 0.05 (0.0005 mM), 0.1 (0.001 mM), 0.5 (0.005 mM), 1 (0.01 mM), 5 (0.005 mM), 10 (0.1 mM), 20 (0.2 mM), and 50 ppm (0.5 mM) of furfural solution; 50, 100, and 200 mM of aniline; and 50 mM of APDS were freshly prepared in a mixture comprising 70% AA and 30% ethanol. Four different condition ((a) 100 μL of furfural + 100 μL of 50 mM aniline, (b) 100 μL of furfural + 100 μL of 100 mM aniline, (c) 100 μL of furfural + 100 μL of 200 mM aniline, and (d) 100 μL of furfural + 100 μL of 50 mM APDS) were placed and mixed in the wells of 96-well microplates at room temperature. The reactions and color development were analyzed in the same way.
Colorimetric and Spectroscopic Analyses of Aliphatic and Aromatic Aldehydes by UV–Vis Spectroscopy
Color development and absorbance shift for the reaction of aldehyde and APDS in acid during the aldehyde-APDS colorimetric assay were monitored at the absorbance range of 200–800 nm by using an UV–vis spectrometer. Next, 50 mM of APDS solution in a mixture comprising 70% AA and 30% ethanol was freshly prepared. Then, 0.2 mM of aliphatic aldehydes (formaldehyde, butanal, and pentanal) and aromatic aldehydes (4-hydroxy-benzylaldehyde, vanillin, benzaldehyde, 5-hydroxymethylfurfural, and furfural) were freshly prepared in a mixture of 70% AA and 30% ethanol. Subsequently, 500 μL each of aldehyde solution and 50 mM of APDS solution were placed and mixed in an UV quartz cuvette (path length 10 × 10 mm). After conducting the reaction for 10 min, the spectra were obtained at the absorbance range of 400–600 nm by using an UV–vis spectrometer. Aldehyde–APDS adducts were analyzed in the mass spectrum range of 0–1700 m/z by Agilent LC TOFMS (6230B TOF LC/MS). The furfural–APDS adducts were characterized by 1H NMR using a Bruker 600 MHz spectrometer. The analytical sample was examined in DMSO-d 6 containing 0.03% (v/v) TMS. Color development for these aldehydes was compared in the reaction involving 150 μL of aldehyde solution and 150 μL of 50 mM APDS in a 96-well microplate, as observed by the naked eye. All the data were obtained from two independent experiments and provided as the average of the replicates.
Furfural–APDS Assay to Determine the Furfural Concentration in a Power Transformer Insulating Fluid
The furfural model samples were prepared at 0, 0.001, 0.0025, 0.005, and 0.01 mM furfural in a power transformer insulating fluid. To evaluate the furfural–APDS assay, 2800 μL of fluid with 700 μL of 50 mM APDS prepared in 70% AA and 30% ethanol was placed in a polystyrene cuvette (external dimensions, 12.5 × 12.5 × 45 mm, path length 10 mm), which is useable in the range of 200–800 nm. The individual sample mixtures were prepared in a polystyrene cuvette and shaken up and down several times to mix. They were then left to stand for 3 min to achieve phase separation. This is an in situ extraction of furfural from the power transformer insulating fluid into an immiscible APDS solution. The reaction was then performed with APDS in the cuvette. After 10 min of phase separation, the lower phase of the solution in the cuvette was directly used to determine the absorbance in the range of 400–600 nm by using an UV–vis spectrometer. The extraction recovery of furfural at different concentrations in fluid was calculated using a standard curve obtained by the ASTM D5387 (HPLC) method.
Formulation of APDS Strip Using PEG and Analysis of Surface Reactions
An APDS strip was prepared using a chromatography paper with the help of polyethylene glycol-3350 (PEG). PEG (250 mg) was dissolved in 1 mL of methanol, followed by the addition and dissolution of 25 mg of APDS (100 mM solution). The 3 × 2.5 cm of a chromatography paper was immersed in 250 μL solution and dried in room temperature for 1 h. Furfural model solutions at 0.0001, 0.0025, 0.005, and 0.01 mM were prepared in a power transformer insulating fluid and extracted at a ratio of 10 (fluid) to 1 (solution of 70% AA and 30% ethanol). Also, furfural standard solutions at 0.001, 0.01, 0.05, 0.1, and 0.2 mM were prepared in a solution comprising 70% AA and 30% ethanol. Furfural samples (250 μL) were extracted and dropped on the 3 × 2.5 cm of an APDS strip. After 4 min, a picture of the strip was taken, and the strip was directly used to measure R % in the range of 350–700 nm by using an UV–vis spectrometer with an integrating sphere. Diffuse reflectance means the intensity of R % described as below
To investigate the precision of the APDS strip, five APDS strips for each concentration were tested on a diffuse reflectance spectrum. A comparison of the average of R % raw data and R % raw data was used to obtain standard deviation and RSD (%).
The Stenhouse reaction on the APDS strip surface was elucidated by XPS using NEXA G2 (Thermo scientific) analysis. The XPS measurements were performed using an Al Kα X-ray source with a 400 μm beam size. A flood gun was used for charge compensation, and peak calibration was carried out against the C 1s peak at 284.8 eV for carbon correction. Due to detection limits and vacuum constraints, the strip was immersed in an excess furfural solution to induce the color-forming reaction. The color-developed strip surface was then analyzed. To avoid potential interference from the organic acid’s O–CO functional group, 30 mM hydrochloric acid was used as the acid catalyst instead of an AA.
Supplementary Material
Acknowledgments
This research was supported by the ministry of Trade, Industry and Energy (MOTEI) under the project (Project Number: 20221A10100011).
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c00572.
Reaction mechanism of APDS and furfural; 1H NMR spectrum of furfural in DMSO-d 6; 1H NMR spectrum of APDS in DMSO-d 6; 1H NMR spectrum of furfural-APDS adducts in DMSO-d 6; Mass spectrum of furfural-APDS; Mass spectrum of HMF-APDS; Mass spectrum of benzaldehyde-APDS; Mass spectrum of 4H-Benzaldehyde-APDS; Mass spectrum of vanilline-APDS; Mass spectrum of formaldehyde-APDS; Mass spectrum of butylaldehyde-APDS; Mass spectrum of pentylaldehyde-APDS; Linear co-relationship between UV absorbance at 550 nm and furfural concentrations, and colorimetric responses obtained from the reaction of furfural (0–0.5 mM) and 50 mM APDS prepared in the mixture of 70% acetic acid and 30% ethanol. Reaction of furfural and 100 μL of 50 mM aniline. Reaction of 100 μL furfural and 100 μL of 100 mM aniline. Reaction of 100 μL furfural and 100 μL of 200 mM aniline. Reaction of 100 μL furfural and 100 μL of 50 mM APDS; Full scan(200–800 nm) spectra of aldehyde-APDS reaction solutions obtained from reaction of aliphatic aldehydes and aromatic with 50 mM APDS aldehyde; UV–Vis spectrum color development of furfural-APDS reaction solutions through in situ extraction model furfural in power transformer insulating fluid with 50 mM APDS; In situ extraction and reaction without shaking; Diffuse reflectance spectrum of APDS-strip furfural and furfural concentration prepared at 0, 0.01, 0.05, 0.1, and 0.2 mM in solution of 70% acetic acid and 30% ethanol. Diffuse reflectance spectrum of APDS-strip furfural and furfural model solution at 0, 0.001, 0.0025, 0.005, and 0.01 mM in power transformer insulating fluid. The extraction was performed at 10:1 ratio (oil: solution of 70% acetic acid and 30% ethanol); Corrected diffuse reflectance value on each furfural concentration. The lines represent average of corrected diffuse reflectance in each group of furfural concentration prepared at 0, 0.01, 0.05, 0.1, and 0.2 mM in solution of 70% acetic acid and 30% ethanol and furfural concentration extracted from furfural model solution at 0, 0.001, 0.0025, 0.005, and 0.01 mM in power transformer insulating fluid; Raw absorbance data and statistical summary; MSDS-based toxicity comparison of APDS and aniline; Mean, standard deviation (SD) and relative standard deviation (RSD) of raw data (R%) reported from furfural concentration prepared at 0, 0.01, 0.05, 0.1, and 0.2 mM in solution of 70% acetic acid and 30% ethanol; Mean, standard deviation (SD) and relative standard deviation (RSD) of raw data (R%) reported from furfural concentration extracted from furfural model solution at 0, 0.001, 0.0025, 0.005, and 0.01 mM in power transformer insulating fluid (PDF)
The authors declare no competing financial interest.
References
- Anastas P. T.. Green chemistry and the role of analytical methodology development. Crit. Rev. Anal. Chem. 1999;29:167–175. doi: 10.1080/10408349891199356. [DOI] [Google Scholar]
- Gałuszka A., Migaszewski Z., Namieśnik J.. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC, Trends Anal. Chem. 2013;50:78–84. doi: 10.1016/j.trac.2013.04.010. [DOI] [Google Scholar]
- Rico-Yuste A., González-Vallejo V., Benito-Peña E., De las Casas Engel T., Orellana G., Moreno-Bondi M. C.. Furfural determination with disposable polymer films and smartphone-based colorimetry for beer freshness assessment. Anal. Chem. 2016;88:3959–3966. doi: 10.1021/acs.analchem.6b00167. [DOI] [PubMed] [Google Scholar]
- Cepeda-Vázquez M., Blumenthal D., Camel V., Rega B.. Multivariate optimization of headspace trap for furan and furfural simultaneous determination in sponge cake. Talanta. 2017;164:708–715. doi: 10.1016/j.talanta.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Xing Q., Ma Y., Fu X., Cao Q., Zhang Y., You C.. Effects of heat treatment, homogenization pressure, and overprocessing on the content of furfural compounds in liquid milk. J. Sci. Food Agric. 2020;100:5276–5282. doi: 10.1002/jsfa.10578. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Bousquières J., Cepeda-Vázquez M., Roux S., Bonazzi C., Rega B.. Kinetic study of furan and furfural generation during baking of cake models. Food Chem. 2018;267:329–336. doi: 10.1016/j.foodchem.2017.06.126. [DOI] [PubMed] [Google Scholar]
- Milani M. I., Rossini E. L., Castoldi K., Pezza L., Pezza H. R.. Paper platform for reflectometric determination of furfural and hydroxymethylfurfural in sugarcane liquor. Microchem. J. 2017;133:286–292. doi: 10.1016/j.microc.2017.03.046. [DOI] [Google Scholar]
- Janzowski C., Glaab V., Samimi E., Schlatter J., Eisenbrand G.. 5-hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000;38:801–809. doi: 10.1016/S0278-6915(00)00070-3. [DOI] [PubMed] [Google Scholar]
- Shi H., Chen W., Wan F., Du L., Zhang S., Zhou W., Zhang J., Huang Y., Zhu C.. Application of self-assembled Raman spectrum-enhanced substrate in detection of dissolved furfural in insulating oil. Nanomaterials. 2018;9:17. doi: 10.3390/nano9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymouri A., Vahidi B.. Power transformer cellulosic insulation destruction assessment using a calculated index composed of CO, CO2, 2-furfural, and acetylene. Cellulose. 2021;28:489–502. doi: 10.1007/s10570-020-03548-1. [DOI] [Google Scholar]
- Kishikawa N., El-Maghrabey M. H., Kuroda N.. Chromatographic methods and sample pretreatment techniques for aldehydes determination in biological, food, and environmental samples. J. Pharm. Biomed. Anal. 2019;175:112782. doi: 10.1016/j.jpba.2019.112782. [DOI] [PubMed] [Google Scholar]
- Li Z., Fang M., LaGasse M. K., Askim J. R., Suslick K. S.. Colorimetric recognition of aldehydes and ketones. Angew. Chem., Int. Ed. 2017;56:9860–9863. doi: 10.1002/anie.201705264. [DOI] [PubMed] [Google Scholar]
- Liang J., Li H., Wang J., Yu H., He Y.. Cascade chromogenic system with exponential signal amplification for visual colorimetric detection of acetone. Anal. Chem. 2020;92:6548–6554. doi: 10.1021/acs.analchem.0c00149. [DOI] [PubMed] [Google Scholar]
- de Almeida J. P. B., dos Santos T. F. F. T., Sabino Júnior J. R., do Amaral E. V. F., Oliveira C. R. S., Maia M. V., Suarez W. T., Ayres L. B., Garcia C. D., dos Santos V. B.. Combining Digital Imaging and Quantum Dots for Analytical Purposes. Anal. Methods. 2025;17(5):916–938. doi: 10.1039/d4ay02097a. [DOI] [PubMed] [Google Scholar]
- de Castro C. M., Olivi P., de Freitas Araújo K. C., Barbosa Segundo I. D., dos Santos E. V., Martínez-Huitle C. A.. Environmental Application of a Cost-Effective Smartphone-Based Method for COD Analysis: Applicability in the Electrochemical Treatment of Real Wastewater. Sci. Total Environ. 2023;855:158816. doi: 10.1016/j.scitotenv.2022.158816. [DOI] [PubMed] [Google Scholar]
- Feng L., Musto C. J., Suslick K. S.. A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. J. Am. Chem. Soc. 2010;132:4046–4047. doi: 10.1021/ja910366p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry M. S., Lee Y. C.. A rapid formaldehyde assay using purpald reagent: application under periodation conditions. Anal. Biochem. 1996;234:50–55. doi: 10.1006/abio.1996.0048. [DOI] [PubMed] [Google Scholar]
- Dickinson R. G., Jacobsen N. W.. A new sensitive and specific test for the detection of aldehydes: formation of 6-mercapto-3-substituted-s-triazolo[4,3-b]-s-tetrazines. J. Chem. Soc. D. 1970;(24):1719–1720. doi: 10.1039/c29700001719. [DOI] [Google Scholar]
- Park H., Kim E., Jun T., Pyo S. H., Kim S. H.. Colorimetric Detection of Furfural with Enhanced Visible Absorption of Furfural-DNPH in Basic Conditions. ACS Omega. 2024;9:2519–2527. doi: 10.1021/acsomega.3c07025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeußel L., Singh S.. Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for specific Amin Identification. Molecules. 2023;28(18):6627. doi: 10.3390/molecules28186627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. ; Fu, Q. ; Lin, M. ; Zhao, Y. ; Qian, Y. ; Li, S. . A novel furfural-detection-method for the aging prediction of paper insulation in power transformer. In IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP); IEEE, 2018, pp 630–633. [Google Scholar]
- de Oliveira Krambeck Franco M. D. O. K., Suarez W. T., Santos V. B. d.. Digital image method smartphone-based for furfural determination in sugarcane spirits. Food Anal. Methods. 2017;10:508–515. doi: 10.1007/s12161-016-0605-4. [DOI] [Google Scholar]
- Stenhouse J. T.. Ueber die Oele, die bei der Einwirkung der Schwefelsäure auf verschiedene Vegetabilien Entstehen. Adv. Cycloaddit. 1850;74(74):278–297. doi: 10.1002/jlac.18500740304. [DOI] [Google Scholar]
- Gomes R. F. A., Coelho J. A. S., Afonso C. A. M.. Synthesis and applications of Stenhouse salts and derivatives. Chemistry. 2018;24:9170–9186. doi: 10.1002/chem.201705851. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Szymański W., Feringa B. L.. The (photo)chemistry of Stenhouse photoswitches: guiding principles and system design. Chem. Soc. Rev. 2018;47:1910–1937. doi: 10.1039/C7CS00772H. [DOI] [PubMed] [Google Scholar]
- Clerc M., Stricker F., Ulrich S., Sroda M., Bruns N., Boesel L. F., Read de Alaniz J.. Promoting the furan ring-opening reaction to access new donor–acceptor Stenhouse adducts with hexafluoroisopropanol. Angew. Chem., Int. Ed. Engl. 2021;60:10219–10227. doi: 10.1002/anie.202100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigma-Aldrich . Safety Data Sheet: 4-Aminophenyl Disulfide (APDS). SDS No. 369462; Sigma-Aldrich: Seoul, Korea, 2024. Available at www.sigmaaldrich.com (accessed March 11, 2025). [Google Scholar]
- Sigma-Aldrich . Safety Data Sheet: Aniline. SDS No. 242284; Sigma-Aldrich: Seoul, Korea, 2024. Available at www.sigmaaldrich.com (accessed March 11, 2025). [Google Scholar]
- Gao W., Xu J., Zuo P., Dong H., Quan Y., Chang P.. Aromatic amine-terminated polysulfide oligomer: synthesis and application in self-healable polyurea. J. Polym. Sci., Part A: Polym. Chem. 2019;57:1460–1466. doi: 10.1002/pola.29410. [DOI] [Google Scholar]
- Li S. W., Batey R. A.. Mild lanthanide(III) catalyzed formation of 4, 5-diaminocyclopent-2-enones from 2-furaldehyde and secondary amines: A domino condensation/ring-opening/electrocyclization process. Chem. Commun. 2007;36:3759–3761. doi: 10.1039/b709337n. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., Gupta V. B.. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011;2:21–25. doi: 10.4103/2229-5186.79345. [DOI] [Google Scholar]
- International Conference on Harmonisation (ICH) . ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2005. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed June 27, 2025). [Google Scholar]
- Angerer, J. ; Bernauer, U. . Opinion on Furfural. SCCS/1461/12; SCCS, 2012. [Google Scholar]
- dos Santos V. B., Ayres L. B., de Sousa H. S., Garcia C. D., Toito Suarez W.. Detection of Surfactants Using a Hydrophobic Natural Deep Eutectic Solvent and Smartphone. Sens. Diagn. 2024;3:1467–1475. doi: 10.1039/d4sd00196f. [DOI] [Google Scholar]
- Pena-Pereira F., Wojnowski W., Tobiszewski M.. AGREEAnalytical GREEnness Metric Approach and Software. Anal. Chem. 2020;92:10076–10082. doi: 10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, J. P. ; Nyeon, H. J. ; Chang, H. N. . Simple Test Method for Furan in Aged Transformer Oil and Its Kit. KR101231586B1, 2013.
- Park H., Kim E., Kwak B. S., Jun T., Kawano R., Pyo S.-H.. Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid. Separations. 2023;10(7):381. doi: 10.3390/separations10070381. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.