Abstract
We previously showed that infiltrating cytotoxic immune cells are reprogrammed to regulatory-like/exhausted cells within accepted kidney allografts through a ‘defensive tolerance’ mechanism. We observed a regulatory B cell (Breg) signature within the accepted kidney. Here we show that despite a Breg phenotype, neither B cell depletion nor the use of μMT recipients which lack B cells, resulted in kidney allograft rejection. Negative regulators of B cell function, Siglecg and Fcgr2b, show increased expression in both accepted kidney and lung allografts. Kidney allografts transplanted in B6.Fcgr2b KO recipients underwent antibody mediated rejection. Hypothesizing that similar mechanisms in a tumor microenvironment may attenuate anti-tumor immunity, we observed that expression of SIGLEC10, the human homolog of Siglecg, was associated with resistance to anti-PD1 therapy in human melanomas. In conclusion, B cell expression of FcγRIIB and Siglec-G appear to play an essential role in maintaining transplant tolerance and in tumor evasion of anti-tumor immunity.
A major goal in the field of transplantation is to identify and implement reliable protocols that induce allograft acceptance in the absence of ongoing immunosuppressive therapy1–3. This ‘holy grail’ would eliminate both the frequent development of chronic rejection and the debilitating side-effects of current standard-of-care treatment regimens4,5. To this end, we and others have studied how tolerance is induced and maintained in various natural biological scenarios, including pregnancy and tumors. Of unique value for defining specific mechanistic pathways are the spontaneous mouse transplant tolerance models, where kidney or liver allografts are accepted in certain strain combinations without the use of immunosuppression6–8. We have previously shown that accepted kidney allografts develop localized, intra-graft aggregates of various lymphocytes around the small vessels of the graft. We refer to these structures as regulatory Tertiary Lymphoid Organs (rTLOs), reminiscent of Tertiary Lymphoid Structures (TLS) found in solid tumors and tolerant lung allografts6,7,9–13. Immunohistochemistry showed that these rTLOs contain Foxp3+ regulatory T cells (Tregs), CD8+ and CD4+ T cells, B cells, conventional and plasmacytoid dendritic cells, and macrophages6. Serial analysis of single cell RNA sequencing (scRNAseq) data from CD45+ cells isolated from accepted kidney allografts revealed a shift from a T cell-dominant to a B cell-rich population by 6 months with an increased regulatory B cell signature14. These findings are consistent with B cell signatures that have been associated with renal transplant tolerance in humans15–17. The functionality of the B cell subsets identified in our kidney transplant model remains unclear. Therefore, the focus of this study was to assess the role that B cells play in kidney graft tolerance.
Here we show that neither depletion of B cells at different times post kidney transplant nor using μMT recipients that lack B cells resulted in rejection. Our scRNAseq data showed a temporal increase of Fcgr2b expression within the B cell clusters. Fcgr2b encodes for FcγRIIb, an inhibitory receptor on B cells, that regulates their function18,19. Using B6.Fcgr2b knockout (KO) recipients, we demonstrate that kidney allografts are rejected with a mean survival time of 21 days when compared to WT recipients. Histological analysis of the rejected kidneys from B6.Fcgr2b KO mice showed thrombotic microangiopathy, glomerulitis and capillaritis, and transplant glomerulopathy, features characteristic of acute and chronic antibody mediated rejection (AbMR).
Complementary to kidney transplantations, analysis of scRNAseq data from re-transplanted tolerized mouse lung allografts20,21 show that newly infiltrating B cells express Siglecg and Fcgr2b while resident B lymphocytes have a regulatory B cell (Breg) signature. Trajectory analysis shows that Breg-like cells arise from Siglecg+Fcgr2b+ B cells. The development of a Breg-like phenotype within a tolerized lung allograft is similar to our previous observations in a model of spontaneous kidney allograft acceptance14.
Lastly, in regard to malignancies, scRNAseq analyses of immune cells from the tolerized tumor microenvironment of spontaneous pancreatic tumors in the KrasLSL.G12D/+; p53R172H/+; PdxCretg/+ (KPC) mouse model for pancreatic ductal adenocarcinoma and human melanoma patients show a similar increased expression of Siglecg and Fcgr2b as well as SIGLEC10 (human homolog of Siglecg) and FCGR2B within the B cell population, respectively. Furthermore, increased SIGLEC10 expression correlated with resistance to anti-PD1 therapy.
Our results suggest that B cells do not play a direct role in the induction and maintenance of kidney allograft tolerance. They rather infiltrate the kidney allografts as pathogenic cells and are reprogrammed to Breg/exhausted cells, first by the induction of Siglecg and Fcgr2b, followed by the increased expression of genes that define Bregs. This extends findings from our recent study that demonstrated that graft infiltrating alloreactive cytotoxic CD8+ T cells are reprogrammed within an accepted kidney allograft to an exhausted/regulatory-like phenotype mediated through a process we call ‘defensive tolerance’22. This intra-graft reprogramming appears to be a secondary, regulatory, and protective mechanism that is characteristic of tolerance, whether it be an accepted kidney or lung allograft in the absence of immunosuppression, or the tumor microenvironment. Overall, our findings further add to our understanding of allotransplant tolerance, with implications that can be expanded to how tumors escape the host’s immune system.
RESULTS
Depletion of B cells does not prevent acceptance of kidney allografts.
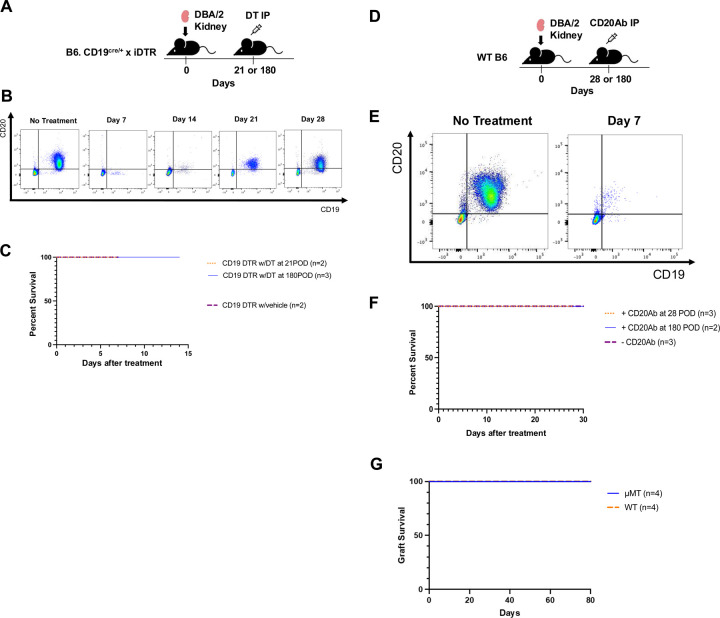
We previously described a temporal increase in a Breg-like phenotype within spontaneously accepted kidney allografts14. To ascertain the significance of this observation and assess the functionality of B cells in the maintenance of renal allotransplant tolerance, we utilized B6.[CD19Cre/+iDTRfl/+] mice as recipients, which express the diphtheria toxin receptor (DTR) on B cells and therefore allow for the depletion of CD19-expressing B cells after systemic treatment with diphtheria toxin (DT)23. Kidney allograft recipients were DT-treated at 28- or 180-days post-transplant (Fig. 1A). Flow cytometric analysis of peripheral blood mononuclear cells (PBMC) showed that B cells were depleted for up to 14 days before they began to recover 21 days post-treatment (Fig. 1B). Unlike the depletion of recipient regulatory T cells (Tregs) that results in the rejection of the DBA/2 kidney allografts 6–9 days post-DT treatment of B6.Foxp3-DTR recipients6, depletion of B cells did not result in the loss of the kidney graft, and maintained a 100% survival rate, comparable to vehicle treated recipients (Fig.1C). We also depleted B cells in B6 WT recipients using anti-CD20 antibodies (Fig.1D), a regimen that results in the depletion of B cells for up to 7days (Fig. 1E). Similar to DT-treated B6.[CD19Cre/+iDTRfl/+] recipients, we observed no rejection of DBA/2 kidney allografts following anti-CD20 mediated B cell depletion (Fig.1F). Furthermore, kidney allografts are spontaneously accepted in B6.μMT recipients with no rejection episodes up to 80 days post-transplantation indicating that B cells are not required for the induction or maintenance of tolerance in this model (Fig. 1G).
Fig. 1. B cells are not required for the induction and maintenance of kidney allograft acceptance.
(A) Schematic showing the experimental design for CD19+ cell depletion using B6. [CD19Cre/+iDTRfl/+] mice and intraperitoneal (IP) injection of diphtheria toxin (DT). (B) Serial flow cytometric analysis of CD19+ and CD20+ cells in peripheral blood mononuclear cells (PBMC) following DT administration. (C) Kidney allograft survival following DT injection at post-operative day (POD) 21 (n = 2) or POD 180 (n = 3), compared to vehicle-treated controls (n = 2). (D) Schematic of the experimental design for CD20+ cells depletion using IP injection of anti-CD20 antibody (CD20Ab) into B6 wildtype (WT) recipients of DBA/2 kidneys. (E) Serial flow cytometric analysis of CD19+ and CD20+ cells in PBMC over time following CD20Ab administration. (F) Kidney allograft survival following CD20Ab injection at POD 28 (n = 3) or POD 180 (n = 2), compared to untreated controls (n = 3). (G) DBA/2 kidney allograft survival in B6.μMT recipients (n=4), which lack mature B cells, compared to B6 WT recipients (n = 4).
Increased intra-graft expression of Fcgr2b and Siglecg within the B cell cluster.
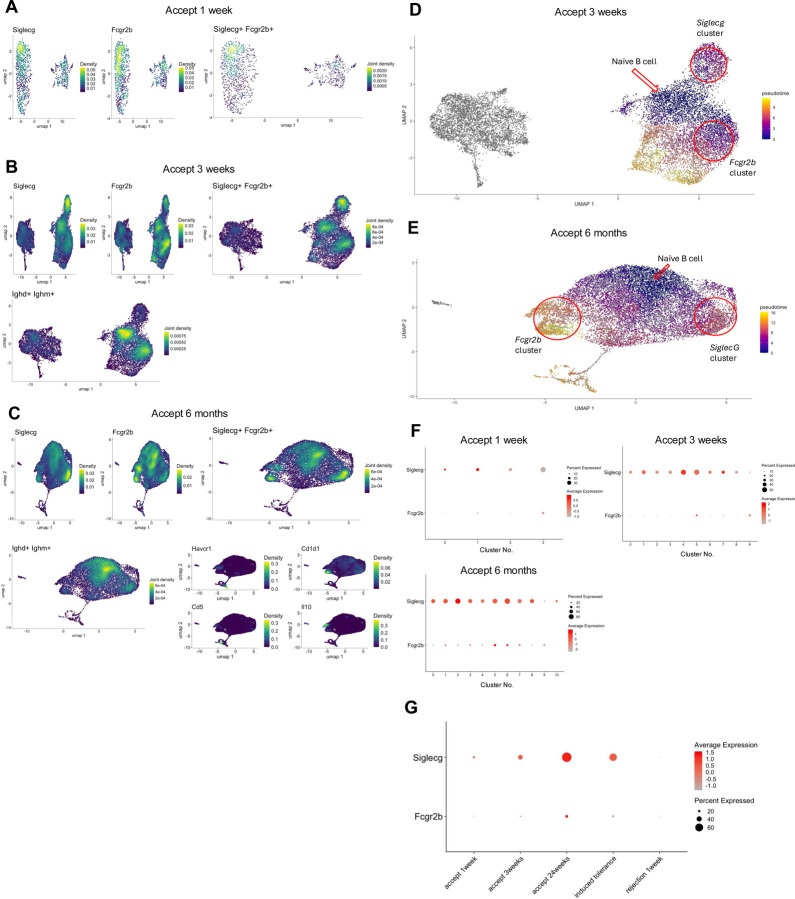
While our data suggest that B cells may not be involved in the induction and maintenance of kidney allograft tolerance, we have previously shown that Siglecg expression is temporarily increased within the B cell cluster in accepted kidney grafts14. Siglecg encodes for Siglec-G, which when expressed on B cells prevents the secretion of antibodies. It can also interact with CD22 on other cell types and induce the upregulation of Siglec-G on their surface24–26. We also showed that infiltrating cytotoxic CD8+ T cells are reprogrammed to a regulatory-like/exhausted phenotype within the tolerized kidney allograft accompanied by an increase in their Fgl2 expression22. Fgl2 encodes fibrinogen-like protein 2 (FGL2), which can be secreted by CD8+ Tregs and functions to induce Bregs and inhibit dendritic cell maturation by interacting with its receptor, FcγRIIB27,28. We next investigated the expression of Fcgr2b (gene encoding FcγRIIB) as an additional Breg marker in the DBA/2 to B6 spontaneous kidney allograft acceptance model. Concurrently, the expression of Siglecg was reassessed to provide a comparative analysis of the expression patterns of regulatory markers. We observed that Fcgr2b and Siglecg share analogous expression patterns within B cell and monocyte populations (Supplemental Fig. 1), with similar levels of expression density in corresponding areas of the B cell cluster (Fig. 2A). At one-week post-transplantation, both markers demonstrated a peak in expression concentration on overlapping subclusters within the B cell population (Fig. 2A). By three weeks post-transplantation, there is a noticeable concentration of expression in analogous regions of the B cell subcluster for both markers. Intriguingly, the peaks occur in complementary regions, with Fcgr2b being more pronounced in the central areas of the right cluster, while Siglecg shows higher intensity in the upper portion of the right cluster (Fig. 2B). To investigate the possibility of intra-graft reprogramming of naïve B cells to Siglecg+ or Fcgr2b+ B cells, we performed trajectory analysis of the scRNAseq data using Monocle329–31. Density plots show that the central region of the right cluster is associated with naïve B cells (Fig. 2B). At six months post-transplantation, Fcgr2b’s expression overlaps with the zones of known Breg markers, such as Havcr1, Cd1d1, Cd5, and Il10 (Fig. 2C). Trajectory analysis suggested that both Fcgr2b+ and Siglecg+ phenotypes mature from naïve B cells (Fig. 2D), and by 6 months post-transplantation, the Fcgr2b+ phenotype occurs later than Siglecg (Fig. 2E). The corresponding dot plots quantify these observations. At one-week post-transplantation, both Siglecg and Fcgr2b are expressed in a smaller subset of cells, with Fcgr2b demonstrating relatively low expression levels within subclusters 0 and 1 (Fig. 2F). At three weeks post-acceptance, Fcgr2b continues to exhibit low expression levels throughout the cluster (Fig. 2F). However, by six months post-transplantation, there is a noticeable increase in the average expression of Fcgr2b within the B cell cluster (Fig. 2F). This expression pattern raises the possibility that FcγRIIB plays a role in the long-term maintenance of tolerance.
Fig. 2. Temporal expression analysis of Fcgr2b and Siglecg in accepted and rejecting kidney allografts.
(A) Density plots of Siglecg+, Fcgr2b+, and Siglecg+Fcgr2b+ cells within B cell clusters in accepted kidney allografts at 1-week post-transplantation. (B) Density plots of Siglecg+, Fcgr2b+, Siglecg+Fcgr2b+, and Ighd+Ighm+ cells in B cell clusters at 3 weeks post-transplantation. (C) Density plots show expression of Siglecg+, Fcgr2b+, Siglecg+Fcgr2b+, Ighd+Ighm+, and Breg markers, including Havcr1, Cd1d1, Cd5, and IL10 at 3 weeks post-transplantation. (D, E) Pseudotime analysis of B cell clusters at 3weeks (D) and 6 months (E) post-transplantation. The red arrow marks the origin point representing naïve B cells, and the red circle highlights a cluster expressing Siglecg or Fcgr2b. Pseudotime graphic is overlaid on the UMAP plot on a gradient color scale. (F) Dot plots show progressive upregulation of Siglecg and Fcgr2b over time (1 week, 3 weeks, and 6 months post-transplantation) within B cell clusters. (G) Dot plot analysis showing higher expression of Siglecg and Fcgr2b in the spontaneous acceptance model (accept 1, 3, 24 weeks) and in an induced kidney acceptance model (where recipients are infused with donor splenocytes treated with the chemical cross-linker ethylenecarbodiimide (ECDI-SP)), when compared to a kidney allograft rejection model (B6 to DBA/2).
To determine whether similar results could be observed in other mouse renal tolerance models, we evaluated the expression of Fcgr2b and Siglecg in an induced tolerance model involving BALB/c to B6 kidney transplantation. In this model, donor-specific tolerance is induced using a well-established regimen involving peri-transplant recipient infusions of donor splenocytes treated with the chemical cross-linker ethylenecarbodiimide (ECDI-SP)32. Dot plot analyses compared Fcgr2b and Siglecg expression across the induced tolerance model (day 15 post-transplantation), our spontaneous DBA/2 to B6 acceptance model (at 1 week, 3 weeks, and 6 months post-transplantation), and a rejection model (B6 to DBA/2) (Fig.2G). In the induced tolerance model, Siglecg was strongly expressed. The percentage expression of Fcgr2b was higher than in the rejection model. Thus, Siglecg and Fcgr2b display similar expression patterns within tolerant grafts across several models.
Early intra-graft expression of Fcgr2b and Siglecg within the B cell cluster in retransplanted tolerant lung allografts.
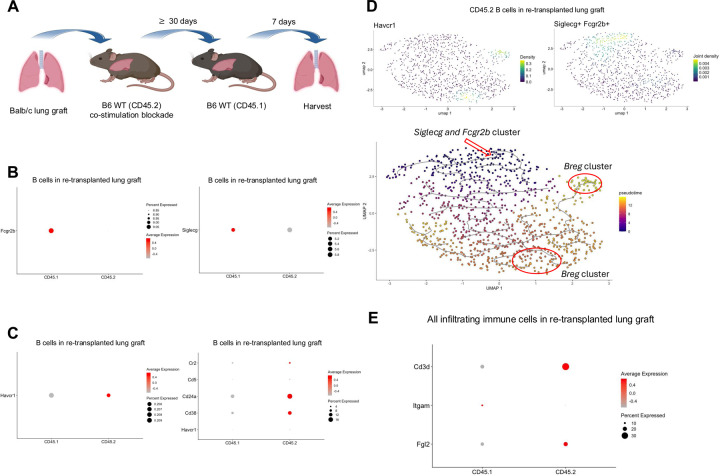
We have shown that peri-operative co-stimulation blockade results in the induction of tolerance after allogeneic lung transplantation in the mouse, which is associated with the development of tertiary lymphoid organs that are enriched in Tregs and also contain abundant B cells20,21. We have also reported that tolerance is maintained after re-transplantation of tolerant lung allografts into non-immunosuppressed secondary hosts. This model affords us the possibility of assessing and characterizing newly infiltrating immune cells and to compare them with immune cells that reside within the tolerant graft at the time of re-transplantation (Fig. 3A). Analysis of scRNAseq data of intra-graft immune cells show that resident B cells (CD45.2+) express lower levels of Siglecg and Fcgr2b when compared to newly infiltrating B cells (CD45.1+) one week after re-transplantation (Fig.3B). Interestingly, resident B cells have a Breg signature, including expression of Cr2, Cd5, Cd24a, Cd38, and Havcr1 not seen in newly infiltrating B cells (Fig. 3C). Trajectory analysis of the CD45.2+ scRNAseq data showed that Havcr1 arose from Siglecg+Fcgr2b+ B cells (Fig. 3D). These findings raise the possibility that Siglecg and Fcgr2b, expressed early within infiltrating B cells, may render them susceptible to reprogramming into Breg-like cells in response to Fgl2. Consistent with our published scRNAseq data from accepted kidney grafts, Fgl2 is expressed in immune cells that reside in accepted lung grafts, specifically within the resident T cell population (Fig. 3E).
Fig. 3. Temporal expression of Fcgr2b, Siglecg, and Breg markers in accepted lung allografts.
(A) Schematic showing transplantation of Balb/c lungs into B6 WT recipients (CD45.2), treated with peri-operative co-stimulatory blockade, followed by re-transplantation into non-immunosuppressed B6 WT (CD45.1) recipients ≥30 days later. Graphic created with BioRender. (B) Dot plots of Fcgr2b and Siglecg expression in the CD45.1+ and CD45.2+ B cell clusters. (C) Dot plot analyses of Breg markers - Havcr1, Cr2, Cd5, Cd24a, Cd38 - within the CD45.1+ and CD45.2+ B cell clusters and Siglecg expression in the CD45.1+ and CD45.2+ B cell clusters. (D) Pseudotime analysis of B cell clusters at seven days after re-transplantation. The red arrow marks the origin point representing Siglecg+Fcgr2b+ B cells, and the red circle highlights clusters expressing the Breg marker Havcr1. Pseudotime graphic is overlaid on the UMAP plot on a gradient color scale. (E) Dot plots show expression of Fgl2 within the T cell cluster.
Antibody-mediated rejection of kidney allografts in B6.Fcgr2b KO recipients.
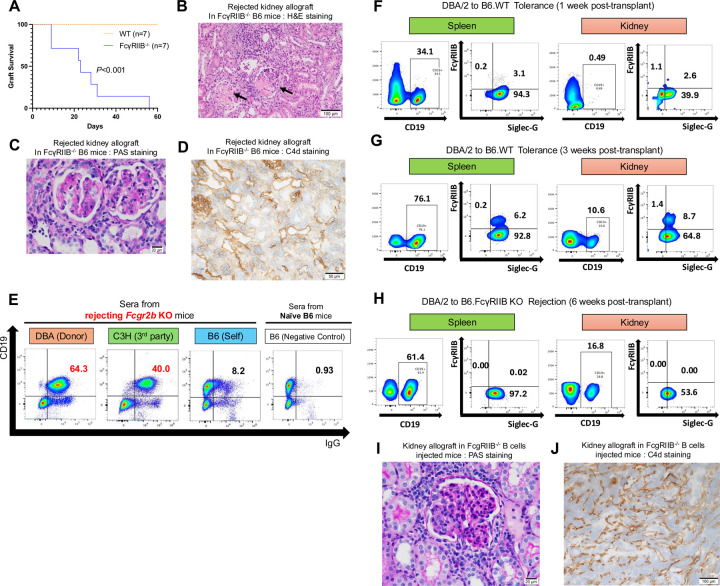
Increased expression levels of Fcgr2b and Siglecg in B cells within accepted kidney allografts, combined with an absence of circulating donor-specific antibodies (DSAs), raises the possibility of a suppressive mechanism against antibody production. To assess if Fcgr2b affects the induction kidney allograft tolerance, we performed survival and immunohistochemical analysis using gene-deficient recipients. The survival of DBA/2 kidneys is significantly shorter after transplantation into B6 FcγRIIB−/− (n=13; MST=31 days) compared to B6 WT mice (n=13) (Fig. 4A). After transplantation into FcγRIIB−/− recipients, kidney allografts exhibit thrombotic microangiopathy, as highlighted by the mononuclear cell infiltration, glomerulitis, and capillaritis (Fig. 4B, C). These pathological changes are accompanied by an increase in circulating DSAs and enhanced C4d deposition (Fig. 4D, E). Collectively, these findings are indicative of antibody-mediated rejection when recipients lack FcγRIIB.
Fig. 4. Antibody-mediated rejection of kidney allografts in FcγRIIB−/− recipients.
(A) DBA/2 kidney allograft survival in FcγRIIB−/− recipients (n=13), compared to wild-type (WT) recipients (n = 13; P<0.001). (B-D) Histopathological analysis of rejected kidney allografts obtained from FcγRIIB −/− recipients. (B) H&E staining shows thrombotic microangiopathy. Arrows indicate mononuclear cell infiltration. Day 35. Scale bars: 100μm. (C) PAS staining shows glomerulitis and capillaritis. Day 35. Scale bars: 20μm. (D) Immunohistchemistory shows increased C4d deposition in FcγRIIB −/− kidney allograft. Day 39. Scale bars: 50μm. (E) Flow cytometric analysis of serum antibodies reactive to mouse spleen cells. Sera from FcγRIIB −/− recipients exhibited higher antibody reactivity against DBA/2 (donor, 64.3%) and C3H (third-party, 40.0%) cells, compared to B6 (self, 8.2%) and negative control (0.93%). (F-H) Flow cytometric analysis of SiglecG+ FcγRIIB+ cells within the CD19+ B cell population isolated from spleens and kidney allografts at 1 week (F) and 3 weeks (G) post-transplantation in the tolerance model and 6 weeks post-transplantation in the FcγRIIB −/− rejection model (DBA/2 to B6 FcγRIIB−/−) (H). The data in (F-H) are representative of triplicate experiments. (I, J) Histopathological analysis of kidney allografts obtained from FcγRIIB −/− B cells injected recipients 4 weeks post-transplantation (n=3). (I) PAS staining shows glomerulitis. Scale bars: 20μm. (J) Immunohistchemistory shows increased C4d deposition in FcγRIIB −/− B cells injected kidney allograft. Scale bars: 100μm.
We observed a temporal increase in FcγRIIB+Siglec-G+ and overall Siglec-G+ B cells in the recipient spleen and kidney allograft after transplantation into WT recipients (Fig. 4F, G). Analysis of splenic and intra-graft B cells from FcγRIIB−/− recipients at the time of rejection shows a similar abundance of Siglec-G+ B cells (Fig. 4H) suggesting that Siglec-G expression on B cells is not sufficient to prevent antibody-mediated rejection observed in FcγRIIB−/− recipients.
Analysis of our scRNAseq dataset shows that Fcgr2b is also expressed in the myeloid clusters in addition to the B cell cluster (Supplemental Fig. 1). To confirm that FcγRIIB−/− B cells can directly result in the induction of rejection in kidney allografts in our model, we adoptively transferred 2×106 purified FcγRIIB−/− B cells (Supplemental Fig. 2A, B) into WT B6 mice prior to transplanting a DBA/2 kidney. Analysis of the kidney allograft at 4 weeks post-adoptive transfer and post-transplantation shows signs of glomerulitis (Fig. 4I) and C4d deposition (Fig. 4K), as well as an increase in circulating DSA (Supplemental Fig. 2C).
The B cell population in tumors exhibits increased expression of Fcgr2b and Siglecg.
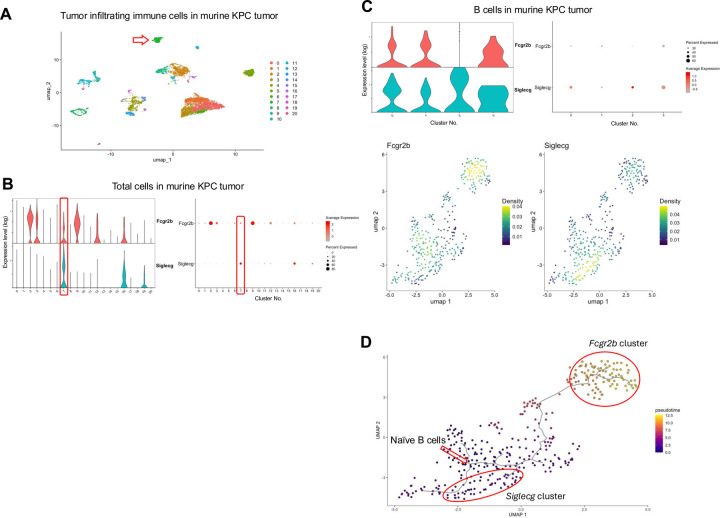
While accepted kidney and lung allografts develop regulatory tertiary lymphoid organs (rTLOs), which are thought to contribute to the maintenance of graft tolerance, the tumor immune microenvironment similarly forms tertiary lymphoid structures (TLSs) that resembles rTLOs in our transplant models6,7,9–13. These TLSs contain B and T cells33 and may play a role in sustaining tumor immune tolerance. We have previously shown that cytotoxic CD8+ T cells are reprogrammed to regulatory-like/exhausted cells in our kidney allograft spontaneous acceptance model and similar regulatory-like/exhausted CD8+ T cells were present in tumors, including KPC tumors22. Given these similarities, we examined whether Siglecg and Fcgr2b are also highly expressed within the B cell population in tumors. Figure 5 summarizes the scRNAseq data from spontaneous pancreatic tumors in the KPC mouse model. Analysis of total cells revealed elevated expression of Fcgr2b and Siglecg in the B cell cluster (Fig. 5A, B). Sub-clustering of B cells further confirmed that both genes are upregulated in a pattern like that observed in tolerant allografts (Fig. 5C). Density plots for each marker show that B cells expressing Fcgr2b or Siglecg are enriched in distinct subclusters (Fig. 5C). Additionally, trajectory analysis of the scRNAseq data via Monocle329–31 shows a cellular transition within this cluster from naïve B cells to Siglecg- and then over time to Fcgr2b-dominated expression (Fig. 5D). This is also demonstrated within the density plot of each respective marker showing that the greatest density of B cells that expresses Fcgr2b or Siglecg is represented by different B cell subclusters (Fig. 5C). These findings raise the possibility of a similar mechanism of immunomodulation taking place within B cells in pancreatic tumors and accepted kidney allografts.
Fig. 5. Expression of Fcgr2b and Siglecg within the B cell cluster in murine KPC tumors.
(A) UMAP plot of all cells isolated from murine KPC tumors. The red arrow indicates the B cell cluster. (B) Violin and dot plots analysis of Fcgr2b and Siglecg expression in total cells. The B cell cluster is boxed in red. (C) Violin, dot and density plots of Fcgr2b and Siglecg expression in the B cell cluster. (D) Pseudotime analysis of the B cell cluster in murine KPC tumors. The red arrow indicates the origin point representing naïve B cells, and the red circle highlights a subpopulation expressing Fcgr2b and Siglecg. Pseudotime graphic is overlaid on the UMAP plot on a gradient color scale.
Increased SIGLEC10 gene expression within the B cell population in human melanomas correlates with poor response to anti-PD1 therapy.
To determine whether B cell signature similar to the one observed in murine tumors and tolerant allografts is present in human cancers, we analyzed publicly available scRNAseq data from human melanoma samples34, focusing on the expression of FCGR2B and SIGLEC10 (the Siglec-G counterpart in humans) in the B cell compartment. Examination of tumor-infiltrating immune cells revealed that SIGLEC10 and FCGR2B are frequently and highly expressed within the B cell cluster (Figure 6A, B). While TLSs may play a role in sustaining tumor immune tolerance, recent data in melanoma demonstrated that TLSs may contribute to the maintenance of anti-tumor immunity, with higher expression of a TLS gene signature associated with greater clinical benefit observed in patients treated with immunotherapy10. First, we investigated whether SIGLEC10 and FCGR2B are highly expressed within the B cell population in human melanomas. Bulk RNA-sequencing35 was performed on resected tumors from patients with metastatic melanoma (n=124). All patients received anti-programmed cell death protein-1 (aPD1) immunotherapy with subsequent clinical follow up. Analysis of CD45+ cells revealed that SIGLEC10- and FCGR2B-expressing cells were densely located within the B cell populations, in addition to macrophage clusters (Fig. 6B). Patient clinical response data were aggregated into progressive disease (PD) and not-progressive disease (notPD). Single-sample gene-set enrichment (ssGSEA) analyses demonstrated enrichment of a B cell signature in notPD tumors (p=0.01) which were effectively patients who responded to aPD1 (Fig. 6C). A multivariate logistic regression analysis further revealed that high expression of SIGLEC10, when evaluated in combination with the B cell signature, was significantly associated with poor clinical response and disease progression (OR 3.80, 95% CI 1,35 – 12.60; p =0.021) (Fig. 6D). These results suggest a potential immunosuppressive role for SIGLEC10 in the context of anti-PD1 resistance.
Fig. 6. Expression of FCGR2B and SIGLEC10 within the B cell cluster in human melanomas.
(A) UMAP plot of tumor-infiltrating immune cells isolated from human melanomas. The red arrow indicates the B cell cluster. (B) Violin, dot and density plots show FCGR2B and SIGLEC10 expression within the B cell cluster among all tumor-infiltrating immune cells. The B cell cluster is boxed in red, and the red arrow indicates its location. (C) Boxplot comparing the B cell normalized enrichment score estimated via ssGSEA in patients treated with anti-PD-1 therapy(aPD1), stratified by clinical response (progressive disease [PD] vs. non-progressive disease [notPD]). The B cell signature includes the following genes: BLK, CD19, MS4A1, TNFRSF17, FCRL2, FAM30A, PNOC, SPIB, and TCL1A. (D) Forest plot showing odds ratios (OR) and 95% confidence intervals (CI) for the association between B cell abundance and SIGLEC10 expression with response to anti-PD1 therapy. ORs are shown on a log scale, with a dotted vertical line indicating the null value (OR = 1). Each square represents the estimated OR for one predictor, and horizontal lines denote the 95% CI. Values were standardized (z-score) prior to model fitting to allow interpretation per 1 standard deviation increase (n=124). Statistically significant associations (p < 0.05) are indicated with asterisks.
DISCUSSION
Kidney allografts are spontaneously accepted without the use of immunosuppression in certain murine strain combinations6–8,36,37. The accepted allografts are characterized by the presence of rTLOs that form perivascularly and contain various immune cell types6,7,14. We have shown through both bulk mRNA and scRNAseq analyses that CD8+ T cells were the abundant intra-graft cell type at 1 and 3 weeks post-transplantation, and observed a shift to a B cell signature by 6 months post-transplantation7,14. Additionally, the B cell signature is reflective of a regulatory one, where we observed an increased expression of Breg markers, both at the transcriptional and protein level14. These findings were corroborated by flow cytometric and immunohistological analyses7,14. Recently, we showed that graft-infiltrating CD8+ T cells expressed cytotoxic markers (Gzmb and Ifng) as early as 1 week after transplantation and by 3 weeks were reprogrammed into exhausted/regulatory-like cells expressing Fgl2, Il2rb, Pdcd1, Tox, and Lag3 through a process we have termed ‘defensive tolerance.’22 In this study we focused on examining the B cell population within the accepted kidney allografts in our transplant model.
We have shown that depletion of B cells, either pharmacologically or genetically, at early and late times post-transplantation did not result renal graft rejection, suggesting that Breg-like cells are not needed for the maintenance of tolerance in our spontaneous kidney acceptance model. This is contrary to our previous observations with Treg depletion in this model6. We further show that spontaneous acceptance of kidney allografts occurs in B6.μMT recipients indicating that B cells are not required for the induction of tolerance.
We have previously shown that one of the genes that was highly expressed within the graft at 3 weeks post-transplantation within the CD8+ T cell cluster is Fgl222, which encodes for Fibrinogen-like protein 2 (Fgl2). It has been shown that regulatory CD8+ T cells secrete Fgl2, which is known to be immunomodulatory12,27. The receptor for Fgl2 is FcγRIIB18,38. In humans and mice, FcγRIIB, also known as CD32B, is the only inhibitory receptor of the Fcγ receptor family. FcγRIIB is encoded by Fcgr2b, and is present on the surface of B cells, dendritic cells, and myeloid effector cells18,38. FcγRIIB can mediate humoral immunity by heightening the BCR activation threshold and inhibiting B cell antigen presentation to helper T cells. FcγRIIB also directly alters antigen presentation by inhibiting DC maturation18. Of relevance, we found a temporal increase in Fcgr2b expression within the B cell clusters in accepted kidney allografts. This increase in expression mirrored a similar pattern in Siglecg14, which encodes for Siglec-G, a molecule that when expressed on B cells prevents the secretion of antibodies. Its interaction with CD22 on other cell types induces the upregulation of Siglec-G on their surface24–26. Flow cytometry data show that by 3 weeks, Siglec-G+ B cells also co-express surface FcγRIIB, both in the graft and in the recipient spleen. To ascertain the importance of FcγRIIB, we transplanted DBA/2 kidneys into B6.FcγRIIB−/− recipients, where we observed antibody-mediated rejection with an increase in circulating DSAs and C4d deposition in the graft. DSA is never observed in our spontaneous acceptance model6. Adoptive transfer of purified FcγRIIB−/− B cells into WT recipients confirmed the role of B cells in the induction of AMR in kidney allograft recipients. These data suggest that not only are B cells not needed for the induction and maintenance of kidney graft acceptance, but their reprogramming from pathogenic B cells to Breg-like cells in the tolerant graft may be a protective mechanism, reminiscent of our observations with graft-infiltrating cytotoxic CD8+ T cells22. Interestingly, in the lung retransplant model, scRNAseq analysis of resident and newly infiltrating B cells showed a Breg signature in the resident B cell population with low expression of Fcgr2b and Siglecg, while newly infiltrating B cells expressed both Fcgr2b and Siglecg with little or no expression of Breg genes. This is suggestive of a mechanism where Fcgr2b and Siglecg are upregulated in newly infiltrating B cells within the tolerant microenvironment, are eventually reprogrammed to Breg-like cells, and maintained as innocuous rather than their original pathogenic state.
We have previously shown that graft-infiltrating cytotoxic CD8+ T cells are reprogrammed to regulatory-like cells, including Cd8+Fgl2+Il2rb+ cells22. Similar cell populations were also observed in spontaneous murine tumor models22. We hypothesized that in the models of both spontaneous acceptance of kidney allografts and spontaneous murine tumors, infiltrating pro-inflammatory cells, such as cytotoxic CD8+ T cells, are reprogrammed to regulatory-like/exhausted cells because of a pro-tolerance microenvironment through a process we called ’defensive tolerance’22. In this study, using a scRNAseq cohort from the KPC mouse tumor model that we used in our previous study where we looked at reprogramming of cytotoxic CD8+ T cells22, we observed a gene expression pattern of Fcgr2b and Siglecg within the B cell population of murine pancreatic tumors that resembled our findings in spontaneously accepted kidney allografts. We observed similar increased expression of FCGR2B and SIGLEC10 (the human homolog of Siglecg) in the B cell cluster from human melanomas34,39. Interestingly, we also observed a correlation between anti-PD1 therapy resistance and progression of disease in human melanoma patients that exhibited increased expression of SIGLEC10. The expression of these negative regulators of B cell function and their association with tumor growth and survival is not unprecedented. For example, studies have shown that expression of FCGR2B and SIGLEC10 in gliomas was higher than in normal tissue where it was associated with a poor prognosis40,41. High expression of Siglec-10 on tumor-associated macrophages in stage 1 lung adenocarcinoma along with expression of tumor CD24 was associated with increased risk of recurrence42, and the Siglec-10-CD24 axis inhibits the phagocytosis of tumor cells by macrophages43. Siglec-10hi tumor-associated macrophages are also associated with poor prognosis in patients with hepatocellular carcinoma44.
In summary, and as we previously showed with infiltrating cytotoxic CD8+ T cells22, pathogenic B cells are reprogrammed to a regulatory-like and innocuous phenotype within tolerant allografts through a process we call ‘defensive tolerance’. What drives the expression of Fcgr2b and Siglecg in B cells within the accepted kidney and in tumors has yet to be determined and will be the focus of future studies. Our further understanding of how this reprogramming is accomplished in tolerant allografts or tumors has implications for the design of novel therapeutics that will be more effective and clinically applicable for achieving allotransplant tolerance and for the efficacious anti-tumor immunity.
ONLINE METHODS
Sex as a biological variable
Our study exclusively examined male mice. We have done some kidney allotransplants using female recipients and have obtained similar results with regards to the induction of tolerance but there was greater variability regarding survival rates, not due to rejection of the renal allograft, but from technical complications resulting from our DBA/2 to B6 kidney transplantation technique when using female recipients.
Mice
The C57BL/6J (B6, H2b), DBA/2J (DBA, H2d), BALB/c (H2d), B6.[CD19Cre/+iDTRfl/+] mice, Ptf1atm2(cre/ESR1)Cvw/J, B6.129P2-Trp53tm1Brn/J, B6.129S4-Krastm4Tyj/J, B6.129S4-Krastm4Tyj/J, and B6.Ptprca Pepcb/BoyJ (CD45.1) mouse strains were purchased from Jackson Laboratories (Bar Harbor, ME). The B6.129S4-Fcgr2btm1TtK N12 (FcgR2b KO) mice were ordered from Taconic Biosciences.
All mice were held throughout the experiment under pathogen-free conditions in filter-top cages, containing an automatic water system. They were cared for according to methods approved by the American Association for the Accreditation of Laboratory Animal Care.
Kidney transplantation
Kidney transplantation was performed as detailed previously37. In brief, a cuff of the aorta and inferior vena cava were anastomosed in an end-to-side manner. The ureter was anastomosed to the urinary bladder. A bilateral nephrectomy was also simultaneously performed.
Lung transplantation
Lungs were transplanted from BALB/c (CD45.2) mice into C57BL/6 CD45.2 (B6) recipients that received treatment with peri-operative co-stimulatory blockade (anti-CD40 ligand (250 μg intraperitoneally (i.p.)) and CTLA4-Ig (200 μg i.p.) on post-operative days 0 and 2, respectively (BioXCell)21. At least 30 days after the primary transplant, these lungs were re-transplanted into non-immunosuppressed B6 CD45.1 mice, as previously described11. Seven days after re-transplantation, lung grafts were harvested, the tissue was digested, and single-cell suspensions prepared, as previously described45. Cells, pooled from two re-transplant recipients, were stained using antibodies for CD45.1, CD45.2 and 4’,6-diamidino-2-phenylindole (DAPI) (BD Biosciences) and were sorted by flow cytometry into DAPI−CD45.1+CD45.2− and DAPI−CD45.1−CD45.2+ cells. Collected cells were centrifuged (300 relative centrifugal force for 5 minutes at 4 °C) and resuspended in collection buffer to a target concentration of 1,000 cells/μL. Cells were counted on a hemocytometer before proceeding. Single-cell suspensions were submitted to the Genome Technology Access Center core facility (Washington University) for single-cell genome-scale metabolic model (GEM) construction and complementary DNA synthesis and library construction. Samples were processed using the Chromium Single Cell 3′ Library & Gel Bead Kit (10X Genomics, v3) following manufacturer’s protocols. The libraries were sequenced on NovaSeq S4 (Illumina) targeting 50,000 reads per cell and 500 million read pairs per library. Cells were aligned to the mouse mm10–2020-A transcriptome using CellRanger (10x Genomics, v6.1.1) to generate feature-barcoded count matrices.
Histological and Immunopathological Analysis
Sagittal sections of allografts were fixed in formalin, and sections were stained for hematoxylin and eosin (H&E) and periodic acid Schiff (PAS). Immunohistochemistry on frozen tissue was done using a rat monoclonal antibody to C4/C4d (16D2, Abcam). Pathologic evaluation was done using an Olympus BX53 microscope (Olympus) equipped with a digital camera (DP76, Olympus).
Isolation of kidney and spleen cells
Prior to tissue collection, the kidney was injected and perfused with a collagenase solution (2ml of 1x HBSS, 1mL of Collagenase A, and 3uL of DNase I). The kidney was removed from the mouse, manually ground down, and digested in the same collagenase solution. The remaining undigested tissue was manually ground down using a 70-um strainer, washed 3 times, and resuspended using FACs buffer. The spleen was collected in RPMI and then manually ground down using an insulin syringe plunger over a 70um strainer. 1 mL of ACK Lysis Buffer (Gibco – ThermoFisher Scientific) was added to remove red blood cells from the spleen. Cells were washed three times and resuspended in FACs Buffer.
Flow cytometry analysis
Cells were plated and stained with viability dye (eFlour 506, 1:1000 dilution, Invitrogen, L34976 A), and incubated in darkness for 30 minutes. After viability staining, CD16/32 Fc Block (1:100 dilution, BioLegend, 101302) was added to each sample for a 5–10 minutes. Cells were then washed and stained with all or some of the following conjugated monoclonal antibodies: CD122 (BV421, BD Biosciences, 562960), CD4 (APC-Cy7, Biolegend, 100413), CD3 (Brilliant Violet 510, Biolegend, 740113), FcγRIIB (Alexa Flour 488, Cell Signaling Technology, 54837), CD27 (Pacific Blue, Biolegend, 124218), CD138 (PE, Biolegend, 112503), CD19 (Brilliant Violet 480, Biolegend, 566107), Siglec-G (Alexa Flour 647, Biolegend, 563336), IgD (Brilliant Violet 605, Biolegend, 406527), IgM (PerCP/Cyanine 5.5, Biolegend, 406512), IgG (PE/Cyanine 7, Biolegend, 405315). Separate panels included I-Ad (PE, Invitrogen, MA5–17780), and H-2kd (PerCP Cyanine 5.5, Biolegend, 1147115). Surface marker antibodies were diluted to 1:200 (or per manufacturers specifications), and 100uL was added to samples and incubated in darkness for 30 minutes. Samples were washed three times with FACS buffer and finally resuspended in order to be analyzed via flow cytometry on Cytek Aurora Flow Cytometer (Cytek Biosciences). Gating strategies were controlled using fluorescent minus one (FMO) controls and universal negatives in the form of unstained cells, as well as cells stained with each isotype-controlled mAb. Samples were then analyzed on FlowJo software (Tree Star). Figures from flow cytometry are representative of the triplicate samples.
Donor-Specific Antibody Detection
The presence of donor- specific antibodies (DSA) were tested for within the recipient’s sera, after its having been isolated from whole blood. Each DSA experiment contained donor splenocytes with an experimental group using recipient sera, a negative control group using the donor’s own sera, and a positive control group consisting of manufacturer antibody staining of the splenocytes. If previously frozen, all sera was thawed per thawing protocol over ice. Otherwise, 1:10 and 1:100 dilutions were prepared of the sera in FACs buffer. 100uL were then plated and incubated over the donor splenocytes (at 500,000 cells/well) for 30 minutes in darkness. Cells were washed with FACs buffer. IgG antibody dilution was prepared (Brilliant Violet 421, Jackson ImmunoResearch, 115–675-071) at 1:200 dilution, added to wells, and incubated for 30 minutes in darkness. Cells were washed twice using FACs buffer. Surface marker antibodies were then added to all samples; CD19 and CD3 (Biolegend, 566107, and 740113, respectively) were added to all wells, while MHC specific antibodies and isotypes (H2-Kd: Biolegend, 1147115; IA-d: Invitrogen, MA5–17780) were added to positive control samples. All wells were incubated for 30 minutes in darkness, and washed twice. Samples were then run on Cytek Aurora Flow Cytometer (Cytek Biosciences). Gating strategies and data analyses were performed as outlined in flow cytometry protocol.
B Cell Isolation for Adoptive Transfer
Following normal cell dissociation protocol, B cells were isolated for using an EasySep B Cell Isolation Kit (Cat:19854) following the manufacturers protocol. In summary, the dissociated total cell mixture was exposed to an EasySep™ Mouse FcR Blocker antibody (Component: 18730), followed by the EasySep™ Mouse B Cell Isolation Cocktail (Component: 19854C). Both were incubated for 10 minutes at room temperature. 50uL of EasySep™ Streptavidin RapidSpheres™ 50001 (Component: 50001) per mL of solution, was added mixture following vortexing for 30 seconds. The solution was then incubated for 2.5 minutes at room temperature. The mixture was then placed in an EasyEights™ EasySep™ Magnet (Cat: 18103) for an additional 2.5 minutes until solution was clear, and all spheres had flushed to the magnet. Remaining B cells were then transferred into a 15mL tube, washed at 1600 RPM for 5 minutes, and a small portion (> a million cells) were set aside for flow cytometry staining and confirmation. The isolation was conducted in sterile conditions, with sterile tubes, under a fume hood for injection. The B cells were resuspended in RPMI at a concentration of 2×106 per dose and injected intravenously through the tail vein into untreated recipients 2 days prior to kidney transplantation. Serum BUN and creatinine levels were regularly monitored to assess kidney function post-transplantation. Sera were also collected at this time for DSA. Pathological examination was performed on kidney allograft samples.
Single cell RNA sequencing of kidney allografts and spleen
Isolated kidney or spleen cells were sorted for viable CD45+ cells via flow cytometry using an anti-CD45+ antibody (BioLegend,103114) and a fixable viability dye ([Invitrogen, L34976 A). These cells were used to construct single-cell RNA-seq libraries at Chromium 10x instrument using Chromium Next GEM Single Cell 3’ kit, which were sequenced in paired-end fashion46 on Illumina HiSeq 2500 instrument to the depth of approximately 100 million read pairs per sample.
Bioinformatics analyses of scRNA-seq data of kidney allografts and spleen
The raw sequencing data underwent initial mapping and processing using the CellRanger package (Chromium Genomics). The resulting read counts were further analyzed using Seurat47,48 and Monocle29–31. This included filtering cells by the number of UMIs, mitochondrial content, and number of expressed genes, as well as further normalization and scaling of read counts. Datasets for various time points (1 week, 3 weeks, and 24 weeks for accepted recipient and 1 week for rejecting recipient) and biological replicates (N = 3 at 1 week, N = 5 at 3 weeks, and N = 3 at 24 weeks) were integrated, followed by principal component analysis (PCA), generation of Uniform Manifold Approximation and Projection (UMAP) plots, and cell clustering using Seurat functions with default parameters. All mouse replicates were integrated using Seurat after scRNAseq was performed. This created a data object for all pooled samples together. While each mouse was a separate sample, replicates in a single time point were pooled and compared across other time points as well. Annotation of cell types was performed manually by investigating canonical markers in literature. Gene expression analysis across time and cluster type was performed using Seurat software47,48 and Nebulosa packages49.
Integrated data from Seurat analysis was then transformed into a Monocle 3 object, including expression data and cell-level metadata using the as.cell_data_set function to allow trajectory analysis. This object underwent further analysis, including unsupervised clustering of cells using the cluster_cells function and updating the Monocle 3 object. Next, the Monocle 3 object underwent analysis using the learn_graph function to determine the biological program of gene expression for the scenario in question and, therefore, learned the trajectory of cells through this higher-dimensional space. Lastly, the order_cells function chooses root states using the interactive Monocle 3 online software and determines the pseudotime values based on the object produced using the learn_graph function. When this was complete, various qualitative graphs were produced to illustrate the trajectories of cell types in a larger population.
scRNAseq data of KPC tumor
A mouse model of pancreatic ductal adenocarcinoma was employed that combined the tamoxifen-inducible pancreatic acinar cell-specific expression of the Cre recombinase (Ptf1a-CreER) with the expression of oncogenic KrasG12D (LSL-KrasG12D) and heterozygous loss of Tp53+/fl 50–52. Mice were administered tamoxifen at 6 weeks of age and tumors were harvested for scRNA-seq analysis at 6 months of age. Approximately 50 mg of tumor was enzymatically disassociated in RPMI containing 0.1 mg/ml DNase I (Roche), 0.2 mg/ml Collagenase P (Roche), 0.1 mg/ml Dispase (Gibco-Thermo Fisher), and 2% fetal bovine serum (FBS). The cell suspension volumes were calculated for a target cell recovery of between 4000 to 8000 cells and loaded on the Chromium using 10x Genomics Chromium Single Cell 3’ Reagents v3 kit (10x Genomics) according to the manufacturer’s guidelines. Purified cDNAs were quantified using High Sensitivity D5000 ScreenTapes and Reagents on an Agilent Tapestation (Agilent). The final single cell 3’ libraries were quantified using an Agilent Tapestation using High Sensitivity D1000 ScreenTapes and Reagents. Libraries were loaded at 160 picomolar on an Illumina cBOT and sequenced on a HiSeq4000 for 28 base pairs on the first read, followed by an 8 base pair index read, and a 91 base pair second read, using 2 HiSeq4000 SBS kits, for 50 cycles. Illumina Real Time Analysis software was employed to generate sequence intensity files which were then demultiplexed and aligned to the human genome, version hg38, using the 10x Genomics CellRanger v3.0.1 software package.
Evaluating B cell Signature enrichment together with SIGLEC10 expression in metastatic melanoma patients treated with anti-PD1
We analyzed publicly available transcriptomic data from Huang and colleagues35. We focused specifically on pre-treatment tumor samples from immunotherapy naïve patients with metastatic cutaneous melanoma treated with anti-PD1(Programmed cell death protein-1) monotherapy. Response was evaluated either by RECIST v1.1 where available, or by best overall response (BOR). Patients with a RECIST or BOR clinical response of complete or partial response (CR, PR) or stable disease (SD) with a progression-free survival (PFS) greater than 6 months were categorized as not progressive disease (notPD). This aggregated group was compared with patients with a RECIST or BOR response of progressive disease (PD). To maintain well-defined cohorts, patients with mixed response (MR) or non-evaluable response (NED) were excluded. From these cohorts, we estimated B cell enrichment using single-sample Gene Set Enrichment Analysis (ssGSEA) implemented in the GSVA R package (R version 4.2.0). The B cell signature gene list was obtained from www.Nanostring.com, defined by the expression of the following genes: BLK, CD19, MS4A1, TNFRSF17, FCRL2, FAM30A, PNOC, SPIB, and TCL1A. The ssGSEA scores were computed on normalized expression data to obtain a B cell enrichment score for each sample. B cell enrichment scores analysis compared samples in response group PD with those in notPD. The ssGSEA scores were then z-score transformed across samples and included in multivariate logistic regression models to evaluate their association with treatment outcome. Expression levels of individual genes, including SIGLEC10, were also assessed independently and in combination with the B cell signature.
Bioinformatics analyses of scRNA-seq data of human melanoma datasets
Human melanoma cancer cell data sets included in GSE11597834 were obtained from the Gene Expression Omnibus (GEO). Cells from tumor samples were included based on the following exclusion criteria (1): cells with greater than 10% mitochondrial genes (2), cells expressing less than 200 genes or greater than 7,000 genes. Following quality control, read counts were normalized and scaled. Datasets were processed by PCA, followed by UMAP visualization and cell clustering using Seurat functions with default parameters. Annotation of cell types was performed manually by investigating canonical markers in literature. Gene expression analysis in T cell subsets was performed using Seurat software47,48, Nebulosa packages49, and Monocle 3 packages29–31.
Statistics
Data is presented as mean ± standard deviation (SD) for technical replicates and mean ± standard error of the mean (SEM) for biological replicates. Variables among groups were compared using the 2-tailed Student's t-test for comparison of 2 conditions; 1-way ANOVA test for comparison of more than 2 conditions. A P value less than 0.05 was considered significant. Allograft survival curves were constructed by the Kaplan and Meier method, and comparisons were performed using the log-rank test. These analyses were performed with Prism v10.0 (GraphPad Software) and IBM SPSS statistic v28.0 (IBMcorp).
Study approval
All research with animal models was subject to prior review and approval and conducted in compliance with institutional guidelines set forth by the Animal Care and Use Committees of the Massachusetts General Hospital.
Supplementary Material
Acknowledgments:
This work was supported by US NIH grant P01-AI123086 (to A.A., R.B.C., and J.C.M.); the Patricia K. Donahoe, MD, Surgeon-Scientist Research Program Incubator Award (A.A.); a generous gift from John Kuhns/Janet Milne (to A.B.C and A.A.); US NIH grants 1P01AI116501, R01HL094601, U01163086-01 (to D.K.); Veterans Administration Merit Review grant 1I01BX002730 (to D.K.); The Cystic Fibrosis Foundation 007731AB324 (to D.K.); The Foundation for Barnes-Jewish Hospital (to D.K.); US NIH grant R01 DK132889 (to X.L.); Henry E. Haller Jr. Foundation (to X.L.); by fellowships from the Mitsukoshi Health and Welfare Foundation (to K.H. and T.Y.); Mihashi Memorial Foundation for International Exchange Grant-in-Aid for study abroad, awarded by“Torin-kai” the Alumni Association of the Department of Surgery, Keio University School of Medicine (to K.H.); and the Uehara Memorial Foundation (to T.Y.). We would like to thank Dr. Mandy Ford for supplying the initial Fcgr2b.KO mice. Subsequently, Fcgr2b.KO mice were purchased from Taconic.
Competing interests:
GMB has sponsored research agreements through her institution with: Olink Proteomics, Teiko Bio, InterVenn Biosciences, Palleon Pharmaceuticals. She served on advisory boards for: Iovance, Merck, Moderna, Nektar Therapeutics, Novartis, Replimune, and Ankyra Therapeutics. She consults for: Merck, InterVenn Biosciences, Iovance, and Ankyra Therapeutics. She holds equity in Ankyra Therapeutics. JCM holds equity in Regatta Bio.
Data availability:
Raw and processed scRNAseq data for accepted and rejecting kidney allografts have been deposited into the Gene Expression Omnibus (GSE252337) database. ECDI-SP induced tolerance model’s data sets, GSM4761001 and GSM4761004, contained in dataset GSE157292 were downloaded from the Gene Expression Omnibus (GEO). Other data are available upon reasonable request from the corresponding authors subject to institutional review and approval.
REFERENCES and NOTES
- 1.Madariaga M.L., Kreisel D. & Madsen J.C. Organ-specific differences in achieving tolerance. Curr Opin Organ Transplant 20, 392–399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leventhal J.R. & Mathew J.M. Outstanding questions in transplantation: Tolerance. Am J Transplant 20, 348–354 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Dai H., Zheng Y., Thomson A.W. & Rogers N.M. Transplant Tolerance Induction: Insights From the Liver. Front Immunol 11, 1044 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wekerle T., Kurtz J., Bigenzahn S., Takeuchi Y. & Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol 14, 592–600 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Mika A. & Stepnowski P. Current methods of the analysis of immunosuppressive agents in clinical materials: A review. J Pharm Biomed Anal 127, 207–231 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Miyajima M., et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol 178, 1635–1645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosales I.A., et al. Novel intragraft regulatory lymphoid structures in kidney allograft tolerance. Am J Transplant 22, 705–716 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Yang C., et al. Kidney-induced systemic tolerance of heart allografts in mice. JCI Insight 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amisaki M., et al. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrita R., et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Li W., et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest 129, 556–568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X.L., et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol 185, 823–833 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Schumacher T.N. & Thommen D.S. Tertiary lymphoid structures in cancer. Science 375, eabf9419 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Guinn M.T., et al. Intragraft B cell differentiation during the development of tolerance to kidney allografts is associated with a regulatory B cell signature revealed by single cell transcriptomics. Am J Transplant 23, 1319–1330 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherukuri A., et al. Transitional B cell cytokines predict renal allograft outcomes. Sci Transl Med 13(2021). [DOI] [PubMed] [Google Scholar]
- 16.Newell K.A., Adams A.B. & Turka L.A. Biomarkers of operational tolerance following kidney transplantation - The immune tolerance network studies of spontaneously tolerant kidney transplant recipients. Hum Immunol 79, 380–387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newell K.A., et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120, 1836–1847 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K.G. & Clatworthy M.R. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 10, 328–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takai T., Ono M., Hikida M., Ohmori H. & Ravetch J.V. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature 379, 346–349 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Li W., et al. Surgical technique for lung retransplantation in the mouse. J Thorac Dis 5, 321–325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okazaki M., et al. A Mouse Model of Orthotopic Vascularized Aerated Lung Transplantation. American Journal of Transplantation 7, 1672–1679 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Yokose T., et al. Dysfunction of infiltrating cytotoxic CD8+ T cells within the graft promotes murine kidney allotransplant tolerance. J Clin Invest 134(2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demircik F., Buch T. & Waisman A. Efficient B cell depletion via diphtheria toxin in CD19-Cre/iDTR mice. PLoS One 8, e60643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W., et al. Siglec-G Deficiency Ameliorates Hyper-Inflammation and Immune Collapse in Sepsis via Regulating Src Activation. Front Immunol 10, 2575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller J., et al. Siglec-G Deficiency Leads to Autoimmunity in Aging C57BL/6 Mice. J Immunol 195, 51–60 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Muller J. & Nitschke L. The role of CD22 and Siglec-G in B-cell tolerance and autoimmune disease. Nat Rev Rheumatol 10, 422–428 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Flippe L., Bezie S., Anegon I. & Guillonneau C. Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol Rev 292, 209–224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H., et al. The FGL2-FcgammaRIIB pathway: a novel mechanism leading to immunosuppression. Eur J Immunol 38, 3114–3126 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Cao J., et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu X., et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14, 979–982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C., et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dangi A., et al. Single cell transcriptomics of mouse kidney transplants reveals a myeloid cell pathway for transplant rejection. JCI Insight 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmink B.A., et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerby-Arnon L., et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 175, 984–997 e924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang A.Y., et al. Stratified analysis identifies HIF-2α as a therapeutic target for highly immune-infiltrated melanomas. bioRxiv, 2024.2010.2029.620300 (2024). [Google Scholar]
- 36.Russell P.S., Chase C.M., Colvin R.B. & Plate J.M. Induced immune destruction of long-surviving, H-2 incompatible kidney transplants in mice. J Exp Med 147, 1469–1486 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell P.S., Chase C.M., Colvin R.B. & Plate J.M. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med 147, 1449–1468 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White A.L., Beers S.A. & Cragg M.S. FcgammaRIIB as a key determinant of agonistic antibody efficacy. Curr Top Microbiol Immunol 382, 355–372 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Tirosh I., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z., et al. FCGR2B as a prognostic and immune microenvironmental marker for gliomas based on transcriptomic analysis. Medicine (Baltimore) 102, e35084 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., et al. Siglec10—An immunosuppressor and negative predictor of survival prognosis in gliomas. Frontiers in Genetics Volume 13 – 2022(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida C., et al. Siglec10 Expression on Tumor-associated Macrophages Is an Independent Prognostic Factor in Stage I Lung Adenocarcinoma. Anticancer Res 44, 1289–1297 (2024). [DOI] [PubMed] [Google Scholar]
- 43.Barkal A.A., et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572, 392–396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao N., et al. Blocking siglec-10hi tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Experimental Hematology & Oncology 10, 36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W., et al. Maintenance of graft tissue-resident Foxp3+ cells is necessary for lung transplant tolerance in mice. J Clin Invest 135(2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haque A., Engel J., Teichmann S.A. & Lonnberg T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med 9, 75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao Y., et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao Y., et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alquicira-Hernandez J. & Powell J.E. Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics 37, 2485–2487 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Jackson E.L., et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15, 3243–3248 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopinke D., et al. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol 362, 57–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marino S., Vooijs M., van Der Gulden H., Jonkers J. & Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 14, 994–1004 (2000). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and processed scRNAseq data for accepted and rejecting kidney allografts have been deposited into the Gene Expression Omnibus (GSE252337) database. ECDI-SP induced tolerance model’s data sets, GSM4761001 and GSM4761004, contained in dataset GSE157292 were downloaded from the Gene Expression Omnibus (GEO). Other data are available upon reasonable request from the corresponding authors subject to institutional review and approval.