Abstract
Identifying and diagnosing early-stage rheumatoid arthritis (RA) has remained an unmet challenge in medicine and a roadblock to identifying treatments at time points when disease-modifying therapies may be most effective. Recent studies have demonstrated that imaging the response of cartilage under mechanical loading, as well as alterations in matrix macro- and micro-molecule composition, could serve as potential biomarkers to identify tissue degeneration. Therefore, the objective of this paper was to identify RA-related cartilage degeneration in human wrists using novel MRI techniques. We applied in vivo displacement-encoded MRI to human wrists during cyclic radioulnar deviation, along with the quantitative MRI methods (T1ρ, T2, T2*) during a static condition, to a small healthy and RA patient cohort (6 healthy, 4 RA). We then used a linear mixed-effects model to identify key factors affecting the results. We found that the RA patients had wrists with higher torsional stiffness by approximately 2-fold compared to the control group. The RA group showed lower intercarpal joint displacements by roughly half of the control group, and some joint regions indicated tissue softening. We also found that the quantitative MRI metrics showed non-significant differences between control and RA groups (the T2 and T2* of the RA group was roughly 10% and 5% more than the control group, respectively), however, differences were detected among regions in T2 and T2* metrics. This study demonstrated that displacement-encoded MRI may be a promising method to distinguish functional and noninvasive metrics between RA and healthy wrists, and may provide a means to distinguish the disease state compared to conventional imaging methods.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-17316-3.
Keywords: Cartilage, Wrist, Cartilage degeneration, qMRI, dualMRI
Subject terms: Biomedical engineering, Magnetic resonance imaging, Cartilage
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that occurs mainly in the synovial joints like hands, wrists, and ankles, resulting in inflammation, swelling, and joint destruction1,2. Changes in advanced disease stages on joint structure can be visualized by radiographs3,4. Recent work suggests that early joint degeneration in RA may also be indicated by examining cartilage structure, molecular composition, and biomechanics5,6. Though few methods to detect the early degeneration state of the joint are available7–9analyzing alterations in tissue structure-function may provide potential biomarkers to detect RA at an earlier stage where pharmacological treatments may be more effective10.
Magnetic resonance imaging (MRI) is emerging as a useful technique to identify and diagnose RA11. Not only has MRI been widely used to capture joint deformities in advanced RA12but it has also been used to visualize synovitis, tenosynovitis, bursitis, and bone marrow edema and erosions in early- and mid-stages of such disease13. In addition to morphometric MRI, compositional MRI markers to detect the integrity of the cartilage have also been developed14–16 using quantitative MRI (qMRI) techniques. For example, T2 value is associated with cartilage water content and water-collagen interactions, and T1ρ is associated with proteoglycan content17,18. Meanwhile, displacement under applied loading MRI (dualMRI), which quantifies mechanical deformation of joint soft tissues, is another emerging technique that could be used to assess biomechanics and softening indicative of intratissue and disease-related functional changes of cartilage19,20.
In this study, we implemented a cyclic loading apparatus that applies radial deviations on the human wrists to assess the joint biomechanics using dualMRI and joint biochemistry using qMRI. We then applied our method to a small cohort by recruiting participants with healthy wrists and patients with RA wrists. We aimed to determine the extent that imaging-based biomarkers like cartilage displacement and strain could be used to identify RA, and compared to qMRI results, using a clinical 3 Tesla (T) MRI scanner. To the best of our knowledge, this is the first study that analyzes the intratissue mechanics of RA wrist cartilage under motion using MRI with their compositional biomarkers.
Methods
This study addresses (1) the design of our radioulnar deviation loading apparatus and experimental layout, (2) dualMRI and qMRI data acquisition composed on two sessions on the patient cohort with healthy and RA wrists, and finally (3) data analysis on the collected data, including displacement and strain, relaxometry, and wrist stiffness on the two participant groups.
Apparatus design
The pneumatic loading apparatus included a loading section along with its associated control unit (Supplementary Table 1). The loading section (Fig. 1A, B) was modified from a previous study21,22. It consisted of a mainframe that interfaces with the MRI scanner (Siemens MAGNETOM Prismafit 3 T) and supports a linear bearing system, pneumatic cylinder that performs a linear reciprocating motion, cylinder connector that joins the cylinder to the main frame, and moving frame that slides on the linear bearing and translated the linear motion to a rotational motion via a hinge. The participant’s hand and distal part of the forearm were secured separately to the wrist fixture by Velcro straps. The two parts of the wrist fixture (hand fixture and arm fixture) were connected via a ball bearing which allows rotational motion. The arm fixture was strapped down to a sandbag, which allowed the hand to rotate with respect to the fixed forearm (radius). When positioning the patient in the MRI scanner, the wrist was placed on top of the ball bearing. Fasteners were used to connect all parts. All materials of the loading section were chosen to be MRI-compatible and structurally robust. In the meantime, the control unit was designed in a preceding study19,23and was placed outside of the scanner room for safety and performance concerns.
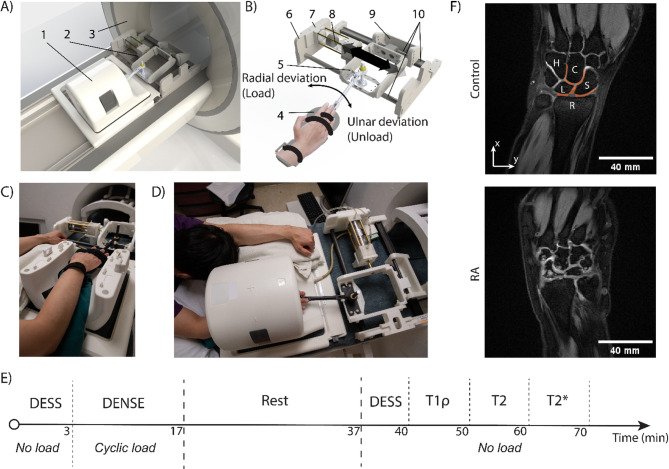
Fig. 1.
Experimental set-up, procedure, and example DESS images. A) Computer aided design rendering of a (1) 15-channel knee coil and the (2) loading apparatus mounted on a (3) 3 T MRI scanner. B) The loading apparatus consists of (4) a hand wrap to fix the hand on the device, (5) the moving structure which translates during radial-ulnar deviation, (6) mainframe, (7) cylinder mount, (8) pneumatic cylinder, (9) rear support of the moving structure, and (10) a set of linear bearing system. Nylon fasteners are used to connect different parts. C, D) Photos of the corresponding computer aided design renderings demonstrate the patient positioned in prone position within the MRI scanner with the loading device. E) Time sequence of the MRI scans. The 70 min of scanning time was separated into two sessions with a 20-minute rest in between. F) DESS images depicting wrist and cartilage anatomy of one representative control participant and one RA participant. Here, hamate (H), capitate (C), lunate (L), scaphoid (S), and radius (R) are labeled at their corresponding location on the DESS image, and selected ROIs are in orange shade. The RA patient has pronounced bone edema and irregular carpal bone shapes compared to the control participant. The DESS images are obtained on a 37-year-old male control participant and a 30-year-old female RA participant.
Participant recruitment
Age-matched RA participants (1 male, 3 females, 43.2 ± 11.0 years old, mean ± standard deviation) and six control participants (3 males, 3 females, 39.5 ± 14.6 years old) were recruited in total by the University of Colorado, Anschutz. We first recruited 4 RA participants and 5 controls in the same age range, but after the initial data processing, we observed motion artifacts in the dualMRI images from one control participant, hence we excluded this person’s dualMRI data from data analysis and recruited an additional control participant. All participants were between 18 and 60 years old. The RA patients met the American College of Rheumatology 2010 criteria24and had active disease activity (e.g., swelling, tenderness, decreased range of motion, etc.) in at least one wrist for scanning. The RA patients’ disease status were not obtained prior to the screening. For the control participants were required to have no history of RA, wrist surgery, joint swelling, or other health conditions affecting the wrist. All experiments and experimental protocols were approved by the Institutional Review Board at the University of Colorado, Anschutz, and were performed in accordance with the relevant guidelines and regulations. The informed consent was obtained from all subjects and/or their legal guardian(s).
MRI data acquisition
After the apparatus was mounted, the patients were then asked to be in a prone position with one or two hands raised over the head (Fig. 1C, D). The targeted wrist was strapped to the device as designed and put in the center of the MRI coil (SIEMENS Tx/Rx Knee 15 Flare). The MRI scan was composed of two roughly 30-minute sessions with a 20-minute rest in between (Fig. 1E). For the RA patients, we scanned the wrist that was most affected by the disease, and for control participants, we scanned the side that they are most comfortable with when being scanned in a prone position.
In the first session of the MRI scan, we collected 3D Double Echo Steady State (DESS) images to visualize joint morphology and establish a common imaging plane for multi-contrast acquisitions. The imaging parameters were as follows: TE/TR = 5/15ms, field of view (FOV) = 125 × 125mm2, spatial resolution = 490 × 490µm2, slice thickness = 1.7 mm, slice number = 28, flip angle = 25°, image average = 1. Subsequently, we performed dualMRI using spiral Displacement ENcoding with Stimulated Echoes (DENSE) MRI on the selected DESS image plane where all the joints between the carpal bones were clearly visible. To apply motion to the wrist, the aforementioned pneumatic loading device was mounted onto the MRI scanner and loading was applied on the hand to generate cyclic (0.5 Hz) radial deviation. The amount of load for each individual was altered so that the cylinder piston could move the same amount of distance (25 mm) during the same time duration (1 s). This resulted in a maximum wrist angle in radial deviation of approximately 5°. DENSE MR images were collected during radial deviation. We synchronized the cyclic motion with the DENSE sequence using an electrocardiogram (ECG) trigger (Siemens PERU Physiol. ECG/Respiratory Unit). DENSE MRI imaging parameters were as follows: TE/TR = 2.5/20ms, FOV = 90 × 90mm2, spatial resolution = 360 × 360µm2, slice thickness = 1.7 mm, slice number = 1, image averages = 8, spiral interleaves = 10, imaging frames = 27, temporal resolution = 40ms, and in-plane displacement encoding gradient = 0.64 cycles/mm.
After 20 min of rest, relaxometry (qMRI) measures were collected while the participant was in a static, unloaded condition. Followed by DESS sequence, T1ρ measurements were made using a magnetization-prepared gradient echo sequence25 with the following parameter setting: TE/TR = 3/6ms, spin-lock frequency = 500 Hz, spin-lock durations = 0, 20, 40, 60, 80ms, FOV = 90 × 90mm2, spatial resolution = 700 × 700µm2, slice thickness = 3.5 mm, slice number = 1 (on the selected DESS plane), flip angle = 10o, image average = 2. T1ρ relaxation maps were acquired by fitting the five MR intensity images to a monoexponential relaxation model using non-linear least squares, while pixels with determination less than 0.66 were rejected26. Subsequently, T1ρ, T2, and T2* measurements were obtained by multi-echo spin-echo and gradient-echo sequences (MapIt, Siemens Healthcare), respectively. The acquisition parameters for T2 were as follows: TEs = 13, 26, 39, 52, 65, 78ms, TR = 1290ms, FOV = 80 × 80mm2, spatial resolution = 420 × 420µm2, slice thickness = 1.7 mm, slice number = 13, flip angle = 180o, image average = 2. For T2*, the following parameters were used: TEs = 4.46, 11.9, 19.94, 26.98, 34.52, 49.68, 64.84, 80ms, TR = 1270ms, FOV = 80 × 80mm2, spatial resolution = 420 × 420µm2, slice thickness = 2 mm, slice number = 11, flip angle = 60o, image average = 1. All measurements used fat suppression. Among the multiple slices acquired for T2 and T2* acquisition, we selected the plane that contained similar morphological features to the DESS image plane for analysis.
Data analysis
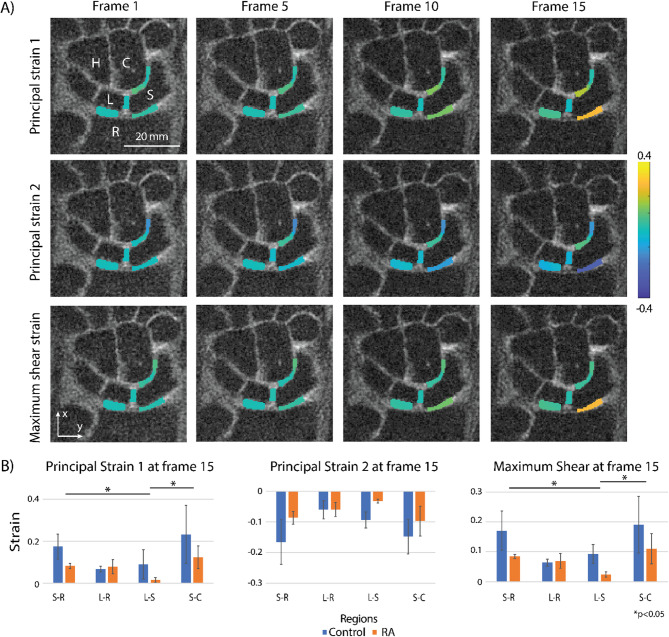
The interosseous space between carpal bones of the wrist, including radius (R), scaphoid (S), lunate (L), capitate (C), and hamate (H), were selected as regions of interest (ROIs) (Fig. 1F). The displacement maps were obtained from the DENSE MRI phase data (Fig. 2A) using custom software (MATLAB, MathWorks, R2019b)19,21. We calculated the absolute value of displacements in x and y for each pixel (Fig. 2B). Principal strain 1 (PS1, maximum principal strain), principal strain 2 (PS2, minimum principal strain), and max shear strain maps (Fig. 3A) were further derived from the displacement maps. Four ROIs between the carpus bones (S-R, L-R, L-S, S-C) were drawn and the displacement and strain values were averaged within each ROI on frame 1–15 (Fig. 2C, Supplementary Fig. 1). We also calculated the change rates of absolute displacement, PS1, PS2, and max shear strain, which were measured from frame 3–15 by finding the slope of a fitted straight line using linear regression. For each parameter (absolute displacement, PS1, PS2, max shear strain), we calculated the ratio of the change rate between the RA (SRA) and control group (SCtrl) on the averaged data, denoted as S (i.e. SAD, SPS1, etc.), where 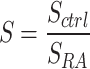 (i.e.
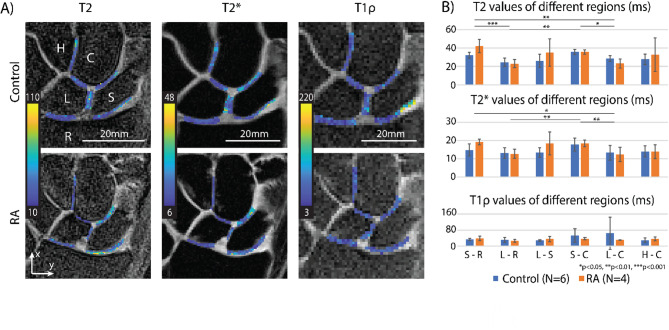
(i.e. 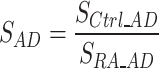 ). We then compared the S value of absolute displacement against the S value of strains to estimate the relative change between the patient groups. For the relaxometry data analysis, a total of six ROIs (S-R, L-R, L-S, S-C, L-C, H-C) were manually drawn on the first echo of the weighted images (Fig. 4A), and the qMRI values were averaged within each ROI (Fig. 4B).
). We then compared the S value of absolute displacement against the S value of strains to estimate the relative change between the patient groups. For the relaxometry data analysis, a total of six ROIs (S-R, L-R, L-S, S-C, L-C, H-C) were manually drawn on the first echo of the weighted images (Fig. 4A), and the qMRI values were averaged within each ROI (Fig. 4B).
Fig. 2.
DENSE MRI and displacement results on four wrist joint regions: the absolute displacement showed significant differences between regions, and the healthy group had more significantly different region pairs compared to the RA group. (A) Raw DENSE MRI time frame 1 (t = 0ms) and frame 15 (t = 560ms), including magnitude, x-phase, and y-phase images on a 29-year-old female control participant. (B) Displacement map calculated from the phase images on four regions at frame 1, 5, 10, 15. Here, we show the displacements in x and y directions, along with the absolute displacements. All displacements showed a gradual increase over time, where x values increased, and y values decreased, representing radial deviation. (C) Absolute displacement values from frame 1 to frame 15 on both control and RA participant groups showed a gradual increase while the control group had more increase than the RA group. (D) We found regional differences of absolute displacements (S-R vs. L-S, p = 0.0104; L-R vs. L-S, p = 0.0182) and the region pairs that showed significant differences were only in the healthy control group (S-R vs. S-C, p = 0.0040; S-R vs. L-S, p = 0.0114; L-R vs. L-S, p = 0.0371; L-R vs. S-C, p = 0.0135). The difference between control and RA groups was approaching significance with p = 0.0677. Error bars = standard error.
Fig. 3.
Dynamic (time-dependent) principal strains 1, 2, and max shear strain, obtained from DENSE MRI, as well as the averaged strain values of the control and RA patient groups among regions at frame 15. (A) A 29-year-old female control participant’s strain maps show the principal strain 1, 2, and maximum shear strain values change along with frames (1, 5, 10, 15). For this participant, the S-R joint has the maximum averaged strain values, which are 0.26, −0.27, and 0.26 for principal strain 1, principal strain 2, and maximum shear, respectively. (B) Averaged strain values within each region at frame 15. Here, we found regional differences in PS1 and max shear strain (PS1: S-R vs. L-S, p = 0.0255, L-S vs. S-C, p = 0.0236; max shear strain: S-R vs. L-S, p = 0.0237, L-S vs. S-C, p = 0.0255), while no significant differences were found in PS2 metric and between the patient groups. Error bars = standard error.
Fig. 4.
Significant regional differences were found in T2 and T2* values, but no significant differences in T1ρ and between the groups were found. (A) T2, T2*, and T1ρ maps of six regions between carpal bones on one control participant and one RA participant. (B) Statistical analysis of relaxometry results where the plots are mean ± standard error on control and RA participants of the 6 regions. Here, we found regional differences in the T2 and T2* values (T2: S-R vs. L-C, p = 0.0067, S-R vs. L-R, p = 0.0005, L-R vs. S-C, p = 0.0011, S-C vs. L-C, p = 0.0129; T2*: S-R vs. L-C, p = 0.0410, L-R vs. S-C, p = 0.0085, S-C vs. L-C, p = 0.0048). No significant differences found in T1ρ metric and between the patient groups. The relaxometry maps were obtained on a 35-year-old female control participant and a 60-year-old male RA participant.
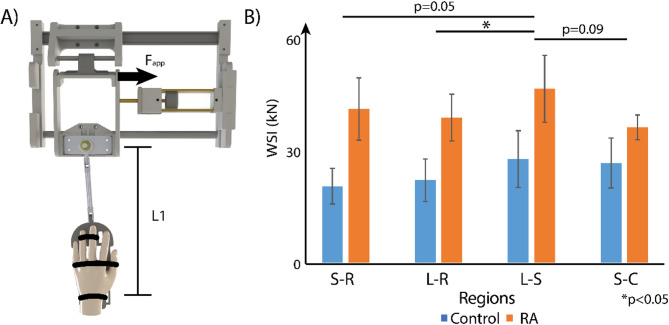
To estimate the ability of the wrist to resist torque in response to the radial deviation, we recorded the applied force (Fapp) and the distance between the hinge and the center of the wrist (L1) for each participant (Fig. 5A), then calculated the torque. Subsequently, we divided the torque by the measured absolute displacement and labeled it Wrist Stiffness Index (WSI), therefore we have the following equation: 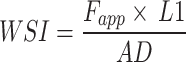 . It is a metric that quantifies the force needed to rotate the wrist, as a higher WSI value would indicate that the wrist has a higher torsional stiffness as it needs more force to achieve the same average angular speed when compared to the wrist with a lower WSI.
. It is a metric that quantifies the force needed to rotate the wrist, as a higher WSI value would indicate that the wrist has a higher torsional stiffness as it needs more force to achieve the same average angular speed when compared to the wrist with a lower WSI.
Fig. 5.
Analysis of a wrist stiffness index (WSI) demonstrated significant regional differences between regions and RA and control patients. (A) To calculate WSI, we measured L1 (341.25 ± 17.50 mm for the RA group, 356.67 ± 9.83 mm for the control group.), recorded Fapp (24.67 ± 5.51 N for the RA group, 25.17 ± 2.40 N for the control group), and then multiplied the two values and divided by absolute displacement, following 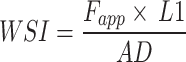 . (B) The comparison of WSI between control and RA groups, as well as among regions. We found regional differences between L-R and L-S regions (p = 0.0373), while the differences between S-R and L-S (p = 0.0500), L-S and S-C (p = 0.0904) regions were approaching significance. Meanwhile, the differences between control and RA groups were also approaching significance with a p-value of 0.0823.
. (B) The comparison of WSI between control and RA groups, as well as among regions. We found regional differences between L-R and L-S regions (p = 0.0373), while the differences between S-R and L-S (p = 0.0500), L-S and S-C (p = 0.0904) regions were approaching significance. Meanwhile, the differences between control and RA groups were also approaching significance with a p-value of 0.0823.
For statistical analyses, we ran linear mixed models where the dependent variables were the MRI measurements (displacement, strain, relaxometry, WSI, S). The regions (S-R, L-R, L-S, S-C, L-C, H-C) and the patient groups (RA/control) were treated as fixed effects, while the patient ID was treated as a random effect. The interaction term between patient groups and regions was added to probe into joint effects. Post-hoc tests were also conducted. Tukey-Kramer tests were applied to adjust for multiple comparisons. Prior to running the linear mixed model, the Shapiro-Wilk test was used to check if the data was normally distributed for each variable and the transformation was conducted on the data that failed the normality test.
Results
The radial-ulnar deviation motion of the wrist was visually confirmed by researchers. The calibration of the pneumatic cylinder was conducted in previous research22. There were no artifacts observed in MR images. The recorded Fapp was 24.67 ± 5.51 N for the RA group and 25.17 ± 2.40 N for the control group; while the recorded L1 was 341.25 ± 17.50 mm for the RA group and 356.67 ± 9.83 mm for the control group.
The DESS images were able to qualitatively show that the RA patients had a more pronounced bone edema and irregular carpal bone shape compared to the control group (Fig. 1F). The substantial difference differed by each participant.
The DENSE MRI results on the wrist joint showed that the displacement and strain values gradually changed during radial deviation. For absolute displacement and strain values, we plotted the values at each frame (Fig. 2C, Supplementary Fig. 1) and extracted the values at frame 15 (t = 560ms) for analysis (Figs. 2D and 3B). For absolute displacement values, we found regional differences between regions (S-R vs. L-S, p = 0.0104; L-R vs. L-S, p = 0.0182), and the regional differences within the healthy group were also observed (S-R vs. S-C, p = 0.0040, S-R vs. L-S, p = 0.0114, L-R vs. L-S, p = 0.0371, L-R vs. S-C, p = 0.0135). We also found the difference between groups was trending towards significance (p = 0.068). For the strain values, we found significant differences between the regions for PS1 (S-R vs. L-S, p = 0.0255; L-S vs. S-C, p = 0.0236) and max shear strain (S-R vs. L-S, p = 0.0237; L-S vs. S-C, p = 0.0255) categories, while no patient group differences were observed.
The relaxometry (qMRI) results (Fig. 4B) showed that the T2 and T2* values only had significant regional differences (T2: S-R vs. L-C, p = 0.0067, S-R vs. L-R, p = 0.0005, L-R vs. S-C, p = 0.0011, S-C vs. L-C, p = 0.0129; T2*: S-R vs. L-C, p = 0.0410, L-R vs. S-C, p = 0.0085, S-C vs. L-C, p = 0.0048). Differences between patient groups, as well as the differences in the T1ρ metric, were not observed.
For S values, we noticed that the SAD values were similar in all regions (Region S-R: SAD=2.30; Region L-R: SAD=2.22; Region L-S: SAD=2.24; Region S-C: SAD=1.73), and the strain S values in S-R, L-R, and S-C regions were smaller than their corresponding SAD values (Region S-R: SPS1=1.65, SPS2=1.99, SMS=1.81; Region L-R: SPS1=0.90, SPS2=1.19, SMS=1.02; Region S-C: SPS1=1.62, SPS2=1.16, SMS=1.43). However, in the L-S region, the SAD (2.24) was smaller than the strain S values (SPS1=183.40, SPS2=3.67, SMS=8.75).
The statistical analysis of WSI (Fig. 5B) showed that there were significant regional differences (L-R vs. L-S, p = 0.0373) and approaching-significance regional differences (S-R vs. L-S, p = 0.0500; L-S vs. S-C, p = 0.0904) for the WSI values, while the difference between the control and RA groups was approaching significance with p = 0.0823.
Discussion
In this study, we explored the extent that cartilage intratissue displacement and strain could be used as new imaging-based biomarkers, in combination with widely-used MR relaxometry metrics, to identify RA. We performed dualMRI measurements along with qMRI accompanied by a customized device on a small healthy and RA patient cohort. We also extracted the WSI of each individual and made a comparison between the two participant groups. Our analysis provided high spatial and temporal resolution displacement and strain maps of human wrists during radial deviation. The major findings were as follows: (1) regional differences were found for absolute displacement metric, with significantly different region pairs limited within the healthy group, and the difference between the patient groups trending towards significance. (2) For strain metrics, only PS1 and max shear strain showed significant regional differences. (3) For qMRI metrics, only T2 and T2* showed significant regional differences. (4) The WSI values showed regional differences, and patient group differences trended towards significance.
The control group had larger absolute displacement values in wrist joints in response to radial deviation compared with the RA group, while the difference was approaching significance. This indicates the motion of the RA wrists was restrained internally even though their external motion range and speed were comparable with the healthy wrists. We only found significant differences between regions in the healthy group, suggesting the RA group underwent a similar level of motion restraining among different regions. Meanwhile, PS1 and mas shear strain showed differences in regions indicating that each local region in the wrist joint experiences a different amount of strain. Interestingly, there was no difference found between regions for PS2 indicating that the joints were compressed at a similar level during radial deviation while the extension applied on each joint was different. This trend was observed in both RA and control groups, and was consistent with other studies showing that the proximal and distal carpal rows are considered to be single functional units with limited intercarpal motion 27,28. Intuitively, the complicated carpal bone kinematics induce different strain values, and due to the high spatial resolution of DENSE MRI, we were able to verify the different strains between regions. This spatially heterogeneous mechanical response can detect the deterioration of tissue and may potentially serve as a biomarker. We utilized absolute displacement since each subject’s wrist was not positioned consistently in the MR images.
We noticed that although the difference in the absolute displacement between patient groups was approaching significance, the difference of the strain values between the patient groups was not significant. To investigate this phenomenon, we decided to probe into the change rate difference between the displacement and strain. Here we made three assumptions: (1) the strain should be proportional to the displacement during deformation, (2) the applied load applied onto joints was consistent in both the control and RA groups, and (3) the original joint space was similar between the two patient groups. Based on these assumptions, the S value was used as an indicator of whether the RA group has harder or softer cartilage compared to the control group. If the S value for strain (for example SPS1) was lower than the S value for displacement (SAD), it indicated that the RA group had a larger strain compared to the control group on a fixed absolute displacement, which implied that the cartilage was softer. Accordingly, higher strain S values in the RA group would indicate harder cartilage compared to the control group. Our results suggest that the RA group has softer cartilage in the S-R, L-R, and S-C regions and harder cartilage in the L-S region when compared to the control group. This is consistent with other literature, as cartilage softening has been widely observed in RA joints29–32. For the opposite trend observed in the scapholunate joint (region L-S), it could potentially be due to the ROI selection including the scapholunate ligament instead of cartilage alone, as the strain within the ROI was restrained by the limited motion of the ligament during radial deviation. Another possible reason is due to the RA joints having subluxation causing the carpal bones to move out of plane during radial deviation and resulting in small strains in our 2D analysis.
The relaxometry results demonstrated that there are regional differences among carpal joints, indicating there are inherent compositional differences as the original matrix composition and biochemical content of wrist cartilage may vary from location to location. Meanwhile, even though other studies have shown that T1ρ is a widely used compositional MRI technique for evaluating the early characteristic changes in the cartilage20,33the lack of statistical significance between the patient groups in this study implies that qMRI techniques may not be sensitive enough to differentiate between RA and healthy wrists in our patient cohort. Since RA is a progressive disease, it could be possible that relaxometry outcomes might reflect only later stages of RA. Besides, our targeted patient pool and the screening criteria (ACR 2010 and MRI-specific requirements) would bias the patient group towards the asymptomatic side, which may have contributed to the non-differentiating feature. Lastly, these findings may also be due to the small ROI size and small number of patient cohorts.
Although the displacement and strain values from DENSE MRI showed meaningful results, the applied load was slightly different among participants resulting in varying displacement and strain values. Therefore, to compare the mechanical response of the wrist joints on a given load, we calculated WSI. These results were interesting where the joint as a whole showed that the RA group has a higher WSI than the healthy wrists, while the difference was approaching significance. These findings are consistent with other studies indicating that RA involves a process of whole joint stiffening34–37 due to synovial hyperplasia, which is a common indicator for RA detection. Meanwhile, the ability to resist torque is different for individual regions, indicating the biomechanical property of the cartilage also varies from location to location. This is a good example showing the biomechanical property could be informative in revealing the cartilage degeneration stat and also demonstrates the complexity of the carpal bone motion38.
Our study included limitations which can be improved in future work. First, as mentioned previously, our initial patient cohort for this proof-of-concept study was small, and additional patients are needed to explore biomechanical and qMRI relationships in healthy and diseased wrists. Due to this limitation, all statistical significances found can only be applied to this exploratory study, and further investigation is needed with a larger sample size to draw more generalizable conclusions. While the small sample size limits statistical power and generalizability, we observed a positive effect size for the WSI value in the S-R region (Cohen’s d = 1.48, 95% CI [0.06, 2.90]) and a positive and moderate effect size for the T2 value in the L-S region (Cohen’s d = 0.77, 95% CI [−0.54, 2.08]). This suggests potential meaningful differences which should be investigated with a larger sample size. Second, while control and RA patients were age-matched, we did not consider the level of RA for the RA patients. Because RA is a progressive disease, and disease duration could affect our outcome, and this will need to be considered as a factor in future experimental designs. Third, the wrist joint is naturally a very thin tissue39 thus the current resolution setting we used limited the number of pixels we used for segmentation. This study was derived directly from our previous work on knee articular cartilage21 with augmented parameters to compensate for the small cartilage size. Reducing the MRI pixel size will result in low SNR and new techniques such as compressed sensing40 can be implemented to compensate for the signal loss. Finally, our current analysis does not provide full insight into a full three-dimensional response of cartilage to loading during naturally complicated motions of the wrist. Our current protocol only selected one slice for DENSE MRI. Instead, 3D DENSE could be used to obtain 3D deformation and hence result in more accurate displacement and strain maps. With this information, further studies can utilize modeling and inverse modeling procedures to obtain the material properties of wrist cartilage.
Conclusion
In this study, we developed an MRI-compatible radial-ulnar deviation loading apparatus for in vivo human wrist, designed a qMRI and dualMRI testing protocol, and then applied this to small healthy and RA patient cohorts. We believe this work is the first to look at the deformation using DENSE MRI of the human wrist cartilage under loading as well as its relaxivity in a clinical 3 T MRI environment. The displacement and strain acquired by this routine will give clinicians and researchers access to the mechanical response of wrist joint cartilage absent from ex vivo examinations. In accompany with the qMRI results, this method has offered us a unique way to probe into the biomechanical and biochemical alternations in the cartilage and hence could provide us with a novel perspective of the onset of RA pathogenesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful for MRI technical support from Teryn S. Wilkes at the Intermountain Neuroimaging Consortium located at the University of Colorado Boulder.
Abbreviations
- RA
Rheumatoid Arthritis
- MRI
Magnetic Resonance Imaging
- qMRI
quantitative MRI
- dualMRI
displacement under applied loading MRI
- DESS
Double Echo Steady State
- DENSE
Displacement Encoding with Stimulated Echoes
- ECG
Electrocardiogram
- ROI
Region of Interest
- WSI
Wrist Stiffness Index
Author contributions
Conceptualization - H.Z., C.P.N.; Device acquisition and manufacturing - H.Z., C.P.N.; MRI Operation and analysis - H.Z., W.L., E.Y.M.; Statistical Analysis, H.Z., W.L; Writing – Original Draft, H.Z., W.L.; Writing – Review & Editing, All authors; Patient recruiting – J.S., A.C., L.M.; Funding Acquisition, L.M., C.P.N.; Supervision, C.P.N.
Funding
Financial support is gratefully acknowledged from Anschutz-Boulder (AB) Nexus.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at the University of Colorado, Anschutz.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongtian Zhu and Woowon Lee: These authors contributed equally to this work.
References
- 1.Ilan, D. I. & Rettig, M. E. Rheumatoid arthritis of the wrist. Bull. NYU Hosp. Jt. Dis.61, 179 (2003). [PubMed] [Google Scholar]
- 2.Trieb, K. Treatment of the wrist in rheumatoid arthritis. J. Hand Surg. Am.33, 113–123 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Genant, H. K. et al. Assessment of rheumatoid arthritis using a modified scoring method on digitized and original radiographs. Arthritis Rheum.41, 1583–1590 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Leak, R. S., Rayan, G. M. & Arthur, R. E. Longitudinal radiographic analysis of rheumatoid arthritis in the hand and wrist. J. Hand Surg. Am.28, 427–434 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Rainbow, M. J., Wolff, A. L., Crisco, J. J. & Wolfe, S. W. Functional kinematics of the wrist. J. Hand Surg. Eur. Vol. 41, 7–21 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Leventhal, E. L. et al. Interfragmentary motion in patients with scaphoid nonunion. J. Hand Surg. Am.33, 1108–1115 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Ostrowska, M., Maśliński, W., Prochorec-Sobieszek, M. & Nieciecki, M. Sudoł-Szopińska, I. Cartilage and bone damage in rheumatoid arthritis. Reumatologia56, 111–120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pap, T. & Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat. Rev. Rheumatol.11, 606–615 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Grassi, W., De Angelis, R., Lamanna, G. & Cervini, C. The clinical features of rheumatoid arthritis. Eur. J. Radiol.27, S18–S24 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Wilsdon, T. D. & Hill, C. L. Managing the drug treatment of rheumatoid arthritis. Aust Prescr. 40, 51–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suter, L. G., Fraenkel, L. & Braithwaite, R. S. Role of magnetic resonance imaging in the diagnosis and prognosis of rheumatoid arthritis. Arthritis Care Res. (Hoboken). 63, 675–688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scutellari, P. N. & Orzincolo, C. Rheumatoid arthritis: sequences. Eur. J. Radiol.27, S31–S38 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Sudoł-Szopińska, I., Jans, L. & Teh, J. Rheumatoid arthritis: what do MRI and ultrasound show. J. Ultrason.17, 5–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein, D., Gray, M. L., Hartman, A. L., Gipe, R. & Foy, B. D. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J. Orthop. Res.11, 465–478 (1993). [DOI] [PubMed] [Google Scholar]
- 15.Bashir, A., Gray, M. L., Hartke, J. & Burstein, D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med.41, 857–865 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Peterfy, C. G. et al. Quantification of articular cartilage in the knee with pulsed saturation transfer Subtraction and fat-suppressed MR imaging: optimization and validation. Radiology192, 485–491 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Guermazi, A. et al. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr. Cartil.23, 1639–1653 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Serai, S. D. Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr. Radiol.52, 217–227 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Chan, D. D. et al. In vivo articular cartilage deformation: noninvasive quantification of intratissue strain during joint contact in the human knee. Sci. Rep.6, 19220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griebel, A. J., Trippel, S. B. & Neu, C. P. Noninvasive dualMRI-based strains vary by depth and region in human Osteoarthritic articular cartilage. Osteoarthr. Cartil.21, 394–400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, W. et al. High frame rate deformation analysis of knee cartilage by spiral DualMRI and relaxation mapping. Magn. Reson. Med.89, 694–709 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, H., Miller, E. Y., Lee, W., Wilson, R. L. & Neu, C. P. In vivo human knee varus-valgus loading apparatus for analysis of MRI-based intratissue strain and relaxometry. J. Biomech.171, 112171 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Zhu, H., Miller, E., Lee, W., Wilson, R. & Neu, C. In vivo human knee Varus-Valgus loading apparatus for analysis of MRI-Based intratissue strain and relaxometry. Social Sci. Res. Network (2023). [DOI] [PubMed]
- 24.Aletaha, D. et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62, 2569–2581 (2010). (2010). [DOI] [PubMed]
- 25.Borthakur, A. et al. T1ρ magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa. 1976). 36, 2190–2196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson, R. L. et al. In vivo intervertebral disc deformation: intratissue strain patterns within adjacent discs during flexion–extension. Sci. Rep.11, 729 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschweiler, J. et al. Anatomy, biomechanics, and loads of the wrist joint. Life12, 188 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamal, R. N., Starr, A. & Akelman, E. Carpal kinematics and kinetics. J. Hand Surg. Am.41, 1011–1018 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Frank, C. B., Rangayyan, R. M. & Bell, G. D. Analysis of knee joint sound signals for non-invasive diagnosis of cartilage pathology. IEEE Eng. Med. Biol. Mag. 9, 65–68 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Shiozawa, S. & Shiozawa, K. A review of the histopathological evidence on the pathogenesis of cartilage destruction in rheumatoid arthritis. Scand. J. Rheumatol.17, 65–72 (1988). [DOI] [PubMed] [Google Scholar]
- 31.Uhl, M. et al. Cartilage destruction in small joints by rheumatoid arthritis: assessment of fat-suppressed three-dimensional gradient-echo MR pulse sequences in vitro. Skeletal Radiol.27, 677–682 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Altman, R. D. & Gray, R. Diagnostic and therapeutic uses of the arthroscope in rheumatoid arthritis and osteoarthritis. Am. J. Med.75, 50–55 (1983). [DOI] [PubMed] [Google Scholar]
- 33.Li, X. et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15, 789–97 (2007). [DOI] [PMC free article] [PubMed]
- 34.Small, C. F. et al. Configuration space analysis of the rheumatoid wrist. Hum. Mov. Sci.16, 309–322 (1997). [Google Scholar]
- 35.Liu, W. et al. Clinical results of a 10-year follow-up of surgical treatment for elbow stiffness in rheumatoid arthritis: A case series. Int. J. Surg.99, 106590 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Fan, Z., Li, J., Liu, J., Jiao, H. & Liu, B. Anti-Inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl. Mater. Interfaces. 10, 23595–23604 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Bajuri, M., Kadir, A., Amin, M. R., Öchsner, A. & I. M. & Biomechanical analysis of rheumatoid arthritis of the wrist joint. Proc. Inst. Mech. Eng. H. 226, 510–520 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Fischli, S., Sellens, R. W., Beek, M. & Pichora, D. R. Simulation of extension, radial and ulnar deviation of the wrist with a rigid body spring model. J. Biomech.42, 1363–1366 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Hayter, C. L., Gold, S. L. & Potter, H. G. Magnetic resonance imaging of the wrist: bone and cartilage injury. J. Magn. Reson. Imaging. 37, 1005–1019 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Lee, W. et al. Multi-frame Biomechanical and relaxometry analysis during in vivo loading of the human knee by spiral DualMRI and compressed sensing. Magn. Reson. Med.90, 995–1009 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.