Summary
Improving nitrogen use efficiency (NUE) of rice plants utilizing a few end-of-season traits poses a severe phenotyping bottleneck in exploring the genetic diversity of a large population and genotype selection accuracy. Therefore, a comprehensive multivariate genotype selection strategy was developed to explore maximum genetic variation of 300 diverse rice genotypes and accurately select promising rice donors with enhanced NUE traits on a multi-year (2019, 2021, and 2022) -trait (126 traits) -environment (2) -temporal (5) –location (3) scale. The multi-trait genotype ideotype distance index (MGIDI) ranked Cauvery, Suweon, RPW9-4 (SSI) and BAM3690 (IC463705) as superior NUE genotypes; Moroberekan, PUSA1121 and BAM8315 (Basmati 370) as low NUE genotypes. The multi-location field performance and molecular analysis of key nitrogen assimilatory genes confirmed the outperformance of the Cauvery genotype in terms of possessing efficient N sensing, uptake, transport and assimilation characteristics under N-limited conditions. Phenome-wide multivariate analysis highlights root-shoot plastic response as a key target trait for breeding rice genotypes resilient to N stress conditions.
Subject areas: Natural sciences, Genotyping, Genomic library, Plant biology, Agricultural science
Graphical abstract

Highlights
-
•
A novel multivariate genotype selection strategy to explore genetic diversity of NUE in rice
-
•
Superior rice donor genotypes discovered for improving nitrogen uptake efficiency
-
•
Multiple cross-validation checks ensured the highest genotype selection accuracy
-
•
Root-shoot carbon allocation is a key breeding trait for improved N stress resilience
Natural sciences; Genotyping; Genomic library; Plant biology; Agricultural science
Introduction
Rice (Oryza sativa L.) is the third most important food crop cultivated worldwide in around 165 million ha with a total production of 776 million tons.1 In order to feed 10 billion people sustainably by 2050, there is a need to increase the productivity of rice by improving input use efficiency, resistance to multiple biotic and abiotic stresses, and resilience to climate change.2,3 Nitrogen (N) dominates among the nutrient inputs used for rice production and pivotally contributed to the green revolution of the 20th century. Nonetheless, it enumerates significant input costs and unfavorably influences natural ecosystems. World utilization of N fertilizer reached approximately 108 million tons in 2022.1 Farmers worldwide are constrained by the affordability of N fertilizers, resulting in poor yields, whereas governments are spending huge exchequer on subsidies, causing global trade imbalance. Excessive N losses through volatilization, denitrification, leaching, surface runoff, and wind erosion cause acidification, water eutrophication, algal blooms, hypoxia, and other public health issues.4,5,6 N is an important building block for many biologically active compounds, and an inadequate supply of N significantly affects growth and productivity.7,8 Rice occupies most of the cultivated land, especially in India, and consumes nearly 50% of all N fertilizers used in agriculture. Rice inherited multiple resilient strategies to cope with the N-deficient environments.9 One of such most prominent N stress tolerance mechanisms is nitrogen use efficiency (NUE).10
NUE is a complex trait; methodologies for dissecting the component traits and exploiting genetic diversity to improve rice with enhanced NUE are thought-provoking. Among the staple crops, rice has the lowest (∼40%) NUE.11 There have been several attempts toward phenotypic characterization of architectural traits such as root length and density,12 dense and erect panicle,13 biomass, flag leaf length, width, leaf thickness and grain yield associated with N response and NUE in rice.14 Vijayalakshmi et al.15 and Srikanth et al.16 estimated NUE for biomass or grain yield production per unit N supplied, similarly for N uptake and utilization efficiency in both fields and hydroponics.17,18,19,20,21,22 Vijayalakshmi et al.15 reported Basmati370 and Ranbir basmati; Panvel,18,21 IR66946-3R-178-1-122 as best NUE genotypes. However, the utility of these novel genetic resources in breeding high NUE rice genotypes is very limited due to the scale and validity of genotype selection. As NUE traits strongly interact with various within-season environmental factors (physical and chemical properties and microbiome activity), selection methodologies based on a few end-of-season traits, estimated from a small population set, would lead to inadvertent selection of genotypes with low breeding value.23 The N-influenced growth performance is known to be highly dynamic at different developmental stages due to the interactome of phytohormones (gibberellin, auxin, cytokinin and strigolactones) at the cellular level.24 Hence, large-scale phenotyping efforts to exploit the genetic diversity of NUE traits are equally important, along with the multivariable trait-based cross-validation and testing of genotypes across multiple phenological stages, seasons and locations.
Traditionally, researchers conduct multi-year or multi-location field trials to overcome the influence of environmental effects on genotype ranking and evaluate the suitability of the genotype for the target environment.15,16 In case of breeding for high NUE, this strategy would not be a cost-effective approach that estimates N in a large population year-on-year and/or locations to select superior genotypes. The alternate way out is to perform the mesocosm assay in a large population set under controlled environmental conditions and select a subset of promising genotypes based on the selection index for further evaluation in the field. Recently, automated high-throughput phenotyping platforms have been used for phenotyping large populations of diverse crops.25 However, such efforts to identify high NUE donors in rice are scarce. Moreover, the NUE selection process in rice for the novel genetic resource has mostly focused on the Basmati type,15 indices focus mainly on yield, overlooking phenology, agronomic traits, and complex physiological components. To address this, the Smith–Hazel Index26 incorporates a phenotypic covariance matrix and economic weights, but multicollinearity can bias index coefficients and genetic gain estimates.27 Rocha et al.27 proposed the FAI-BLUP, using factor analysis to design ideotype scores based on desirable and undesirable traits. More recently, Olivoto et al.28 developed the MGIDI index, a multi-trait genotype–ideotype distance approach, implemented in the R metan package with a step-by-step guide for genotype selection. With this background, it was hypothesized to utilize both selection index and multivariate strategy to identify the superior rice donor using a large-scale control environmental phenotyping experiment and select a subset of best-performing rice ideotypes with the highest breeding value for NUE. To evaluate the superior NUE genotypes in the control and multi-location field trials. Finally, molecular analysis and characterization of key NUE genes in contrasting NUE genotypes were performed to validate the genotypic performance at the cellular level.
Results
Large-scale phenotyping of NUE and genotype selection
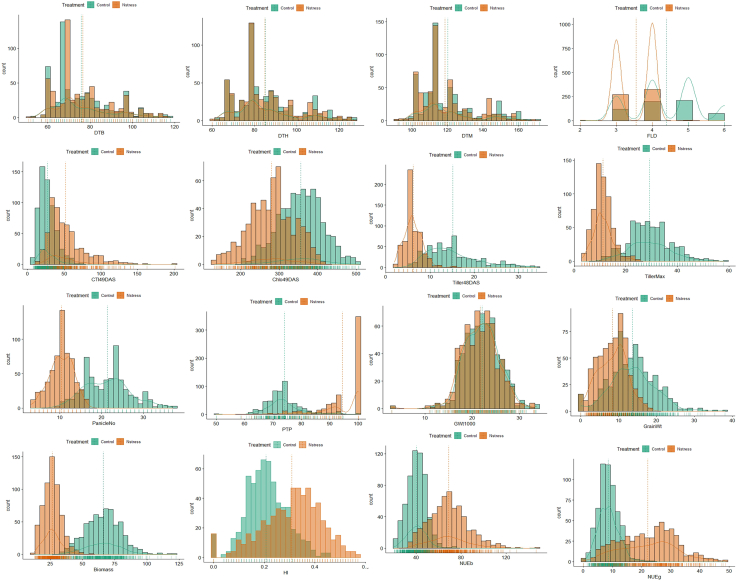
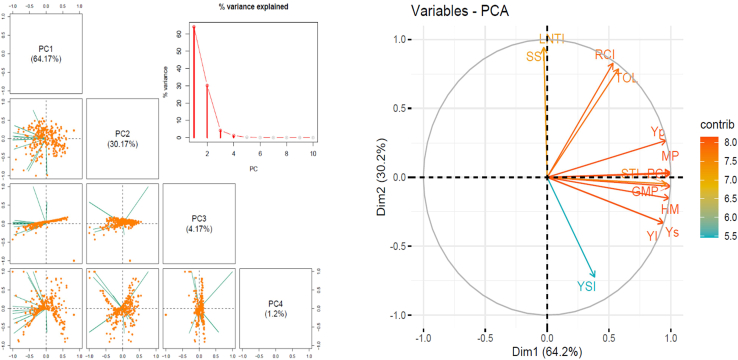
A large phenotypic variance was recorded in this experiment, and most of the phenotypic variance was contributed to by the genetic component of the variance (Table 1). The heritability of the traits varied from medium to high (0.74–1), and the coefficient of variation was within the admissible limit (<30%) for the large-scale control environmental experiment. The analysis of variance (ANOVA) showed a statistically significant difference between genotypes among the treatments (at p-value <0.001). The trait value distribution (histogram) of 16 major performance-related traits (Figure 1). The principal component analysis (PCA) was performed to reduce traits’ dimensionality and find the most important trait features associated with the maximum phenotypic variation. The PCA results and scree plot showed that there were three most informative PCs and were able to explain most of the (98.51%) phenotypic variance presented in the experimental data (Figure 2). The biplot showed the distribution of the loading vectors in two-dimensional PC space and figured out two superior features (MP and HM stress indexes), capturing a maximum phenotypic variance of 64.17% in the first PC itself. The pair’s panel matrix on Pearson correlation coefficient (r at p-value <0.001) clearly showed a very high positive correlation of 0.98 between MP and HM indexes (Figure 3). Also, MP and HM showed a strong correlation of up to 0.97 and 0.98 with grain yield in control (Yp) and N stress condition (Ys), respectively. Finally, a subset of 15 rice genotypes was selected based on common ranking on the MP and HM index and found suitable for comprehensive characterization and NUE field trials under control and N stress conditions (Table S10).
Table 1.
Genetic analysis and estimated variance components of 300 diverse rice genotypes
| Traits | Gen |
Gen |
Res |
Res |
Phen |
CVg | CVr | CVg/ |
p-value | h2 |
|---|---|---|---|---|---|---|---|---|---|---|
| var | (%) | var | (%) | var | CVr | |||||
| DTB.C | 187.26 | 99.99 | 0.02 | 0.01 | 187.28 | 18.04 | 0.19 | 96.65 | 0.00E+00 | 0.99 |
| DTH.C | 208.54 | 99.99 | 0.03 | 0.01 | 208.57 | 16.96 | 0.20 | 85.66 | 0.00E+00 | 0.98 |
| DTM.C | 256.62 | 100.00 | 0.01 | 0.00 | 256.62 | 13.33 | 0.06 | 226.29 | 0.00E+00 | 0.99 |
| FLD.C | 0.87 | 97.92 | 0.02 | 2.08 | 0.88 | 21.19 | 3.09 | 6.86 | 1.80E-209 | 0.98 |
| CTI49DAS.C | 103.57 | 78.97 | 27.58 | 21.03 | 131.16 | 27.41 | 19.31 | 1.94 | 1.69E-65 | 0.97 |
| Chlo49DAS.C | 2696.40 | 84.31 | 501.79 | 15.69 | 3198.19 | 14.60 | 6.30 | 2.32 | 1.15E-82 | 1.00 |
| Tiller48DAS.C | 27.65 | 78.00 | 7.80 | 22.00 | 35.45 | 34.77 | 18.47 | 1.88 | 6.49E-63 | 1.00 |
| TillerMax.C | 55.75 | 84.57 | 10.17 | 15.43 | 65.92 | 25.56 | 10.92 | 2.34 | 1.19E-83 | 0.91 |
| PanicleNo.C | 26.03 | 85.80 | 4.31 | 14.20 | 30.34 | 23.85 | 9.70 | 2.46 | 1.23E-88 | 0.93 |
| PTP.C | 25.59 | 58.84 | 17.90 | 41.16 | 43.49 | 6.83 | 5.72 | 1.20 | 1.80E-29 | 0.94 |
| GWt1000.C | 14.34 | 99.95 | 0.01 | 0.05 | 14.35 | 17.13 | 0.37 | 46.37 | 0.00E+00 | 0.84 |
| GrainWt.C | 35.14 | 98.54 | 0.52 | 1.46 | 35.66 | 23.34 | 5.28 | 8.21 | 4.25E-232 | 0.91 |
| Biomass.C | 152.21 | 83.83 | 29.35 | 16.17 | 181.56 | 18.69 | 8.21 | 2.28 | 6.85E-81 | 0.91 |
| HI.C | 0.01 | 95.26 | 0.00 | 4.74 | 0.01 | 29.23 | 8.75 | 4.48 | 8.06E-157 | 0.88 |
| NUEb.C | 57.29 | 83.83 | 11.05 | 16.17 | 68.34 | 18.69 | 8.21 | 2.28 | 6.85E-81 | 0.89 |
| NUEg.C | 13.23 | 98.54 | 0.20 | 1.46 | 13.42 | 23.34 | 5.28 | 8.21 | 4.25E-232 | 1.00 |
| DTB.Ns | 178.00 | 99.99 | 0.02 | 0.01 | 178.01 | 17.43 | 0.17 | 103.24 | 0.00E+00 | 0.98 |
| DTH.Ns | 198.61 | 99.96 | 0.07 | 0.04 | 198.68 | 16.59 | 0.32 | 51.62 | 0.00E+00 | 1.00 |
| DTM.Ns | 234.79 | 99.99 | 0.02 | 0.01 | 234.81 | 12.93 | 0.11 | 113.83 | 0.00E+00 | 1.00 |
| FLD.Ns | 0.24 | 91.20 | 0.02 | 8.80 | 0.26 | 13.78 | 4.28 | 3.22 | 7.11E-118 | 1.00 |
| CTI49DAS.Ns | 527.03 | 84.49 | 96.77 | 15.51 | 623.81 | 24.30 | 18.98 | 2.33 | 2.44E-83 | 1.00 |
| Chlo49DAS.Ns | 3225.35 | 91.83 | 286.93 | 8.17 | 3512.28 | 20.29 | 6.05 | 3.35 | 1.57E-122 | 1.00 |
| Tiller48DAS.Ns | 3.69 | 84.15 | 0.69 | 15.85 | 4.38 | 21.06 | 13.48 | 2.30 | 4.50E-82 | 0.92 |
| TillerMax.Ns | 12.57 | 87.52 | 1.79 | 12.48 | 14.36 | 21.71 | 11.97 | 2.65 | 1.95E-96 | 0.91 |
| PanicleNo.Ns | 7.14 | 87.92 | 0.98 | 12.08 | 8.12 | 25.81 | 9.57 | 2.70 | 2.10E-98 | 0.96 |
| PTP.Ns | 46.98 | 72.01 | 18.26 | 27.99 | 65.24 | 7.26 | 4.52 | 1.60 | 1.90E-49 | 0.88 |
| GWt1000.Ns | 14.25 | 99.96 | 0.01 | 0.04 | 14.25 | 17.39 | 0.36 | 48.21 | 0.00E+00 | 0.88 |
| GrainWt.Ns | 14.52 | 97.01 | 0.45 | 2.99 | 14.97 | 25.63 | 8.02 | 5.69 | 4.60E-186 | 0.92 |
| Biomass.Ns | 34.93 | 78.25 | 9.71 | 21.75 | 44.64 | 22.23 | 11.72 | 1.90 | 1.47E-63 | 0.92 |
| HI.Ns | 0.01 | 93.69 | 0.00 | 6.31 | 0.01 | 26.72 | 9.53 | 3.85 | 9.55E-139 | 0.74 |
| NUEb.Ns | 238.76 | 79.38 | 62.03 | 20.62 | 300.79 | 22.37 | 11.40 | 1.96 | 1.25E-66 | 0.99 |
| NUEg.Ns | 97.99 | 97.01 | 3.02 | 2.99 | 101.01 | 25.63 | 8.02 | 5.69 | 4.60E-186 | 0.95 |
Note: h2: broad-sense heritability on a genotype mean basis. # Suffix with trait name-C denotes control (N sufficient treatment) and Ns refers to N deficit stress treatment.
Figure 1.
Histogram frequency distribution of sixteen growth performance-related traits associated with 300 diverse rice genotypes
Green bars represent the Control group. Orange bars represent the N stress group. The x axis denotes the trait metric, while the y axis indicates the frequency (count = 300) of observations. Vertical dashed lines mark the mean values of the trait for each group: Green dashed line for the Control group, Orange dashed line for the N stress group. The small bar lines below the x axis in the histogram are rug plots, which represent individual data points for each treatment group—Control and N stress. These lines provide a visual cue about the density, spread of the data, showing the raw data distribution in a compact form.
Figure 2.
Principal component analysis, scree plot and biplot of 11 stress indexes calculated using 300 diverse rice genotypes
(A) Scatterplots visualize the distribution of sample values in the reduced-dimensional spaces, highlighting clusters and separation patterns based on the principal components of stress indexes. The percentage of total phenotypic variance explained by the first four PCs is 99.17.
(B) Scree plot (right panel) showed the % of phenotypic variance explained by the first ten principal components. A sharp decline (angled elbow) in variance after PC2 indicates that the first two components capture most of the data’s variability and the presence of three major clusters within the studied population group.
(C) Biplot showed the first two dimensions represent the first (64.2%) and second (30.2%) principal components, capturing most of the variance in the dataset. Arrows represent individual variables, with their direction indicating the correlation with the principal components and their length reflecting the strength of the contribution. The color gradient (ranging from 5.5 to 8.0) indicates the magnitude of each variable’s contribution, with warmer colors (e.g., red) signifying higher contributions.
Figure 3.
Pairs panel on Pearson correlation coefficient matrix of 11 stress indices and grain yield at p ≤ 0.05 (∗), p ≤ 0.01 (∗∗), and p ≤ 0.001 (∗∗∗) significance level
The figure represents a correlation matrix visualizing pairwise relationships among multiple variables related to yield and stress indices. Diagonal cells display histograms representing the distribution of individual variables. Lower triangle cells contain scatterplots showing the bivariate relationships between variable pairs. Upper triangle cells display the Pearson correlation coefficients (Corr) quantifying the strength and direction of linear relationships between variables.
Comprehensive characterization of selected genotypes
A comprehensive performance evaluation strategy was adopted for two consecutive seasons for the selected genotypes (Season II: 2021 and III: 2022). 126 traits were measured across four temporal time points in this experiment and categorized into morphological, physiological, yield and NUE-related traits (Table S7). The ANOVA using two-season data showed a statistically significant genotypic variation among treatments (Table S11). A significant genotype × treatment (G×T) interaction effect was also noticed for all the traits analyzed. However, most of the major traits (except, CCI_30, SA 30, and TGW) showed a nonsignificant difference between the two seasons (Table S11). This result supports the hypothesis on the benefits of conducting mesocosm experiments inside climate-simulated GH for phenotyping the most complex physiological traits like NUE. Therefore, all six replications of two-season data were pooled together and considered as single experimental data for further analysis (Table S12). The ANOVA of pooled data showed a statistically significant genotypic variation across treatments. As expected, a significant interaction between G×T among all the traits analyzed after pooling two season data together was observed. A large phenotypic variation was found in morpho-physiological, yield, and NUE traits analyzed in control and N stress treatment (Table S13). The phenotypic variation of the traits was higher in the N stress treatment than in the control treatment, except for a few yield traits. The coefficient variation (CV) of the traits varied up to 60% in two-season pooled data. The trait with more than 30% CV was removed from the further analysis (Table S13). The genetic component of phenotypic variance was always higher (>50%) than the residual error components for all three categories of traits irrespective of treatment condition (Figure S2). Finally, the traits with <50% genetic variance contribution (COND_30, AD_60, AGR_30-0 and RGR_30_0) were excluded from further analysis. Therefore, 122 selected traits were regrouped and analyzed based on temporal scale (early season, mid-season and end of season) to exploit the within-season genotypic performance for novel donor identification. Further analysis showed a high heritability of the traits with an average of 0.81 (Table S14). The maximum heritability of 1 was observed in DTB, DTH, and DTM irrespective of treatment conditions; it showed a strong genetic control of the trait, independent of environmental influence. The highest reduction in heritability values was noticed in early establishment traits like SDW_30 (35.4%), LN_30 (36.9%) and TN_30 (23.1%) measured at 30DAT. The heritability of NUE-related traits ranged (from medium to high) from 0.54 to 0.96 in control and 0.73 to 0.84 in the N stress condition. The negative influence of the N stress effect on heritability was also observed in NUEb (26.3% reduction), NHI (15.3%), NUpE (11.5%), GrainN (11.3%) and TotalNinPlant (10.8%).
Early season performances
The effect of N stress on early-season growth performance was comprehensively studied by analyzing a group of 22 traits associated with leaf system architecture, root system architecture, biomass, photosynthesis etc. (Table S12). The results showed that N stress negatively reduced most above-ground canopy traits at 30DAT. Among the traits, maximum trait value reduction due to N stress was higher in above-ground canopy traits like SDW_30 (76.1%) and TN_30 (63.3%). In contrast, N stress significantly increased most of the below-ground root morphological traits (RL_30, PA_30, SA_30, AD_30, LPV_30, RV_30 and TPS_30) to the tune of up to 19.8% at 30DAT. On the other hand, the physiological trait values viz., N_30, photo_30, and QY_30 were drastically reduced (up to 38.4%) due to N stress. The one-way post hoc analysis results clearly showed that Cauvery and RPW-9-4(SSI) ranked as top genotypes with the highest trait values for TN_30 and SDW_30, N_30 and NUpE_30 in the control condition. Under the N deficit condition, the trait value for SDW_30 ranged from 1.6 (Moroberreken) to 2.82 (Cauvery). Shoot N content and QY_30 were also relatively high in Cauvery in N stress conditions. This showed that the Cauvery genotype was well adapted to have early canopy cover even under low levels of soil N availability. Similarly, the TN_30 varied from 3 (Suweon) to 7 (Moroberreken); showing a wide genetic variation among the genotypes to rapidly sense the N stress and adjust the number of tillers based on soil N availability. A large variation in root architecture traits was noticed among the genotypes, where the RDW_30, RL_30 and RV_30 trait values were significantly higher in Moroberreken (2.73, 736, and 8.57) than in Cauvery (1.01, 396, 3.45), respectively. This suggested that the tolerant genotypes put forth early canopy cover, optimum tillering and enhanced root N uptake efficiency per unit root length rather than increasing the RL to forage the soil N. Hence, these traits must be considered more important than the increased root length strategy as it promotes the plant to spend more carbon below ground under N stress-induced carbon-starved conditions.
Mid-season performances
A total of 22 morpho-physiological traits were measured at 60 DAT to evaluate the maximum vegetative growth potential of the genotypes under contrasting N stress conditions. The results showed a consistent reduction in canopy trait values of TN_60 (65.7%), LN_60 (61.9%) and SDW_60 (34.1%) as observed in the early season (Table S15). Interestingly, the positive effect of N stress on root trait was much more at 60DAT than at 30DAT. The N stress-mediated increase in the root morphological traits was at a maximum in RV_60 (74.1%), AD_60 (66.2%), and RL_60 (56.7%) at 60DAT. The canopy physiological trait values related to CCI_60, QY_60, and Tr_60 were significantly reduced (up to 25.12%) due to the N stress condition. However, the NUpE_60 value was observed to increase by 27.9% and reached up to 60% in Cauvery and RPW9-4(SSI) at 60 DAT compared to a reduction of NUpE_60 by 29.65% at early season (Figures S3 and S4). This showed some time-dependent adaptive response of the tolerant genotypes to N stress resilience over the growth period. Among the genotypes, Cauvery was identified as a genotype with the highest trait value for TN (33) and SDW (48.5) than Moroberreken, which possesses the lowest trait value for TN (14.3) and SDW (33.6). A large genetic variation for RL_60 (645–1538), RV_60 (1749–5211) and AD_60 (1.59–7.67) was found in the N stress condition (Figure S4). Genotypes like Cauvery, RPW9-4(SSI) and Suweon were found to possess very low trait values for RL (1242, 856, 1159), RV (4398, 4148, 1749) and AD (7.69, 3.91, 4.26), respectively. At the same time, the trait values for RL, RV and AD were relatively high in Moroberreken (792, 2513, 4.03), BAM8315 (952, 4198, 5.65) and PUSA 1121 (1145, 2703, 5.99), respectively. These results suggested focusing future research toward harnessing more genetic diversity of root system architecture traits and breeding for N efficient root system in rice.
End of season yield traits
To understand the effect of N stress on flowering behavior and yield trait, 14 traits were recorded at the end of the season. The negative effect of N stress was observed in all 14 traits. The greater reduction in trait values was observed in TN (59.4%), PT (53.8%), GWPP (37.91%) and SDW (32.4%) (Table S15). The trait value for TN ranged between 4 and 16 in Moroberrekken and Cauvery, respectively, in the N stress condition. Cauvery genotype was also found to possess very high trait values for PT (12), GWPP (14.5) and SDW (28.5) compared to Moroberekken; which showed much lower traits values for PT (3), GWPP (6.22) and SDW (22.8). Like Cauvery, RPW9-4 and Suweon also possessed high trait values for TN (8.6 and 8.6, PT (6 and 6), GWPP (13.1 and 12) and SDW (28.5 and 26.7) in N stress conditions, respectively. BAM 8315 and PUSA 1121 genotypes showed less trait values like Moroberreken under N stress environment (Figure S5). These results suggested that genetic variation in maximum tiller number and flower abortion rates under stress conditions can be potentially exploited as a very important trait for breeding N-tolerant rice genotypes in the future.
NUE related traits
10 component traits associated with NUE were derived from the N estimated from the shoot and grain samples. A greater reduction in Grain N content (67.86%) and TotalNinPlant (64.2%) was observed under N stress treatment. In contrast, N deficiency significantly increased the component traits of NUE viz., NUEb (87.9%), NUpE (57.4%) and NUtE (42.7%) (Table S15). Under the N deficit condition, the trait value for grain N per cent ranged from 0.05 in Moroberrekon to 0.14 in Cauvery. Similarly, the NPP varied from 0.68 (Moroberrekan) to 1.13 (Suweon), and the total N content ranged from 0.15 to 0.30 in Moroberrekken and Suweon, respectively. The important component traits of NUE viz., NUpE and NUtE ranged from 11 to 53 and 41.2 to 76.8%, respectively (Figure S6). The NUEb trait ranged from 33.9 to 50.08 under N-stress environmental conditions. The genotypes like Suweon, Cauvery and RPW9-4(SS1) were found to possess the highest NUpE (53, 51.9, 46.4%), NUtE (41.2, 49.2, 49.6) and NUEb (46.8, 49.8, 50.0), respectively. In contrast, NUpE (27.6, 32.7, 30.2), NUtE (39.7, 51.4, 43.6), NUEb (39.9, 40.7, 48.4) was much lower in Moroberreken, BAM8315 and PUSA1121, respectively. Overall, Cauvery, BAM3690, Suweon and RPW9-4(SS1) are shown to possess the highest NPGrain, GrainN, TotalNinPlant, NPP, NUpE, NUtE and NUEb compared to Moroberrekan, PUSA 1121 and BAM8315 in N stress condition. However, ranking among the genotypes in the treatment groups was noticed to change due to high genotype and environmental interaction as described earlier. This ranking problem was observed when selecting genotypes from early, mid-, late-, and end-season traits. Therefore, the hypothesis is to exploit the multi-trait-temporal based selection strategy to counter the genotype ranking issue, achieve the maximum genetic gain and improve the NUE of rice.
Multi-trait genotype ideotype distance index (MGIDI)
To address the genotype ranking problem and identify the superior genotype stable across multi-temporal timepoints, the MGIDI index was calculated based on 16 end-of-season traits directly contributing to NUE. When estimating the MGIDI index value, the breeder goal was “high” for ideotypes of all 16 traits, particularly for NUpE and NUtE in the N stress condition. The results of the MGIDI index ranged between 1.42 and 4.33 for the end-of-season NUE traits under N stress conditions (Figure 4). Based on the MGIDI value, Cauvery (1.33) and BAM 3690 (1.42) and RPW9-4(SS1) (1.46) were identified as superior genotypes against Moroberrekan (4.33), BAM8315 (3.75) and PUSA1121 (3.16). To reduce the dimensionality of 16 variables and identify the most significant feature, traits were categorized into three principal components (FA1, FA2 and FA3) based on the contribution of phenotypic variation in N stress conditions. The first principal component (FA1) was related to N uptake (NPS, NPG, SN, TNP, NPP, NUpE and PNUE). The second principal component traits were limited to N utilization (GW, HI, GN, NUtE, NUEg and NHI). The third principal component traits were related to biomass production, viz., SDW, SFW and NUEb in N-starved conditions. The most important traits contributing to the phenotypic variation in the FA1 component were NPP (0.97) followed by NPS (0.94), TNP (0.74) and NUpE (0.74). These results suggested that the Cauvery genotype outperformed all other genotypes with enhanced N uptake-related traits under N-starved conditions and can be potentially used for studying the component traits of N uptake efficiency in future.
Figure 4.
Multi-trait genotype ideotype distance index (MGIDI) radar plot
(A and B) MGIDI plot based on 16 NUE-related traits in control (A) and N stress (B) treatment. Radar plots visualize the relative performance of genotypes across multiple traits in relation to the ideotype. Selected genotypes are shown in red color. Gray circles represent non-selected genotypes. The red color circle represents selected genotypes with the lowest MGIDI values, indicating closer proximity to the ideotype based on 15% selection pressure.
The holistic impact study of all three FAs on the MGIDI index is shown in Figure S7. This analysis uses a ranking system to show the relative contribution of each factor, with the most influential factors being closer to the center of the plot and the less influential factors being placed toward the plot’s edge. The superior genotypes (Cauvery and BAM3690), in terms of NUE traits, exhibit notable strengths in FA1, indicating commendable performance under low nitrogen conditions. This suggests that Cauvery and BAM3690 excel across various traits, displaying favorable characteristics even in restricted nitrogen environments. In contrast, Moroberrekan and BAM8315 revealed weaknesses in FA1, particularly in N uptake-related traits. While PUSA1121 demonstrated weaknesses in FA2 (NUtE-related parameter). The same interpretation can be extended to other factors in different stages. The multi-temporal MGIDI analysis was performed for early, mid and end-of-season traits in control and N stress conditions (Figure S10). To our surprise, the MGIDI index-based selection value was invariably low and stable for Cauvery, found superior genotype across genotype, treatment, temporal time and season (Figures 5 and S8). Next to Cauvery, BAM 3690, Suweon, and RPW-9-(SSI) were identified as another promising line for breeding NUE-efficient rice. In contrast, Moroberrekan, BAM 8315 and PUSA 1121 were identified as sensitive genotypes with very high MGIDI index across genotype, treatment, time and season.
Figure 5.
Hierarchical clustering of 15 promising rice genotypes in N stress treatment
A dendrogram represents the clustering of 15 genotypes based on their similarity across multiple traits, useful for selection and breeding decisions. The vertical axis indicates the dissimilarity between clusters, with values ranging from 10 to 22. Two major branches found in the tree represent genotypes grouped based on their similarity, with shorter branch lengths indicating higher similarity.
Cross-validation
The cross-validation strategy has been overwhelmingly common in the recent past when handling many variables for developing models for selection, classification, or prediction. According to the breeders’ equation, the correct selection of superior donors is critical to achieving greater genetic gain in crop improvement programs with the shortest possible product life cycle time. This validated the methodological error in the selection strategy due to inadvertent manual intentions. The issue was addressed by revalidating the MGIDI selection process with (1) genotype classification based on unsupervised clustering algorithms, (2) comparative analysis of key N assimilatory enzyme and gene activity at the tissue level, and (3) multi-location field trial evaluation.
Hierarchical clustering analysis
The MGIDI index-based selection was performed on a dataset of 16–22 variables categorized into early, mid, late and end-of-season traits for genotype selection at a multi-temporal scale. To validate the multi-temporal MGIDI analysis, we performed an unsupervised hierarchical clustering approach that calculated the similarity coefficient and distance matrix of all 126 traits measured throughout the study period. Additionally, the two independent unsupervised hierarchical clustering algorithms (average and complete linkage) were used to cross-validate the classification algorithm is given in Figure S9). The confusion matrix (Table S17) showed that the genotypes were grouped into three categories in the control and N stress treatment. Group 1 comprised sensitive genotypes, group 2 comprised moderately tolerant, and group III categorized tolerant genotypes. The results of the average linkage and complete linkage methods of hierarchical clustering for determining N stress tolerance showed a 100% accurate prediction of the genotype category. That is, Cauvery and RPW9-(SS1) were grouped into category III; BAM4521, Black Gora, Suweon, Kunjukunju were grouped into group II as moderately tolerant; PUSA1121, BAM8315, and others were grouped into group I as sensitive genotypes (Figure 5).
Nitrogen assimilatory enzyme activity
The REML analysis of all five N assimilatory enzymes showed a very high genotypic variance (>50%) contribution to the phenotypic variance and the variance contribution due to treatment, G × T interaction, rep × treatment and residual was much less than the genetic variance contribution (Table S18). The statistical analysis of variance showed that N stress significantly reduced the NR, NiR, GS, GOGAT and GDH activity irrespective of rice genotypes. However, the percentage reduction in the enzyme activity due to N stress was significantly lower in tolerant genotypes than in sensitive genotypes (Figure 6). Cauvery showed a lesser reduction in N assimilatory enzyme activity among the genotypes throughout the phenological stages. In contrast, the percent reduction in enzyme activity was higher (up to 67%) in Moroberrekan and BAM 8315. The heatmap showed contrasting enzyme activity in two categories of genotypes. The negative effect of N stress in tolerant genotypes was much lower in tolerant genotypes (up to 30%) than in sensitive genotypes (up to 67%).
Figure 6.
Heatmap on N stress-induced reduction (%) of key N assimilatory enzyme activity in the selected four rice genotypes contrasting for NUE traits
Heatmap illustrates the % reduction in enzymatic activity of five nitrogen metabolism (NR, NiR, GS, GOGAT, and GDH) across different time points and genotypes. Row represents gene expression at specific time points (30, 60 DAT & Flag leaves). The column represents four rice genotypes with contrasting NUE traits. The color gradient indicates % reduction, blue-low expression and Red-high expression.
Expression profile of N assimilatory genes
The relative mRNA expression profile of four key genes, namely OsNLP4, OsNRT1.1b, OsGS1.1 and OsNADHGOGAT1, were studied in distinct rice genotypes classified as N stress tolerant (Cauvery and BAM3690) and sensitive (Moroberekan and BAM8315) at two pivotal time points: 30DAT and 60DAT (Figure 7). The expression level of each gene in the control treatment was considered as the baseline expression level (value 1) to calculate the fold change expression of the corresponding gene in the N stress treatment. At early season (30DAT), a 5- to 6-fold increase in the mRNA expression level of the OsNLP4 gene was observed in Cauvery and BAM3690 (Tolerant genotypes) subjected to N stress conditions. These expression levels were found to be maintained up to ∼6–7 times during later (60DAT) stages. Similarly, the expression level of the OsGS1.1 gene increased 2 to 4-fold in tolerant genotypes than in sensitive genotypes. In contrast, we could not find any significant change in the expression level of OsNLP4 and OsGS1.1 genes in sensitive genotypes like Moroberekan and BAM8315, respectively, under the N stress condition. OsNRT1.1b showed a 3-4-time increase in expression level in sensitive genotypes rather than tolerant genotypes. At 30DAT, OsNADHGOGAT1 expression level ranged 2–4 times in all genotypes and was found to increase up to 9-fold during 60DAT (Table S19).
Figure 7.
Heatmap on N stress-induced fold change in gene expression of key N assimilatory genes in the selected four rice genotypes contrasting for NUE trait
Heatmap illustrates gene expression levels of five nitrogen metabolism-related genes (OsNLP4, OsNRT1.1b, OsGS1.1, OsNADHGOGAT1) across different time points and genotypes. Row represents gene expression at specific time points (30, 60 DAT & Flag leaves). The column represents four rice genotypes with contrasting NUE traits. The color gradient represents expression intensity, Purple-low expression and Yellow-high expression.
Multi location field trials
The ANOVA showed a statistically significant genotypic variance across two environments (C and N Stress) and three locations (Table S20). The AMMI (Additive main effect and multiplicative interaction) analysis of grain yield showed that all three environments differed in main and interaction effects (Figure 8). AMMI plot showed a high PCI (56.9%) and PC2 (43.1%) score for single plant yield across locations. The PC1 of any environment or genotypes scored positive and closer to zero are considered stable with less interaction and adaptable to specific environments. In our case, Cauvery (G14), (RPW9-4 (SSI) (G15), and Suweon (G12) are identified as the most stable genotypes across two locations (E2 and E3). The genotypes Suweon (12g), Cauvery (10.6g) and RPW9-4 (SSI) (10.4g) also showed to have the highest single plant yield average across locations (Figure S11). This indicated that these genotypes are stable and favorably adapted to N stress environments of all three locations tested with different dates of sowing, season and year. Which-won-where polygon plots displayed the winning genotypes at the vertex by forming a polygon linking the farthest genotypes from the biplot origin. Seven sectors are identified in the plot of the “which won where” biplot space. Out of all fifteen genotypes tested across 3 locations, Suweon (12), Cauvery (14) and RPW9-4 (SSI) (15) were identified as the winners in the sector where at least 2 environments (TNAU (E2) and IIRR (E3)) were located (Figure 9). Multiple divergence, convergence and crossover interactions in the multi-environmental data analysis can also be observed (Figure 10). The nominal yield plot shows the location-wise GEI and clearly shows that G12, G14, and G15 are much more stable across the environment in terms of yield under N stress conditions. In contrast, G2, G1 (Moroberreken), G4 (PUSA1121), and G5 (Basmati 370) are shown to have very high GEI (Diverging interaction) across three locations. We also attempted to use multivariate trait data collected at three temporal time points in each location for multivariate-based genotype selection. A total of 32 traits has been collected in E1 and E2 locations; seven traits were collected at the end of the season in the E3 location (Table S20). The genotype based on (the MGIDI index) calculated using pooled multi-temporal (early, mid, end of season) data in control and N stress conditions, was selected separately. The results revealed the identification of the superior rice genotypes (Suweon, Cauvery, RPW-9(SSI) and BAM3690) in all three locations, irrespective of treatment group (Figure S10A). However, ranking among the genotypes varied among locations as the year, season, sowing dates and cultivation practices were different in all locations. Contrasting rice genotypes (Moroberreken, BAM8315 and PUSA1121 based on high MGIDI index value across treatment and location was also confirmed (Figure S10A).
Figure 8.
AMMI biplot showing the mean yield performance of fifteen rice genotypes in three locations
AMMI biplot (Additive Main effects and Multiplicative Interaction model) illustrates the interaction between genotypes and environments based on two principal components, PC1 (56.9%) and PC2 (43.1%) represent the first two interaction principal component axes (IPCA), together explaining 100% of the genotype × environment interaction variance. Green points and lines represent environments (E1, E2, E3). Blue points and dashed lines represent genotypes (G1 to G14). The proximity of genotypes to environments indicates their specific adaptability, while genotypes near the origin are considered more stable across environments.
Figure 9.
Nominal yield plot showing the genotype-environment interaction (GEI) of 15 rice genotypes in three multi-location trials
Nominal yield plot illustrates the performance of 15 genotypes (G1 to G15) across a range of environmental conditions, identifying genotypes with high yield potential and stability under varying environmental conditions. The x axis represents the environmental principal component 1 (PC1), expressed as the square root of yield in Mg/ha, indicating environmental quality or productivity. The y axis shows the Nominal Yield (Mg/ha) for each genotype. Each line corresponds to a specific genotype (G1–G15), distinguished by unique colors and line patterns. The slope of each line reflects the sensitivity or responsiveness of the genotype to environmental variation. Steeper slopes suggest higher responsiveness across the environment. Flatter slopes indicate greater stability across environments.
Figure 10.
GGE biplot showing the relationship between environment and yield performance of 15 rice genotypes in three locations
GGE biplot in the “Which-won-where” view is used to visualize the performance of genotypes across multiple environments, identify mega-environments and the best-performing genotypes within each, supporting genotype selection and recommendation. The x axis (PC1: 65.5%) and y axis (PC2: 22.81%) represent the first two principal components derived from genotype and genotype × environment interaction effects. Points labeled G1 to G16 represent different genotypes. Points labeled E1 to E3 represent different environments. Polygons connect the outermost genotypes, forming sectors that help identify which genotype performed best in which environment. Dotted lines (rays) divide the plot into sectors, each associated with a specific environment. The genotype at the vertex of each sector is considered the “winner” in that environment.
Discussion
N is a major macronutrient required for rice’s growth and development. N acts as a critical structural element for many biologically active compounds such as nucleotides, amino acids, proteins, ATP, coenzymes, and chlorophyll at a cellular level.29,30 At the canopy level, N directly influences the overall growth performance of plants in terms of producing more lush green leaves, a higher number of tillers, rapid growth rate, biomass and grain yield. However, the natural nitrogen content in the soil is often insufficient to meet the demands of intensive rice cultivation practices. As a result, applying N fertilizers has become an indispensable practice to enhance rice productivity to keep pace with the ever-increasing demand from the growing global population. Over the past five decades, global rice production has significantly increased due to the introduction of modern-era fertilizer-responsive rice varieties vis-à-vis utilization of chemical nitrogenous fertilizers. Conventionally, N is applied mostly through broadcasting methods, with or without assessing the soil and/or plant requirement. This way, excessive use of N fertilizers in rice cultivation has led to environmental problems such as soil degradation, eutrophication, and GH gas emissions, apart from increasing the cost of production and global food prices. Moreover, under the changing global climatic conditions, excessive N and N deprivation are considered major abiotic stresses affecting rice productivity. Thus, it is imperative to optimize crop productivity while minimizing environmental impact and conserving non-renewable natural resources through effective management of nitrogen inputs.31,32
Rice has evolved several strategies to optimize N uptake through alterations in the canopy and root architecture traits, functional physiological traits, regulation of assimilatory enzymes and expression of high-affinity transporters to cope with the N-starved conditions.33,34 However, an inadequate supply of N significantly affects rice productivity through multiple complex interactions of component traits.7 Particularly, efficient N uptake and assimilation under limited N availability impact nutrient remobilization, quick senescence and yield loss due to early maturity.35 Currently, the NUE of rice is very low among other cereals, and it is estimated to be around 30%–50%36,37 due to ammonia volatilization, denitrification, surface runoff, and leaching of the applied nitrogen fertilizer in the wetland field. Achieving maximum NUE in cereals is essential for agricultural growth and meeting global food demands and food insecurity, with a target of reaching 67% NUE by 2050.36 Various natural resource management strategies aim to improve NUE through precision N application, split doses of N, slow-release fertilizers and biofertilizers, nitrification inhibitors, legume-based crop rotations etc.38
Recently, rice genotypes showed improved performance under low N fertilization conditions and have been developed through transgenic approaches, indicating better nitrogen uptake and utilization mechanisms to address the issue of low NUE.37 However, despite progress, NUE has not reached the desired level. As a sustainable and alternative approach, identifying new genetic resources/rice donor genotypes with enhanced NUE is crucial for sustainable rice production.39 Moreover, there is a need for comprehensive efforts and a multidimensional approach to enhancing NUE in rice by selecting a superior genotype with multiple contributing traits for higher NUE.
Comprehensive genotype selection strategy for enhancing NUE in rice
Traditionally, breeding rice for improved NUE starts with exploring genetic variation present in the diverse population and identifying contrasting genotypes for traits of interest. The success of the entire program of downstream utility (viz., development of bi-parental mapping population, QTL mapping, haplotype/gene identification and marker-assisted breeding or gene editing processes) is perpetually dependent upon the accurate recognition of superior genotypes. Rapid exploration of large genetic diversity and accurate identification of novel genetic resources with high breeding value is critical for achieving the highest genetic gain according to the breeder’s equation. However, the large-scale phenotyping efforts for several studies focused on N stress response and N tolerance under N-deficient conditions. This is mainly due to the non-availability of a target environment (long-term nutrient evaluation field) for screening a large set of diverse populations. Very few isolated studies have reported the identification of superior rice genotypes exhibiting higher NUE but mostly using either end-of-season yield or NUE-related traits estimated from highly heterogeneous field conditions under varied N levels. Many researchers have screened out rice genotypes; for instance, Ray et al.40 screened contrasting rice genotypes differing in leaf mass ratio (LMR) and leaf nitrogen content and discovered IET 12989 and IET 13567 genotypes with higher net photosynthetic rate and lower rubisco content. Haefele et al.41 screened under limited and sufficient nitrogen conditions and identified the best cultivars like CT6510-24-1-2, IR55423-01, IR72, and IR57514-PMI5-B-1-2. Singh et al.17 characterized rice varieties based on the physiological response under different nitrogen levels and discovered the Krishna Hamsa rice genotype with high NUE. Vijayalakshmi et al.15 characterized 78 aromatic rice genotypes based on agronomical performance and NUE-related traits. This group identified Basmati370 and Ranbir basmati as high NUE genotypes, while Kolajoha-3 and Ratnasundari as low NUE genotypes. However, very few of these genotypes have been used in breeding pipelines due to limitations in genetic diversity and a lack of confirmation efforts on the stability of the genotypes and traits of interest across years and locations.
The other major limitation in the lack of prior art information on large-scale phenotyping efforts and subsequent validation was the phenotyping bottleneck in estimating N concentration in plant samples. The low-throughput Kjeldahl method has been widely used to quantify the N concentration and is a time and labor-intensive process. Conversely, accurate high-throughput methodologies like CHNS elemental analysis and remote sensing techniques are known for their costly initial investments and high running costs. In the study, a large population set of 300 diverse rice genotypes from wide geographical locations (Figure S1) was selected, and N from a large number of shoot and grain samples (∼8000 Nos.) were estimated using a CHNS elemental analyzer within one season (2019). Several strategies were reported in the literature for validating the superior performance of selected donors to avoid inadvertent selection of the wrong genotype that drastically reduces the genetic gain. The confirmation strategies reported are studying the trait of interest in two different sets of populations, repetition of the experiment for two or more seasons, multi-location trials, confirming the genotype performance at different phenological stages (within season variation: seedling vs. end of season); compare the genotype performance in mesocosm (hydroponics/pot) versus field study and comprehensive characterization of donor genotypes at cellular (gene and protein expression) level. Among these tactics, the strategy to repeat the large-scale experiment on a multi-environment-location-year scale would be presumably less viable, considering the cost and time involved in the analysis of N in plant and soil samples. Moreover, the availability of target environments (long-term N stress field) in multiple locations is a major concern associated with large-scale NUE phenotyping and validation. Therefore, more weightage was given to all other validation strategies rather than repeating the large-scale phenotyping experiments. At the same time, we aimed to capture the highest proportion of genotypic variation in a large-scale phenotyping study by conducting the mesocosm/pot culture experiment in controlled environmental GH conditions, where soil fertility gradients, variation due to moisture heterogeneity and environmental interactions are relatively very low. In this way, our comprehensive genotype selection strategy can be a cost-effective and inclusive solution for accurately identifying and validating NUE donors.
N stress impact on canopy growth and yield performance
Plants commonly demonstrate performance alterations in reaction to varying levels of N supply.42 Zhang and Shangguan43 observed that N application significantly increased the Fv/Fm ratio during the flowering and grain-filling stages. The results of the current study revealed a significant genetic variation in growth and yield performance among the genotypes. N deficiency negatively affected the canopy and yield traits (plant height, tiller number, biomass, growth rate, flowering behavior and grain) during the active growth period. The N stress-induced reduction in leave greenness index, photochemical efficiency (Fv/Fm), and gas exchange rate were reported in this study. In line with previous findings by Cassman et al.,44 applying N resulted in increased grain yield compared to low N application. The current results align with these observations and showed higher grain yield and total dry matter in the control treatment compared to the N stress treatment. Contrary to canopy traits, the root system plays a vital role in responding and adapting to changes in nitrogen availability, as it can act as a signaling mechanism for the plant to initiate modifications in its root system.45 Foulkes et al.46 identified the traits for improved NUE, including increased root length density at depth, higher stem N accumulation, reduced leaf N concentration, efficient post-anthesis nitrogen remobilization, and lower grain N concentration in wheat. The N starvation-induced root proliferation is typically documented in our experiment.
Promising donors for improving NUE in rice
The pot culture method is used to screen a large population in an environmentally controlled GH facility to control heterogeneity in soil fertility and climatic conditions, as NUE is a polygenic trait. The results showed a typical N stress-mediated increase in NUE (irrespective of genotype tolerance level), as observed by Vijayalakshmi et al.15 and Srikanth et al.16 A high positive correlation was observed between the grain yield (Yp and Ys), and NUEg in stress. Yp and Ys are the most phenotypic variance contributing index selected among the stress indexes. Hence, the large-scale phenotyping experiment efforts selected the subset of 15 genotypes based on grain yield (breeders’ choice) as one of the criteria of selection under control and N stress conditions, respectively.
A total of six promising rice donor genotypes (Cauvery, Suweon RPW-9(SSI) and BAM3690), while Moroberreken, BAM8315 (Basmati 370) and PUSA1121 genotypes were identified as poor NUE genotypes, Cauvery and RPW9-4 (SSI) are Indica-type rice that possess very high NUE compared with other rice types, viz., Aus, Japonica, Basmati, etc., However, we also identified one Japanica rice genotype (Suweon) as the best donor with high NUE and maximum productivity under N-starved conditions. BAM3690 (high NUE) and BAM8315 alias Basmati 370 (Low NUE) are basmati rice and are found as contrasting donors for NUE. Vijayalakshmi et al.15 reported Basmati 370 as a high NUE genotype based on the field evaluation. In our study, PUSA1121 was also identified as one of the poor NUE genotypes along with BAM8315 (Basmati 370). Interestingly, Basmati 370 was one of the donor parents (extra-long slender grain) used to develop the PUSA Basmati 1121 rice variety in India.47 Recently, PUSA Basmati 1 was used as one of the Low NUE donors for studying the N signaling factors in rice.48 This suggests that aromatic basmati rice genotypes can be used as recipient donors for the NUE crop improvement program. It has very high commercial potential if basmati varieties are used as recipients, as they are widely cultivated in Southeast Asian countries for their high-yielding export-quality grains. The contrasting results on the Basmati 370 genotype may be due to the difference in the scale of the phenotyping efforts and environment and the number of traits used for genotype selection.15 The potential utilization of these genetic resources includes, breeding pipeline for improving NUE traits in rice, mapping population development for understanding genetic mechanisms of NUE.
Cauvery: Rice donor with enhanced nitrogen uptake efficiency
The results showed that high NUE donors demonstrated a 20% higher grain yield than low NUE genotypes under N-limited conditions. Additionally, high NUE genotypes exhibited significantly higher total nitrogen uptake than low NUE genotypes in both N treatments. This indicates a better yield performance of high NUE donors in low N fields. These findings support the notion that high NUE genotypes have improved NUE, as evidenced by their higher nitrogen uptake and better overall performance in terms of grain yield. Singh et al.17 assessed rice varieties across different nitrogen levels, pinpointing the Krishna Hamsa rice genotype as possessing high NUEb (51.6%). These studies collectively contribute valuable insights into identifying genotypes with enhanced NUE, establishing a strong foundation for our investigation.
The superior performance of the Cauvery genotype was observed throughout growth stages (early vegetative, maximum tillering, and maturity) and NUE traits, which can likely be attributed to its inherent genetic makeup, which promotes efficient nutrient uptake, utilization and allocation around the clock on phenological stages. During the genotype selection process, the MGIDI index value was invariably the lowest minimum for Cauvery donors across treatment, stages, and trait groups, suggesting the suitability of the donor for further utilization in a breeding program. The MGIDI selection differential (SD in %) is calculated based on the difference between the population mean (Xo) of the trait and the mean of the superior genotype (Xs). The SD values depicted in Table S16 showed the superior performance of the Cauvery genotype, particularly NUpE (10.52%), NUEb (6.55%) and NUEg (5.47%). Notably, the superior genotype Cauvery showed a very low SD for the major agronomic performance indicators like biomass (0.82) and grain yield (3.11). However, the SD for NUEb and NUEg was 6.55 and 5.47, respectively. This means the selection advantage for selecting the Cauvery genotype is low per se for enhanced biomass or grain yield over the population mean in the mesocosm experiment (2021 and 2022). But one can expect the yield advantage of 5.47% and biomass increment of 6.55% per unit of N available in the soil through 10.52% increased NUpE. In addition, a very low (−0.96%) SD was recorded for NUtE, suggesting that the Cauvery genotype can be recognized as a superior donor for future studies on uptake efficiency (NUpE), one of the major component traits of NUE. The grain yield performance of Cauvery, RPW9-4(SSI) and Suweon under N-stress environments was stable and very high in multilocation trials across three locations. This confirmed that these genotypes could be selected for higher NUpE to achieve the highest grain yield per unit N supplied in the main field. However, this result can be revalidated by developing recombinant inbred lines between Cauvery and BAM 8315 and characterizing the mapping population using the same strategy described in this article. The relatively higher NUpE in the Cauvery genotype might have helped to improve photosynthesis, root architecture, nutrient absorption mechanisms, and stress tolerance, allowing it to maintain robust growth and productivity across different developmental phases and enabling optimal nitrogen utilization, resulting in exceptional NUE performance.
Footprints of N stress tolerance at sub-cellular level
The canopy level estimation of yield and NUE-related traits is mostly a resultant phenotypic expression of a complex genetic and environmental interaction. Thus, an inclusive strategy of donor validation must include the expression profile of key genes and proteins involved in N uptake and assimilatory pathways. N is absorbed by plants as nitrates and ammoniacal forms through root transporter systems, and it is then assimilated by several nitrate assimilatory enzymes (NR, NiR, GS and GOGAT). NR is one of the critical cytoplasmic enzymes in plants involved in the metabolic conversion of nitrate to nitrite.49 When exposed to varied quantities of N, the NR enzyme exhibit a wide range of activity.50,51 GS is a vital enzyme that plays a crucial role in metabolizing ammonia by converting ammonium ions into glutamine, facilitating the efficient utilization of nitrogen in various biological processes. N stress alters the transcriptional levels of enzymes involved in N-metabolism (GS, GOGAT and GDH) responsible for N assimilation.52 Significant variation was found in NR, NiR, GS, GOGAT, and GDH activity between high-NUE genotypes and low-NUE genotypes. The NR activity peaks at its highest levels during the periods of 30DAT and 60DAT in N stress conditions. Hakeem et al.50 found that high NUE genotypes displayed consistent and increased NR activity with elevated nitrogen levels. The same trend followed that high NUE genotypes have higher NR activity than low NUE genotypes. The NR activity followed the same path, i.e., higher activity is seen in higher NUE genotypes than in lower NUE genotypes. The activity of NR and NiR is regulated by applying nitrate-based fertilizer.53 Our results showed a similar pattern for NR and NiR activity.
The peak activities of the enzymes were noted during the flag leaf stage, which is attributed to the efficient remobilization processes at this stage. In situations with low N availability, the activity of GS experienced an increase primarily attributed to the more significant buildup of cytosolic GS1.54 Our findings align with these prior observations, as they reveal a higher level of GS activity in genotypes characterized by high NUE under N-deprived conditions (N0) compared to conditions with ample nitrogen supply (N100). Consequently, it can be deduced that genotypes possessing superior NUE can effectively harness ammonia as an alternative nitrogen source, facilitated by augmented GS activity during nitrogen stress circumstances. Cauvery showed a strong ability to process N due to its continuous transformation of nitrate to nitrite, then to ammonia and amino acids, even when nitrate levels are low.15,53 Additionally, the reason for Cauvery’s efficient use of nitrogen might be its well-coordinated system for the uptake and utilization of nitrogen. This system keeps ammonia levels low in the plant tissues,55 linked to higher levels of NR and NiR enzymes.
NIN-LIKE PROTEIN (NLP) family transcription factors are known to play a major role in N- regulated expression of downstream genes. Recently, Wu et al.56 reported the pivotal role of OsNLP4 in N uptake, assimilation and yield. Os NRT1.1b was particularly selected as it is a low-affinity nitrate transporter reported to affect the NUE of indica and japonica under sufficient and deficit N supply conditions. The OsGS1.1 gene encodes a cytosolic GS and catalyzes the conversion of ammonium into glutamine. This gene is primarily involved in improving the assimilation and recycling of N within plants. Expression analyses of OsNLP4, OsNRT1.1b, OsGS1.1 and OsNADH GOGAT1 genes are studied in the root at 30 DAT and 60 DAT. Gene expression of OsNLP4 is seen more in high NUE genotypes than in low NUE genotypes. Genotype Cauvery showed a general upregulation of OsNLP4 expression as early as 30 and 60DAT, correlated with higher transcript abundance of nitrate uptake genes. OsNRT1.1b also has a vital role in regulating nitrate signaling and contributes to regulating NUpE and NUtE.57 OsNRT1.1b, which was found to be highly upregulated in the high NUE genotypes.48 The expressed protein of OsNRT1.1b effectively increases N uptake in both control and N stress conditions. This study found that the OsNRT1.1b gene is upregulated in low NUE genotypes than in high NUE genotypes. The primary reason for these contrasting results could be the form (urea/ammoniacal) of N supplied. The high NUE genotypes might have been utilized as high-affinity ammonium transporters rather than nitrate transporters. This must be validated using the gene expression study of other significant genes involved in the pathway. The relative mRNA expression profile of other N assimilation genes showed conclusive trends. Genotypes BAM3690 and Cauvery showed a high OsGS1.1 gene expression under 30DAT; Moroberekan and BAM8315 showed no such increase in the expression in 30DAT and 60DAT. This showed that tolerant genotypes might have tolerated N stress through early sensing of N availability through OsNLP4, assimilated the current ammoniacal form of N, and remobilized plant N through higher activity of OsGS1.1 in the early seedling stage. In contrast, the poor NUE genotypes with much lesser N sensing capabilities selectively rely on OsNRT1.1b gene expression for N uptake and assimilation without having any remobilization of Plant N through enhanced OsGS1.1 activity.
Physiological mechanism of NUE
NUE traits are complex and highly dynamic during the plant growing season. Some genotypes are adopted to show the plasticity in trait function at very early stages and reach the maximum trait value at the end of the season. Some other genotypes may show the plasticity of traits at the later season and reach the maximum trait value of earlier genotypes at the end of the season. In the case of NUE, very little is known about the plasticity (early or late response) requirements for N tolerance. Large-scale phenotyping techniques on a temporal scale must be explored to check the plasticity variation. These results must be very interesting to come out with our data, though expectations are beyond the scope of this paper. Nevertheless, improving plants for efficient use of N is essential to maintain high productivity while using less nitrogen.58 This helps us to understand the reasons behind the efficiency of using N and how plants respond to different N conditions.59 Another peculiar root plastic response noticed in this experiment was early season root length, where the Cauvery (tolerant) genotypes exhibited better canopy traits with less root length than the Moroberrakan (sensitive) genotypes in response to the N stress condition. The correlation coefficient between early season root traits (RDW_30) and end of season NUE traits (Total N in plant.g) clearly showed a contrasting relationship in tolerant genotypes (−0.48 at P value < 0.05) than in sensitive (nonsignificant) genotypes (Figure S11). This contrasting phenotypic plasticity in root traits was also noticed in control conditions, where RDW_30 in tolerant groups of genotypes showed a positive correlation (0.44 at P value < 0.05) with end-of-season NUpE. The sensitive genotypes showed a significant negative correlation (−0.64, p < 0.01) between RDW_30 and end-of-season NUpE. Literature suggests that N stress negatively impacts the growth and development of canopy traits like tiller number, biomass and N content; positively enhances the root traits as an adaptive response during early vegetative growth stages. At the same time, the NUE traits are always observed to be relatively higher at the end of the season in N stress compared to control conditions, irrespective of genotypes. Here, the Cauvery genotype with enhanced NUE mechanism did not increase the root surface area/length in contrast to Moroberrekan, which soon after N stress sensing invested more canopy carbon to root growth. In contrast, Cauvery exhibited focused canopy cover establishment during early stages and which might have supported the overall N stress resilience in tolerant genotypes. This contrasting root developmental pattern (root and shoot carbon allocation) noticed during early season correlates very well with the enhanced reproductive stage NUE of tolerant genotypes in N stress conditions. These results suggest that the temporal dynamics of root/shoot ratio (root-shoot communication) play a major role in N stress tolerance and need further investigation to harness the genetic diversity of root traits for improving NUE in rice. The recent developments in high-throughput sensors can capture all the above phenome-wide phenotypic variation at a temporal scale and are well-suited to advance rice NUE breeding efforts soon.
Limitations of the study
For genotype, selection strategy, Tyagi et al.60 successfully used stress indexes to select N stress-tolerant wheat cultivars. Since genotype selection based on NUE-associated component traits has more advantages than end-of-season yield (the breeder’s choice). MGIDI methodology is used to select the best genotypes using multidimensional temporal data collected during the early season, mid-season, and end-of-season traits along with NUE traits. The advantage of MGIDI (ideotype) based genotype selection is the manual weightage (breeder goal) assigned to multiple contributing traits of interest. Hence, the genotypes identified in this experiment were stable across phenological stages, treatments (environment), location, and years and were conclusively revalidated. However, major limitations of this study are related to manual ground truth data collection on a multi-temporal scale, that are laborious, time-consuming, error-prone and a low-throughput process. Recently, non-invasive imaging sensors were used for repeatedly estimating the dynamic physiological traits of the same plant.25,61,62,63 In this scenario, high-throughput phenotyping technologies, machine learning, and AI models are overwhelmingly advantageous in combining all different NUE-associated traits in one super genotype. The major limitation in the MGIDI strategy is related to manually setting goals for the trait of interest during index calculation. The manual intervention to set the ideotype goal is sometimes inadvertently selected based on a linear relationship between the dependent and independent variables. The genotype selection for a complex physiological trait requires the automatic selection of ideotype goals based on non-linear trait relationships rather than manual intervention in setting goals. Recent developments in machine learning and AI-based genotype selection at the multi-trait-temporal-treatment-location level are the most promising efforts needed in the future.
Rice faces many challenges under limited soil N availability, which causes yield losses in the field. The discovery of novel genetic resources with enhanced NUE is critical for improving the N stress tolerance and producing more crops from less N. In this connection, a comprehensive multivariate genotype selection strategy was adopted to study the phenome-wide list of traits (126) on the multi-temporal-environment-year-location scale. Cauvery, BAM3690 (alias IC463705), RPW-9-4 (SSI) and Suweon are identified as superior donors with high NUE across the explored scale. In contrast, Moroberekan, BAM8315 (or Basmati 370) and PUSA 1121 were identified as low NUE genotypes. The superior genotype selection based on multiple variables was always meritorious compared to a single trait-based (yield or NUE collected at the end of the season) selection strategy. The elevated expression profile of key N assimilatory genes in the Cauvery genotype confirms the superiority among the genotypes studied. The donors selected based on multiple interactome traits will be used further to understand the genetic basis of NUE in rice. Furthermore, future research may focus more on studying the component traits of NUE, the temporal dynamics of root-shoot carbon partition, and the reproductive physiology of rice plants to improve nitrogen use efficiency in rice.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, DR (r.dhandapani@icar.gov.in).
Materials availability
Materials are available in NDPPC (Nanaji Deshmukh Plant Phenomics Center), Division of Plant Physiology, New Delhi, India.
Data and code availability
-
•
Data: The sources of the datasets supporting the current study are presented in the key resources table and the method details sections.
-
•
Code: Requests for the data and code that support the findings of this study should be directed to the lead contact.
-
•
Additional information: Any additional information required to reanalyze the data reported in this article or reproduce the results is available from the lead contact upon request.
Acknowledgments
N.T.D. and A.H. are grateful to the Division of Plant Physiology, Nanaji Deshmukh Plant Phenomics Center (NDPPC), for providing research facilities and to the Indian Agricultural Research Institute (ICARI-IARI), New Delhi, India, for providing the PG scholarship. The NDPPC was established and is supported by the National Agriculture Science Fund (NASF), ICAR, Grant No. NASF/Phen-6005/2016–17, and the National Agricultural Higher Education Project-Centre for Advanced Agricultural Science and Technology (NAHEP-CAAST), ICAR-IARI, Grant No. NAHEP/CAAST/2018-19/07. This research work was funded by the iHub Drishti Foundation, IIT, Jodhpur, and Grant No. TIH/iHub Drishti/Project/2022-23/36. N.T.D. and A.H. also acknowledge the technical assistance provided by Ayyagari Ramlal, School of Biological Sciences, Universiti Sains Malaysia, Malaysia, for coordination in manuscript communication and review process. Figures (as applicable) are created using BioRender. https://BioRender.com/j72c721.
Author contributions
Conceptualization, N.T.D., A.H., D.R., and V.C.; data curation, N.T.D., A.H., and M.A.I.; supervision., D.R., S.K., R.N.S., and V.C.; investigation, N.T.D., A.H., C.N.N., and A.S.; methodology and Validation; R.P., L.S., C.N.N., and A.S.; resources, E.A., B.S., R.K.P., and V.C.; writing – original draft, N.T.D., A.H., and D.R.; seed material, C.V., R.K.E., and V.C.; statistical analysis, N.T.D., A.H., A.R., and M.A.I.; writing – review and editing, D.R. and V.C.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| IRRI 3K panel | International Rice Research Institute (IRRI) | https://gigadb.org/dataset/200001 |
| 300 diverse rice genotypes (150 NE landraces, 150 IRRI 3K panel) | Division of Genetics and Plant Physiology, IARI, New Delhi, India | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Urea (46% N) | Indian Farmers Fertiliser Cooperative (IFFCO) | Cat# N/A |
| KH2PO4, KCl, Micronutrient mix | Sigma-Aldrich or Local Agricultural Supplier | Various; standard grade |
| Trizol reagent | Invitrogen (Thermo Fisher Scientific) | Cat# 15596018 |
| DNase I | Thermo Fisher Scientific | Cat# EN0521 |
| SYBR Green Master Mix | Applied Biosystems (Thermo Fisher Scientific) | Cat# 4367659 |
| Superscript III Reverse Transcriptase | Invitrogen (Thermo Fisher Scientific) | Cat# 18080044 |
| Deposited data | ||
| Raw phenotypic and physiological data | This paper (Supplementary Tables S1, S2–S6, S7, S8, and S9) | N/A |
| Gene expression Ct values and ΔΔCt output | This paper (Table S9) | N/A |
| Software and algorithms | ||
| WinRHIZO Pro v.2009 | Regent Instruments Inc., Montreal, Canada | https://www.regentinstruments.com |
| LI-COR 6400XT software | LI-COR Biosciences | https://www.licor.com |
| FluorPen FP 100 | Photon Systems Instruments (PSI) | https://www.psi.cz |
| MC-100 Chlorophyll Meter Software | Apogee Instruments | https://www.apogeeinstruments.com |
| R v4.2.0 and packages for MGIDI | R Core Team; Selection Index Package by Olivoto et al.28 | https://cran.r-project.org |
| GraphPad Prism 9 | GraphPad Software | https://www.graphpad.com |
Method details
Experiment I
Experimental model
The large-scale phenotyping experiments include 300 diverse rice genotypes (a subset of 150 landraces collected from northeast India and 150 subsets of 3K IRRI diverse germplasm lines). The genotypes were procured from the Division of Genetics and Plant Physiology, Indian Agricultural Research Institute (IARI), New Delhi, India. Rasi and Varadhan were used as an N stress-tolerant check and PUSA Basmati 1 as a susceptible check (Table S1).
Experimental design and location
A pot culture experiment was laid out in randomized block design (RBD) with three replications. The experiment was conducted in a climate-controlled greenhouse (GH) at Nanaji Deshmukh Plant Phenomics Centre (NDPPC), IARI, in Kharif 2019.
Soil profile, seedling germination and treatments
Seeds were sown in the field nursery plots, and then 28-day-old seedlings were transplanted in two sets of three pots (capacity: 36 × 24 cm with ∼15 kg of field soil). One set was designated as the control supplemented with the recommended dose of N (120 kg/ha) in the form of Urea, and the other set was designated as N stress pots (N0: with no Urea supply). The recommended dose of fertilizers for control and N stress pots were 120-60-80 kg/ha N-P-K 964 and 0-60-80 kg/ha N-P-K, respectively. 1.245g/pot containing 15 kg of field soil was used, with the assumption that 1 ha of 15 cm depth soil equals 1.95 million kg of soil with a 1.3 bulk density; 46% of N per unit of Urea applied. The calculated ∼1.245 g/pot full dose was equally split into 3 split doses, each split of ∼400 mg was applied at transplantation, tillering and panicle initiation stages to create the mesocosm. A total of 1800 (300 genotypes × 2 treatments × 3 replications) pots were used for the study (Figure S1). The available soil N status prior to the experiment was measured using the Kjeldahl method65 as indicated in Table S2. Two weeks after transplantation, uniformly established plants were selected, and pots were placed on the conveyors inside the climate-controlled greenhouse (GH). The growth conditions were set to 32 °C and 28°C during day and night, respectively, with a sinusoidal gradient coinciding with local sunrise and set timings. The daily greenhouse climatic conditions (temperature, RH and CO2) are given in Table S21.
Traits measurement
Sixteen agronomic growth performance traits and end-of-season NUE traits were calculated to select the best rice genotypes (Table S3). There were three groups of traits 1) phenology-related traits: days to booting (DTB), days to heading (DTH), days to maturity (DTM) and flowering duration (FLD), 2) early vigor-related traits: chlorophyll content at 49 DAS (Chlo49DAS), and tiller number at 48 DAS (Tiller48DAS) to calculate chlorophyll tiller index (CTI.49DAS). Maximum tiller number (TillerMax) and panicle number (PanicleNo) were measured at the heading stage. Productive tiller percentage (PTP), biomass (Biomass), grain yield (GrainWt) and 1000 grain weight (GWt1000) were measured at the maturity stage. The NUE of rice biomass and grain per pot was calculated using the formula modified by Crasswell and Godwin.66
WBiomass and WGrain are biomass per pot and grain yield per pot at maturity, respectively. W (N inputs + N available in initial soil) is the total and available nitrogen supply in the initial soil. The promising genotypes were selected based on 11 stress indexes (Table S4).
Experiment II
Plant materials, experimental design, location and treatments
A subset of 15 promising rice genotypes with contrasting NUE traits was selected from Experiment I and was repeated for two seasons (2021 and 2022) in a completely RBD inside a controlled environmental phenotyping facility at the NDPPC, IARI (Table S5). Soil N status (Table S6), treatments and GH growth conditions are maintained as in experiment I. A total of 450 (15 genotypes × 2 treatments × 15 replications) pots were used for this study. Three plants, each out of 15 replications of genotypes, were destructively sampled for comprehensive characterization at early-season (30DAT), mid-season (60 DAT), late-season (∼90 DAT) and end-of-season (Maturity).
Traits measurement and biochemical analyses
Here, a total of 126 morpho-physiological traits were measured (Table S7).
Chlorophyll content: The leaf chlorophyll content was measured using an MC-100 chlorophyll concentration meter (Apogee Instruments, Inc., Logan, UT, USA). A field-of-view reducer is included to reduce the sampling area to approximately 20 mm2 (circle with 5 mm diameter) for leaves narrower than 9 mm.
Chlorophyll fluorescence (Chl-F): The photosynthetic light reaction efficiency was measured using a fluorometer (FluorPen FP 100, PSI, Czech Republic). The effective quantum yield (QY) equivalent to Fv/Fm was derived, where Fv represents the variable fluorescence and Fm represents the maximum fluorescence.
Photosynthetic dark reaction efficiency: Leaf gas exchange parameters were measured using a portable photosynthetic system (LI-6400 XT; LI-COR Inc. Lincoln, Nebraska, USA). The major parameters included for analysis were the net rate of photosynthesis (Photo; μmol CO2 mˆ2 sˆ(-1)), stomatal conductance (Cond: mol H2O mˆ2 sˆ(-1)), transpiration rate (Tr: mmol H2O mˆ2 sˆ(-1)) and measured at a light intensity of 1000 μmol mˆ(-2) sˆ(-1) PAR, a leaf temperature of 30 °C, RH of 60%, and a constant CO2 concentration of 400 μmol. The measurement for each treatment was made on the fully expanded top and bottom leaves during the morning on sunny days to avoid the effects of photo-inhibition and was repeated at least three times using different plants.
Shoot and Grain N content: At 30, 60 and physiological maturity, biomass and grain samples were dried and powdered to determine N content using a CHNS analyzer (EuroVector S.p.A. - EuroEA3000 CHNS-O Analyzer) using the methodology of Jimenez et al.67
NUE traits: Nitrogen uptake efficiency (NUpE), nitrogen utilization efficiency (NUtE), nitrogen harvest index (NHI), physiological nitrogen use efficiency (PNUE), NUE calculated in terms of biomass per pot (NUEb) and NUE per pot in terms of grain yield (NUEg) were determined (Table S8).
Root system architecture traits: Total root length (RL; cm), projected area (PA; cm2), root surface area (RSA; cm2), root average diameter (RD; mm), length/volume (LPV; cm/m3), total root volume (RV; m3), and root tips (number) were measured using Epson flatbed scanner and WinRHIZO Pro (V: 2009, Regent Instruments, Montreal, QC, Canada).
Genotype selection: Multi-trait genotype ideotype distance index (MGIDI)
The 126 morpho-physiological traits measured in this experiment were grouped based on the temporal scale of data collection and treatment (C & N). The multivariate-based selection index (MGIDI) was calculated based on factor analysis and genotype-ideotype distance.28 The genotype selection intensity of 15% was selected while ranking the genotype. The breeder’s goal for multiple contributing traits was mostly set as “high” for positive traits and “low” for negative influence traits (plant height, days to booting, heading and maturity) on biomass and grain yield.
N assimilatory enzyme activity
For N assimilatory enzymatic activity, the leaf samples were collected at different phenological stages: early vegetative stage (30DAT), maximum tillering stage (60 DAT) and flag leaf stage (∼90DAT) and stored at -80°C for further analyses. Nitrate reductase (NR) and Nitrite reductase (NiR): The enzyme was isolated according to Hageman and Hucklesby68 and Joy and Hageman,69 respectively. Glutamine synthetase (GS), glutamate oxoglutarate aminotransferase (GOGAT) and glutamate dehydrogenase (GDH): Extraction of enzyme and assays of GS, GOGAT and GDH were done following the method of Mohanty and Fletcher.70
Gene expression analysis
Four key genes involved in N sensing (OsNLP4), uptake (OsNRT1.1b) and assimilation and remobilization (OsGS1.1 and OsNADH-GOGAT) were selected for studying the expression pattern in contrasting genotypes (Table S9). Total RNA was extracted from the root samples collected at 30 & 60 DAT using a Trizol method, followed by DNaseI treatment to obtain DNA-free RNA using the standard protocol. RNA was quantified using a Thermo nanodrop 2000c spectrophotometer, and purity was confirmed by checking the ratio of A260/A280. First-strand cDNA was synthesized from 1 μg of total RNA using superscript–III reverse transcriptase (Invitrogen, USA) with the reaction mixture: 7.5 μl of SYBR Green master mix, 0.7 μl forward and reverse primers, 1.3 μl cDNA and 4.8 μl of nuclease-free water. qRT-PCR was carried out using Power SYBR® Green Master Mix (Applied Biosystems, USA) on a real-time PCR detection system (Applied Biosystems). Normalization of the data for each transcript was carried out, and the expression level was analyzed using the 2-ΔΔCt method.71
Experiment III
15 selected rice genotypes were evaluated under long-term N stress condition in multi-location field trials during 2023 and 2024 at three locations in India at IARI, New Delhi (Kharif, 2023; 28° 38′ 23″ N & 77″ 09′ 27′ E; E1), marginal land at TNAU, Coimbatore, Tamil Nadu, India (Rabi, 2023; 28° 38′ 23″ N & 77″ 09′ 27′ E; E2) and IIRR, Hyderabad, Telangana, India (Rabi, 2024; 17.53″ N and 78.27″ E; E3). In E1, E2 & E3, the experiments were conducted using RBD with three replications. In E1, 25-day-old seedlings were transplanted in a plot size of 2 × 2 m2 with a row spacing of 20 × 15 cm with four lines per replicate of each genotype. The recommended dose of NPK is normal, and stress is kept as mentioned previously. The N was applied as urea with three equal splits of doses as basal at transplanting, tillering and booting stages. In E2, soil N level was ∼172 kg/ha for N stress and a recommended dose of NPK was applied for the control field. A plot size of 1 × 1m2 with 20 × 15 cm spacing was maintained for each genotype. In E3, each entry was planted in three rows of 15 plants with a 20 × 15 cm spacing. The available soil N was estimated to be ∼177 kg/ha in the N0 field and 286 kg/ha in the N120 field. The growth and yield performance of genotypes in all three locations was estimated as per plot (kg/m2) and on a single-plant basis. In all the locations, standard management practices were followed to cultivate rice.72 For MGIDI-based genotype selection, 32 traits have been collected in the E1 and E2 locations; seven traits were collected at the end of the season in the E3 location (Table S20). Similarly, a group of 12, 12, and 7 traits were collected in E2 at 30, 60DAT, and the end of the season. The pooled data on temporal time snapshots were used for MGIDI index calculation under control and N stress conditions to select novel NUE genotypes.
Quantification and statistical analysis
All statistical analyses and graphs were prepared using R v.4.0.2.73 The descriptive statistical analysis, analysis of variance, and calculation of Tukey’s HSD were performed using the “statix” packages. The level of significance is indexed at p ≤ 0.05 (∗), p ≤ 0.01 (∗∗), and p ≤ 0.001 (∗∗∗). The observed variance in a particular phenotypic variable (trait) was partitioned into components attributable to different sources of variation, such as variation due to genotype effect (G), environment effect (E), and their interaction (G∗E). A joint analysis of variance was performed with a linear mixed model using a restricted maximum likelihood (REML) approach as implemented in ASReml-R v.3.0. Genetic analysis component variance and broad-sense heritability were estimated using the variability package in R. The principal component analysis was performed using the FactoMineR library, and the scree plot was constructed using the fviz_screeplot function. The first three principal components were selected based on the elbow angle of the scree plot. The PCA biplot was constructed with the facto extra command in the fviz_pca_biplot function. The pair’s panel analysis for visualizing Pearson’s correlation coefficient (r) was executed using the Psych library in R. Hierarchical clustering analysis was performed for unsupervised clustering of genotypes using the hclust command. Two complementary methods, i, the average linkage & ii the complete linkage methods, were adopted for hierarchical clustering of genotypes based on the distance index. A confusion matrix was formed to confirm the clustering of member genotypes in two methods. The multi-trait selection index was estimated step by step proposed by Olivoto et al.28 with the “metan” package in R. The ggplot2 package was used for plotting graphs and preparing images. The AMMI and GGE multivariate models were constructed using the performs_ammi & gge functions in the “metan” package.28
Published: August 7, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113280.
Contributor Information
Dhandapani Raju, Email: dandyman2k6@gmail.com.
Viswanathan Chinnusamy, Email: viswa.chinnusamy@gmail.com.
Supplemental information
References
- 1.FAOSAT . FAO Statistical Database; 2022. Food and Agricultural Organization of the United Nation.https://www.fao.org/faostat/en/#data/QCL [Google Scholar]
- 2.Hickey L.T., N Hafeez A., Robinson H., Jackson S.A., Leal-Bertioli S.C.M., Tester M., Gao C., Godwin I.D., Hayes B.J., Wulff B.B.H., Wulff B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019;37:744–754. doi: 10.1038/s41587-019-0152-9. [DOI] [PubMed] [Google Scholar]
- 3.Elangovan A., Duc N.T., Raju D., Kumar S., Singh B., Vishwakarma C., Gopala Krishnan S., Ellur R.K., Dalal M., Swain P., et al. Imaging sensor-based high-throughput measurement of biomass using machine learning models in rice. Agriculture. 2023;13:852. [Google Scholar]
- 4.Nolan B.T., Hitt K.J. Vulnerability of Shallow Groundwater and Drinking-Water Wells to Nitrate in the United States. Environ. Sci. Technol. 2006;40:7834–7840. doi: 10.1021/es060911u. [DOI] [PubMed] [Google Scholar]
- 5.Good A.G., Beatty P.H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson C.R., Berdalet E., Kudela R.M., Cusack C.K., Silke J., O’Rourke E., Dugan D., McCammon M., Newton J.A., Moore S.K., et al. Scaling Up From Regional Case Studies to a Global Harmful Algal Bloom Observing System. Front. Mar. Sci. 2019;6 doi: 10.3389/fmars.2019.00250. [DOI] [Google Scholar]
- 7.Rao A.N., Wani S.P., Ramesha M.S., Ladha J.K. In: Rice production worldwide. Chauhan B., Jabran K., Mahajan G., editors. Springer; 2017. Rice production systems; pp. 185–205. [Google Scholar]
- 8.Harika A., Dhandapani R., Kumar S., Lekshmy S., Vinutha T., Ranjith K.E., Kumar M., D M., Sahoo R., C V., Viswanathan C. Understanding the physiological, genetic and molecular basis of nitrogen deficiency tolerance and their application in rice improvement. Oryza. 2023;60:45–52. [Google Scholar]
- 9.Abrol Y.P., Adhya T.K., Aneja V.P., Raghuram N., Pathak H., Kuleshrestha H., Sharma C., Singh B., editors. Indian Nitrogen Assessment, Sources of Reactive Nitrogen Environmental and Climate Effort, Management, Options and Policies. Vol. 538. Elsevier; 2017. http://airea.net/page/65/statistical-data/world-rice-production-consumption-and-stocks [Google Scholar]
- 10.Xing Y., Jiang W., He X., Fiaz S., Ahmad S., Lei X., Wang W., Wang Y., Wang X., Wang X. A review of nitrogen translocation and nitrogen-use efficiency. J. Plant Nutr. 2019;42:2624–2641. [Google Scholar]
- 11.Norton R., Davidson E., Roberts T. Position paper from the GPNM’s Task Team Workshop, December 8, 2014 held at Washington, DC. 2015. Nitrogen use efficiency and nutrient performance indicators. [Google Scholar]
- 12.Anis G.B., Zhang Y., Islam A., Zhang Y., Cao Y., Wu W., Cao L., Cheng S., Cheng S. RDWN6XB, a major quantitative trait locus positively enhances root system architecture under nitrogen deficiency in rice. BMC Plant Biol. 2019;19:12. doi: 10.1186/s12870-018-1620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H., Qian Q., Wu K., Luo J., Wang S., Zhang C., Ma Y., Liu Q., Huang X., Yuan Q., et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014;46:652–656. doi: 10.1038/ng.2958. [DOI] [PubMed] [Google Scholar]
- 14.Rao I.S., Neeraja C.N., Srikanth B., Subrahmanyam D., Swamy K.N., Rajesh K., Vijayalakshmi P., Kiran T.V., Sailaja N., Revathi P., et al. Identification of rice landraces with promising yield and the associated genomic regions under low nitrogen. Sci. Rep. 2018;8:9200. doi: 10.1038/s41598-018-27484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayalakshmi P., Vishnukiran T., Kumari B.R., Srikanth B., Rao I.S., Swamy K.N., Surekha K., Sailaja N., Subbarao L.V., Raghuveer Rao P., et al. Biochemical and physiological characterization for nitrogen use efficiency in aromatic rice genotypes. Field Crop Res. 2015;179:132–143. [Google Scholar]
- 16.Srikanth B., Subrahmanyam D., Sanjeeva Rao D., Narender Reddy S., Supriya K., Raghuveer Rao P., Surekha K., Sundaram R.M., Neeraja C.N., Neeraja C.N. Promising physiological traits associated with nitrogen use efficiency in rice under reduced N application. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1268739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh H., Verma A., Ansari M.W., Shukla A. Physiological response of rice (Oryza sativa L.) genotypes to elevated nitrogen applied under field conditions. Plant Signal. Behav. 2014;9 doi: 10.4161/psb.29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari S., Sharma N., Raghuram N. Meta-analysis of yield-related and N-responsive genes reveals chromosomal hotspots, key processes and candidate genes for nitrogen-use efficiency in rice. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.627955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin W., Liu H., Zhao H., Wang J., Zheng H., Jia Y., Yang L., Wang X., Li J., Li X., et al. The response of grain yield and root morphological and physiological traits to nitrogen levels in paddy rice. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.713814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma N., Sinha V.B., Gupta N., Rajpal S., Kuchi S., Sitaramam V., Parsad R., Raghuram N., Raghuram N. Phenotyping for nitrogen use efficiency: rice genotypes differ in N-responsive germination, oxygen consumption, seed urease activities, root growth, crop duration, and yield at low N. Front. Plant Sci. 2018;9:1452. doi: 10.3389/fpls.2018.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma N., Kumari S., Jaiswal D.K., Raghuram N. Comparative transcriptomic analyses of nitrate-response in rice genotypes with contrasting nitrogen use efficiency reveals common and genotype-specific processes, molecular targets and nitrogen use efficiency-candidates. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.881204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan N.T.H., Heymans A., Bonnave M., Lutts S., Pham C.V., Bertin P. Nitrogen use efficiency of rice cultivars (Oryza sativa l.) under salt stress and low nitrogen conditions. J. Plant Growth Regul. 2023;42:1789–1803. [Google Scholar]
- 23.Lammerts van Bueren E.T., Struik P.C. Diverse concepts of breeding for nitrogen use efficiency. A review. Agron. Sustain. Develop. 2017;37:1–24. [Google Scholar]
- 24.Luo L., Zhang Y., Xu G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020;71:4415–4427. doi: 10.1093/jxb/eraa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duc N.T., Harika A., Raju D., Kumar S., Pandey R., Ellur R.K., Krishnan S G., Allimuthu E., Singh B., Ramlal A., et al. Maximizing Nitrogen Stress Tolerance Through High-throughput Phenotyping in Rice. Plant Stress. 2025;15 [Google Scholar]
- 26.Bhering L.L., Laviola B.G., Salgado C.C., Sanchez C.F.B., Rosado T.B., Alves A.A. Genetic gains in physic nut using selection indexes. Pesq. agropec. bras. 2012;47:402–408. [Google Scholar]
- 27.Rocha J.R.d.A.S.d.C., Machado J.C., Carneiro P.C.S. Multitrait index based on factor analysis and ideotype-design: proposal and application on elephant grass breeding for bioenergy. Gcb Bioenerg. 2018;10:52–60. doi: 10.1111/gcbb.12443. [DOI] [Google Scholar]
- 28.Olivoto T., Diel M.I., Schmidt D., Lúcio A.D. MGIDI: a powerful tool to analyze plant multivariate data. Plant Methods. 2022;18:121. doi: 10.1186/s13007-022-00952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh U.M., Sareen P., Sengar R.S., Kumar A. Plant economics: a newer approach to study mineral transport and its regulation. Acta Physiol. Plant. 2013;35:2641–2653. [Google Scholar]
- 30.Agnihotri A., Seth C.S. Exogenously applied nitrate improves the photosynthetic performance and nitrogen metabolism in tomatoes (Solanum lycopersicum L. cv Pusa Rohini) under arsenic (V) toxicity. Physiol. Mol. Biol. Plants. 2016;22:341–349. doi: 10.1007/s12298-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyu Y., Yang X., Pan H., Zhang X., Cao H., Ulgiati S., Wu J., Zhang Y., Wang G., Xiao Y., Xiao Y. Impact of fertilization schemes with different ratios of urea to controlled release nitrogen fertilizer on environmental sustainability, nitrogen use efficiency and economic benefit of rice production: A study case from Southwest China. J. Clean. Prod. 2021;293 [Google Scholar]
- 32.Javed T., Singhal R.K., Shabbir R., Shah A.N., Kumar P., Jinger D., Dharmappa P.M., Shad M.A., Saha D., Anuragi H., et al. Recent advances in agronomic and physio-molecular approaches for improving nitrogen use efficiency in crop plants. Front. Plant Sci. 2022;13:917. doi: 10.3389/fpls.2022.877544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirel B., Tétu T., Lea P.J., Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability. 2011;3:1452–1485. [Google Scholar]
- 34.Mahboob W., Yang G., Irfan M. Crop nitrogen (N) utilization mechanism and strategies to improve N use efficiency. Acta Physiol. Plant. 2023;45:52. [Google Scholar]
- 35.Alam I., Zhang H., Du H., Rehman N.U., Manghwar H., Lei X., Zaid Khan, Batool K., Ge L., Ge L. Bioengineering Techniques to Improve Nitrogen Transformation and Utilization: Implications for Nitrogen Use Efficiency and Future Sustainable Crop Production. J. Agric. Food Chem. 2023;71:3921–3938. doi: 10.1021/acs.jafc.2c08051. [DOI] [PubMed] [Google Scholar]
- 36.Ladha J.K., Jat M.L., Stirling C.M., Chakraborty D., Pradhan P., Krupnik T.J., Gerard B. Achieving the sustainable development goals in agriculture: The crucial role of nitrogen in cereal-based systems. Adv. Agron. 2020;163:39–116. [Google Scholar]
- 37.Neeraja C.N., Voleti S.R., Desiraju S., Kuchi S., Bej S., Talapanti K., R. Puskur R. In: Molecular breeding for rice abiotic stress tolerance and nutritional quality. Hossain M.A., Hassan L., Ifterkharuddaula K.M., Kumar A., Henry R., editors. John Wiley & Sons, Ltd; 2021. Molecular breeding for improving nitrogen use efficiency in rice: Progress and perspectives; pp. 234–248. [Google Scholar]
- 38.Cui Z., Zhang H., Chen X., Zhang C., Ma W., Huang C., Zhang W., Mi G., Miao Y., Li X., et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature. 2018;555:363–366. doi: 10.1038/nature25785. [DOI] [PubMed] [Google Scholar]
- 39.Fiaz S., Wang X., Khan S.A., Ahmar S., Noor M.A., Riaz A., Ali K., Abbas F., Mora-Poblete F., Figueroa C.R., et al. Novel plant breeding techniques to advance nitrogen use efficiency in rice: A review. GM Crops Food. 2021;12:627–646. doi: 10.1080/21645698.2021.1921545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray D., Sheshshayee M.S., Mukhopadhyay K., Bindumadhava H., Prasad T.G., Udaya Kumar M. High nitrogen use efficiency in rice genotypes is associated with a higher net photosynthetic rate at lower Rubisco content. Biol. Plant. (Prague) 2003;46:251–256. [Google Scholar]
- 41.Haefele S.M., Jabbar S.M.A., Siopongco J.D.L.C., Tirol-Padre A., Amarante S.T., Sta Cruz P.C., Cosico W.C. Nitrogen use efficiency in selected rice (Oryza sativa L.) genotypes under different water regimes and nitrogen levels. Field Crops Res. 2008;107:137–146. [Google Scholar]
- 42.Iqbal A., Qiang D., Alamzeb M., Xiangru W., Huiping G., Hengheng Z., Nianchang P., Xiling Z., Meizhen S., Meizhen S. Untangling the molecular mechanisms and functions of nitrate to improve nitrogen use efficiency. J. Sci. Food Agric. 2020;100:904–914. doi: 10.1002/jsfa.10085. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X.C., Shangguan Z.P. Effect of nitrogen fertilization on photosynthetic pigment and fluorescence characteristics in leaves of winter wheat cultivars on dryland. J Nucl Agric Sci. 2007;21:299–304. [Google Scholar]
- 44.Cassman K.G., De Datta S.K., Amarante S.T., Liboon S.P., Samson M.I., Dizon M.A. Long-term comparison of the agronomic efficiency and residual benefits of organic and inorganic nitrogen sources for tropical lowland rice. Exp. Agric. 1996;32:427–444. [Google Scholar]
- 45.Zhang W., Zhang T., Zhang J., Lei W., Zhao L., Wang S., Shi M., Wei M., Wei M. Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae. 2023;9:249. [Google Scholar]
- 46.Foulkes M.J., Hawkesford M.J., Barraclough P.B., Holdsworth M.J., Kerr S., Kightley S., Shewry P.R. Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crop Res. 2009;114:329–342. [Google Scholar]
- 47.Singh V., Singh A.K., Mohapatra T., Ellur R.K., Ellur R.K. Pusa Basmati 1121–a rice variety with exceptional kernel elongation and volume expansion after cooking. Rice. 2018;11:19. doi: 10.1186/s12284-018-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagadhesan B., Sathee L., Meena H.S., Jha S.K., Chinnusamy V., Kumar A., Kumar S. Genome wide analysis of NLP transcription factors reveals their role in nitrogen stress tolerance of rice. Sci. Rep. 2020;10:9368. doi: 10.1038/s41598-020-66338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandna R., Hakeem K.R. In: Crop Improvement: New Approaches and Modern Techniques. Hakeem K., Ahmad P., Ozturk M., editors. Springer; 2013. From agronomy to molecular genetics and proteomics to improve nitrogen use efficiency in crops; pp. 345–362. [Google Scholar]
- 50.Hakeem K.R., Chandna R., Ahmad A., Iqbal M. Physiological and molecular analysis of applied nitrogen in rice genotypes. Rice Sci. 2012;19:213–222. [Google Scholar]
- 51.Gupta P., Seth C.S. Nitrate supplementation attenuates As (V) toxicity in Solanum lycopersicum L. cv Pusa Rohini: Insights into As (V) sub-cellular distribution, photosynthesis, nitrogen assimilation, and DNA damage. Plant Physiol. Biochem. 2019;139:44–55. doi: 10.1016/j.plaphy.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Sahay S., Robledo-Arratia L., Glowacka K., Gupta M. Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA-mediated drought tolerance in Brassica juncea L. Sci. Rep. 2021;11:7992. doi: 10.1038/s41598-021-86401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali A., Sivakami S., Raghuram N. Effect of nitrate, nitrite, ammonium, glutamate, glutamine and 2-oxoglutarate on the RNA levels and enzyme activities of nitrate reductase and nitrite reductase in rice. Physiol. Mol. Biol. Plants. 2007;13:17. [Google Scholar]
- 54.Guan M., de Bang T.C., Pedersen C., Schjoerring J.K. Cytosolic glutamine synthetase Gln1; 2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant Physiol. 2016;171:1921–1933. doi: 10.1104/pp.16.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yun C.A.O., Xiao-Rong F.A.N., Shu-Bin S.U.N., Guo-Hua X.U., Jiang H.U., Qi-Rong S.H.E.N. Effect of nitrate on activities and transcript levels of nitrate reductase and glutamine synthetase in rice. Pedosphere. 2008;18:664–673. [Google Scholar]
- 56.Wu J., Zhang Z.S., Xia J.Q., Alfatih A., Song Y., Huang Y.J., Wan G.Y., Sun L.Q., Tang H., Liu Y., et al. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 2021;19:448–461. doi: 10.1111/pbi.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu C., Xia X., Chen Y., Qiao Y., Liu D., Fan J., Li S. Yield, nitrogen use efficiency and balance response to thirty-five years of fertilization in paddy rice-upland wheat cropping system. Plant Soil Environ. 2019;65:55–62. [Google Scholar]
- 58.Garnett T., Conn V., Kaiser B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009;32:1272–1283. doi: 10.1111/j.1365-3040.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- 59.Kant S., Bi Y.M., Rothstein S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011;62:1499–1509. doi: 10.1093/jxb/erq297. [DOI] [PubMed] [Google Scholar]
- 60.Tyagi B.S., Foulkes J., Singh G., Sareen S., Kumar P., Broadley M.R., Gupta V., Krishnappa G., Ojha A., Khokhar J.S., et al. Identification of wheat cultivars for low nitrogen tolerance using multivariable screening approaches. Agronomy. 2020;10:417. [Google Scholar]
- 61.Dodig D., Božinović S., Nikolić A., Zorić M., Vančetović J., Ignjatović-Micić D., Delić N., Weigelt-Fischer K., Junker A., Altmann T., Altmann T. Image-derived traits related to mid-season growth performance of maize under nitrogen and water stress. Front. Plant Sci. 2019;10:814. doi: 10.3389/fpls.2019.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen G.N., Maharjan P., Maphosa L., Vakani J., Thoday-Kennedy E., Kant S. A robust automated image-based phenotyping method for rapid vegetative screening of wheat germplasm for nitrogen use efficiency. Front. Plant Sci. 2019;10:1372. doi: 10.3389/fpls.2019.01372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Shi P., Ji R., Min J., Shi W., Wang D. Development of a model using the nitrogen nutrition index to estimate in-season rice nitrogen requirement. Field Crops Res. 2020;245 [Google Scholar]
- 64.Murthy K.M.D., Rao A.U., Vijay D., Sridhar T.V. Effect of levels of nitrogen, phosphorus and potassium on performance of rice. Indian J. Agric. Res. 2015;49:83–87. [Google Scholar]
- 65.Kjeldahl J. A new method for the determination of nitrogen in organic matter. J. Anal. Chem. 1883;22:366–382. [Google Scholar]
- 66.Craswell E.T., Godwin D.C. The efficiency of nitrogen fertilizers applied to cereals grown in different climates. Adv. Plant Nutrition. 1984;1:1–55. [Google Scholar]
- 67.Jiménez J., Wray A.A., Saffman P.G., Rogallo R.S. The structure of intense vorticity in isotropic turbulence. J. Fluid Mech. 1993;255:65–90. [Google Scholar]
- 68.Hageman R.H., Hucklesby D.P. In: Pietro A.S., editor. Vol. 23. Academic Press; 1971. Nitrate reductase from higher plants; pp. 491–503. (Methods in Enzymology). [Google Scholar]
- 69.Joy K.W., Hageman R.H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem. J. 1966;100:263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohanty B., Fletcher J.S. Ammonium influence on nitrogen assimilating enzymes and protein accumulation in suspension cultures of Paul's Scarlet rose. Physiol. Plant. 1980;48:453–459. [Google Scholar]
- 71.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 72.De Datta S.K. John Wiley and Sons; 1981. Principles and practices of rice production. [Google Scholar]
- 73.R Core Team . R Foundation for Statistical Computing; 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: The sources of the datasets supporting the current study are presented in the key resources table and the method details sections.
-
•
Code: Requests for the data and code that support the findings of this study should be directed to the lead contact.
-
•
Additional information: Any additional information required to reanalyze the data reported in this article or reproduce the results is available from the lead contact upon request.