Abstract
Background
The ongoing burden of mortality and morbidity associated with Streptococcus pneumoniae infections requires that monitoring of carriage epidemiology continues. Here, we present data from the annual, cross-sectional surveillance study in Southampton UK on serotype epidemiology and diversity, as well as carriage of other frequent colonisers of the respiratory tract in over 7000 children over a period of seventeen years (2006–2023).
Methods
Children were recruited from two sites: Site 1 - Southampton General Hospital, administered by University Hospital Southampton (UHS) NHS Foundation Trust and Site 2– a collection of community health care facilities within the Solent NHS Trust region. Recruitment was limited to children < 5-years-old. Pneumococcal serotyping was done using whole genome sequence data.
Results
A total of 7,686 swabs were collected from which 2,386 (31%) pneumococci were recovered. Carriage of pneumococci has remained consistent (median carriage prevalence 31.4%) even with the almost complete removal of vaccine-type (VT) serotypes. Examining three PCV13 periods separately (pre, early and late), carriage was not significantly different at 27.7%, 35.3% and 39.3% respectively. A decrease in carriage of Haemophilus influenzae, Staphylococcus aureus and Moraxella catarrhalis was seen pre-PCV13 (following PCV7 implementation) but has since stabilised. Continued, low-level persistence of VTs 3, 19A and 19F was noted. PCV13 did not impact the pneumococcal serotype rank abundance despite clear reductions in targeted serotypes and fluctuations in other non-VT serotypes such as 15A, 23B and 23B1. Non-PCV13 PCV20 serotypes 10A and 11A, in addition to paired prevalence of 15B and 15C (15B/C) were in the five most isolated serotypes in the late-PCV13 period (2012 to present). Non-PCV13 PCV20 serotypes now account for approximately 40% of all carriage. By contrast, the serotypes only included in PCV15 (22F and 33F) represented just 7% in the same period.
Conclusion
With consistent carriage prevalence in this UK paediatric population since PCV13 introduction, serotype epidemiology is now dominated by non-PCV13 serotypes that are in higher valency vaccines.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-025-00174-y.
Keywords: Streptococcus pneumoniae, PCV, Carriage epidemiology
Background
The importance of monitoring community-level prevalence of respiratory pathogens has been clearly recognised in recent years. Whilst attention has shifted to viruses, pervasive challenges remain to reduce respiratory infections caused by bacterial pathogens e.g., Streptococcus pneumoniae. The most recent modelling estimates from the Global Burden of Disease Study indicated that S. pneumoniae was responsible for ~ 829,000 deaths in 2019 alone, was the main pathogen associated with lower respiratory tract infections and caused 225,000 deaths (95%CI: 180 000–281 000) in children under age 5 years [1]. Invasive pneumococcal disease (IPD) therefore remains a problem, even in countries with longstanding pneumococcal conjugate vaccine (PCV) programmes. In the UK the most recent estimates of annual childhood IPD incidences in 2022/23 were 15.88/100,000 in < 1 year-old and 8.15/100,000 in 1–4 years-old [2]. Notwithstanding the effectiveness of PCVs to prevent disease [3–8], this ongoing global disease burden requires continual vigilance.
PCVs target the primary virulence determinant of the pneumococcus, the polysaccharide capsule from which the serotype is derived and which now number over 100, with new types continuing to be described [9, 10]. A7-valent PCV (targeting serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) was introduced into the UK’s national childhood immunisation programme (NIP) in 2006 and was replaced in 2010 with a 13-valent PCV (PCV7 + additional serotypes 1, 3, 5, 6A, 7F, and 19A). Since then, there was a schedule alteration in 2020 from 2 + 1 (at age 2 months, 4 months, and 12 months) to 1 + 1 (at 12 weeks and 1 year of age). However, with recent recommendations for either PCV15 (PCV13 serotypes plus 22F and 33F) or PCV20 (PCV13 serotypes plus 22F, 33F, 8, 10A, 11A, 12F and 15B) for children in the USA and several European countries, if and when one is introduced into the UK carriage epidemiology will likely alter. Alongside this, there is the issue of serotype replacement where disease burden associated with non-vaccine serotypes increases, a phenomenon observed following both PCV7 and PCV13 introductions in some countries [11, 12], although much less so in the United States [13].
The pneumococcus is a frequent coloniser of the upper respiratory tract (URT) of children, who are considered the primary group responsible for transmission [14]. PCVs in children then are important not just for direct protection but also for indirect (herd) protection as it is well established that PCVs reduce vaccine-type colonisation [15]. As such, studies that examine pneumococcal epidemiology in this demographic, particularly in the context of changes in vaccine valency and/or schedule, are important barometers for population-level outcomes [3, 16, 17]. For example, we have previously revealed expansions of serotype 6C following PCV7 introduction and 22F following PCV13 [18, 19], in addition to continued persistence of serotype 3 linked to Clade Iα CC180 GPSC12 [20].
The URT is a diverse ecological niche, and the microbiome found there includes many bacterial species of particular interest with respect to human health [21]. For example, the pneumococcus has frequently been observed to co-colonise with Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus [22–26]. With known inter-species interactions which may shape the community of this niche [23–25, 27, 28], there is a need to consider impacts of PCVs on these additional species. Although the evidence is mixed, there are studies which report both increases of S. aureus and H. influenzae carriage following PCV introduction, with the reverse for M. catarrhalis [29–34]. Additionally, there is the growing body of evidence demonstrating that non-pneumococcal Streptococci (e.g., S. mitis) can acquire capsule biosynthesis loci and express that capsule [9]. These species are often found in the upper airways, particularly the oral cavity, with pneumococci. How these may impact our efforts to capture vaccine impact has not been fully investigated. Therefore, the wider impact of PCVs on respiratory microbiota should be examined.
Here we present follow-on data from the long-running paediatric pneumococcal carriage study in Southampton, UK. We present data on pneumococcal serotype epidemiology, serotype diversity, as well as carriage of other frequent colonisers of the child URT over a period of seventeen years in the UK. This period begins with the introduction of PCV7 into the UK NIP in 2006 and spans the PCV13 period until 2023, the verge of an era which may see the introduction of higher valency vaccines. This unique, annual, cross-sectional surveillance study has enabled us to examine the impact of PCV introductions on the URT microbiota of children < 5 years of age and provides important baseline data for consideration in future changes in vaccines and vaccination schedules.
Methods
Paediatric Population: The population and study sites have been extensively described previously [4, 19, 35]. In brief, children were recruited from two UK sites: Site 1 - Southampton General Hospital, administered by University Hospital Southampton (UHS) NHS Foundation Trust and Site 2– a collection of community health care facilities within the Solent NHS Trust region. Site 1 recruitment has been on-going since 2006, whereas Site 2 began in 2017. Recruitment was limited to children less than five years of age. The only other exclusion criterion was that only one child per family was swabbed, irrespective of whether the child was the one attending the hospital or a sibling, and that child was swabbed only once. No exclusion criteria based on health were used. For the analysis of pneumococcal serotype diversity, an annual target of n = 100 isolates was determined to enable the detection ~ 50% relative reduction with 80% power at a 5% significance level.
Nasopharyngeal Swab Samples and Laboratory Processing: Nasopharyngeal Rayon Tip Transwabs (Medical Wire, Corsham, UK) in charcoal Amies media were used for swabbing and then plated onto several media (Oxoid, Basingstoke, UK) within 9 h of swabbing, including Columbia Colistin Naladixic Acid agar (CNA), Columbia Agar with Chocolated Horse Blood and Bacitracin (BAC) and Columbia Agar with Chocolated Horse Blood (CHOC). Confirmation of presumptive S. pneumoniae was done on CNA using optochin sensitivity indicated by a ⩾14 mm diameter inhibition zone around the disc (Thermo Scientific™, Loughborough, UK). Only one colony of S. pneumoniae per participant swab was selected for further analysis. H. influenzae was identified as characteristic colonies requiring X + V on BAC. M. catarrhalis was identified as Gram-negative, oxidase-positive, tributyrin-positive and DNase-positive diplococci on CHOC. S. aureus was identified as characteristic coagulase-positive colonies on CNA using a Pastorex Staph Plus Kit (Bio-Rad, 56353).
Pneumococcal serotyping: Isolates from skim milk, tryptone, glucose, and glycerin (STGG) stocks were cultured on CNA plates and incubated overnight at 37oC in 5% CO2 prior to DNA extraction. Extraction was carried out using QIAamp® DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The DNA extracts were sent to the Wellcome Sanger Institute (WSI) for whole genome sequencing (WGS) using Illumina HiSeq or 10X platforms generating initially 2 × 75 bp and later 2 × 100 bp paired-end reads from libraries prepared using TruSeq chemistry. Pneumococcal serotype was inferred using SeroBA version 1.0.5 [36].
Statistical analysis: All statistical analysis was done in R version 4.2.2 (2022-10-31) using RStudio version 2022.12.0 + 353 with graphics built using the grammar of graphics package, ggplot2 [37, 38]. Participants were characterised by whether they were recruited in the pre-PCV13, early PCV13 and late PCV13 era and were defined as having swabbing dates of 01/01/2006-30/06/2010 inclusive, 01/07/2010-30/06/2012 inclusive, and 01/07/2012 to the end of the study period (31/03/2023), respectively. Serotype diversity was calculated using diversity(,“simpson”) from the R package Vegan, with serotype rank-abundance model fitting done using radfit() from the same package.
Results
Since the start of the study, 7,686 swabs were collected from which 2,386 (31%) pneumococci have been recovered. In total, 72.5% (5,575) of children were recruited at Site 1 (Hospital), a consequence of this being the longest running site. Here, recruitment numbers ranged from a low of n = 31 in 2022/23 to n = 542 in 2018/19. At Site 2 (community clinics), where recruitment started in 2017/18, the lowest recruitment was in 2020/21 with n = 228 and the highest of n = 470 in 2019/20. Recruitment at Site 1 has remained challenging since the SARS-CoV2 pandemic and therefore recently most children (n = 1,784, 60.1%) were recruited from Site 2.
Participant recruitment numbers are given in Supplementary Table 1. There was no significant difference between sites in the ratio of males to females (p = 0.35, row-wise z-test of proportions) with males accounting for 43% (n = 513/1,182) and 46% (n = 820/1,784) of the populations respectively at sites 1 and 2 The mean age of children recruited in site 1 was 18.1 months (± 16.37; range: 0-59.9) compared to 11.1 months (± 10.5; range: 0.4–57.1) at site 2, and this difference was statistically significant (Wilcoxon, p < 0.001). The median age of all children recruited was 13.0 months (IQR: 22) with 49.3% male, 42.8% female and 7.9% for whom accurate gender was not available or not recorded. We previously showed that a recent RTI (within the previous 30 days) resulted in an increased risk of carriage [35]. Whilst we do not know whether a child had an RTI at the time of swabbing, the proportion of children with a self-reported, prior cold was significantly different between the two sites (site 1 52.1% vs. site 2 46.8%, p < 0.001), but not for other RTIs (throat/chest infection or flu-like illness).
Carriage of S. pneumoniae between 2006/07 and 2022/23 is shown in Fig. 1. Median carriage was 31.4% (IQR: 6.7) and ranged from a low of 19.2% (95%CI 14.9–24.3) in 2020/21 to a high in 2014/15 of 37.9% (95%CI 32.8–43.2) across both sites. A lower carriage prevalence was evident in 2020/21 which corresponded to the period of non-pharmaceutical intervention introductions, e.g., lockdowns, in response to the SARS-CoV2 pandemic. As previously described this reduction was not statistically significantly [39].
Fig. 1.
Carriage prevalence of S. pneumoniae across all years (left) and by PCV13 period (right). Left - Error bars represent 95% CI. From 2017/18 (year 12) onwards data has been split into Site 1 (Hospital) and Site 2 (community clinics). Combined mean carriage was 31.2%, ranging from a low of 19.2% in 2020/21 (year 15) to 37.9% in 2014/15 (year 9). Year 15 denotes a period when the UK was under various forms of non-pharmaceutical intervention (NPI). PCV13 introduction is shown as vertical dashed line; Right– Box and whisker plot showing a comparison of overall pneumococcal carriage prevalence (%) by PCV13 period. Error bars represent 95% CI. Age groups are illustrated by colour with connecting lines across PCV13 periods. No statistically significant difference was found between the pre-PCV13 and either early or late PCV13 periods
Examining the three PCV13 periods (pre, early and late), carriage in the pre-PCV13 period was 27.7% (IQR: 16.5), 35.3% (IQR: 14.4) in the early-PCV13 period and 39.3% (IQR:10.0) in the late-PCV period (Fig. 1). This apparent increase was, however, not significant. Assessing carriage in age groups between these periods, we observed an increase in carriage over time in children aged 24 to 35 months, however this did not reach the significance threshold.
We continued to monitor the relative prevalence of pneumococcal serotypes in this paediatric survey. In total we detected 48 distinct serotypes, not including the various non-typeable genotypes that have been described. Clear decreases in both PCV7 and PCV13 serotype groups were evident, with a concomitant increase in the carriage (as a proportion of total carriage) of non-vaccine type (NVT) serotypes and those included in PCV20 (Fig. 2).
Fig. 2.
Prevalence of serotypes grouped by inclusion in PCVs. The reduction in PCV7 serotypes post-PCV7 from >50% is shown in black and of PCV13 serotypes (purple) from a high of ~20% post-PCV13. NVTs, here split to illustrate those that may be targeted by PCV20, equates to 94.0% of carriage in 2022/23
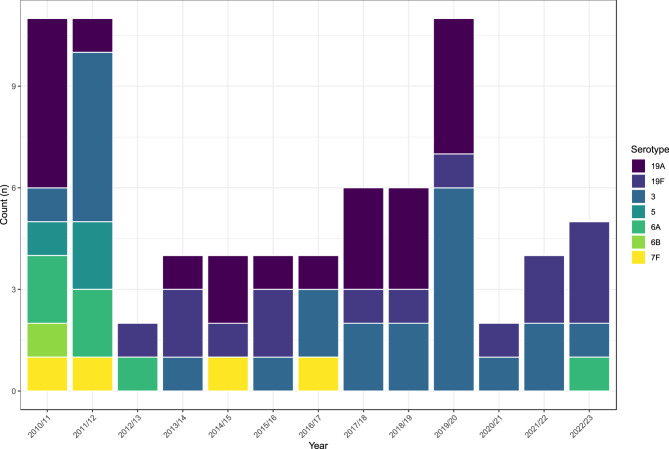
Residual carriage of PCV7 and PCV13 serotypes persisted, though carriage was relatively low and limited to serotypes 3, 19A, 19F, 5, 6A, 6B and 7F (Fig. 3). The most frequently carried between 2010/11 (the point of PCV13 introduction) and 2022/23 (years 5 to 17) were serotypes 19A (n=21), 3 (n=24) and 19F (n=15). Serotype 6B was last detected in 2010/11 (year 5), serotype 5 in 2011/12 (year 6), and serotype 7F in 2016/17 (year 11). A single serotype 6A carriage in the last year of the study (2022/23) was the first occurrence since 2012/13 (year 7). Serotype 19A has not been detected since 2019/20 despite being relatively prevalent previously.
Fig. 3.
Isolation of PCV-type serotypes following PCV13 introduction in 2010. In recent years, 19F and 3 have been the most frequently isolated PCV targeted serotypes
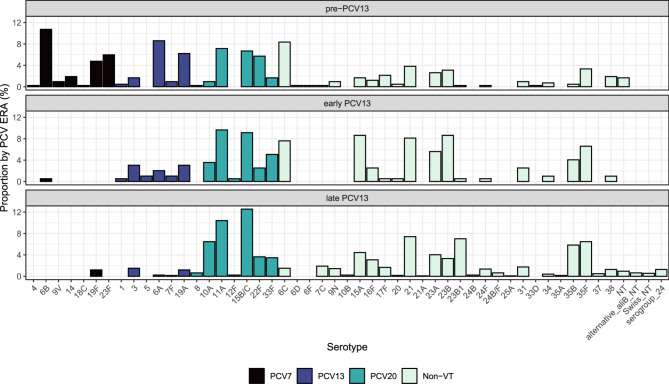
The relative frequency of carriage of each individual serotype within PCV13 periods is shown in Fig. 4. Of those additional PCV20 serotypes it is notable that 8 and 12F were rare, however three (10A, 11A and 15B/C) were all in the five most isolated serotypes in the late-PCV13 period. Cumulatively, the most common serotypes (by carriage prevalence) across the 17 years of this study were: 11A (n=181, 9.8%), 15B/C (n=168, 9.1%), 21 (n=126, 6.8%), 35F (n=109, 5.9%), 10A (n=93, 5.0%) and 23B1 (n=91, 4.9%). In the most recent year (2022/23, year 17) 23A was the most common serotype (n=13, 15.5%) followed by 15B/C (n=8, 9.5%), 11A (n=7, 8.3%), 23B1 and 22F (both n=6, 7.1%) (data not shown).
Fig. 4.
Bar plot showing serotype proportions by PCV era from the pre-PCV13 period (top) to early-PCV13 period (middle) and late-PCV13 period (bottom). Pre-PCV13: 01/01/2006-30/06/2010 inclusive; early PCV13: 01/07/2010-30/06/2012 inclusive; late PCV13: 01/07/2012 to the end of the study period. Serotypes are coloured according to vaccine type status: PCV7– black bars, PCV13– blue bars, PCV20– green, and NVT– pale green. Noticeable decreases in VT serotypes can be seen, with residual serotype 3 and 19A carriage. PCV20 serotypes 11A and 15B/C are the two most common serotypes now observed
Comparisons of serotype prevalence between the early-PCV13 and late-PCV13 period are given in Table 1. Carriage of serotypes included in PCV7 were infrequent in either period, as were serotypes 1 and 5 of PCV13. The OR for carriage prevalence of serotype 3 did not decrease, however 5, 6A and 19A all decreased significantly in the late-PCV13 period. Of the PCV20 serotypes only 22F prevalence increased significantly in the late compared to early PCV13 periods (OR: 4.60, 95%CI 1.86–11.35 p = 0.009). Although this was a consequence of a transient expansion on which we have previously reported [19, 40] 22F continues to be frequently isolated in carriage. This has implications for PCV15 where this serotype, along with 33F, are the only additional serotypes included. In contrast, serotype 33F was not increased between the two periods examined (OR 0.67; 95%CI: 0.333–1.361, p = 0.2706), and whilst 22F accounted for 7% of carriage isolates in the final year of the study, no 33F isolates were seen. Similarly to 22F, whilst a post-PCV7 expansion of 6C was previously reported [18] this increase has proved to be transient and is further reflected in the significant decreases observed between early and late-PCV13 periods reported here. Of the remaining NVTs only serotype 15A carriage prevalence changed significantly (p = 0.0133) in the late-PCV13 period, dropping from 8.63 to 4.42% of all serotyped isolates with an OR of carriage of 0.49 (95%CI: 0.28–0.86). Finally, previous replacement of serotype 23B by 23B1 was noted in disease surveillance [41], with an upsurge of the latter from 2011. Carriage data reflected this, where 23B decreased significantly (p = 0.0007) from 9% of all pneumococci in the early PCV13 period to 3% in the late. This was reflected by an increased odds of carriage for 23B1 (OR: 14.82, 95%CI 2.05–107.00, p = 0.0075) which in the late PCV13 period represented 7% of pneumococcal serotypes examined.
Table 1.
Frequency and odds ratio for serotypes isolated in the early versus late-PCV13 periods. Serotypes are grouped according to the vaccine by which they are targeted (PCV7, PCV13 and PCV20). Vaccine serotypes that were not identified in either period are not listed. *Significant p-values are shown in bold following Benjamini–Hochberg adjustment for FDR correction
| Group | Serotype | Early PCV§ % (n) | Late PCV§ % (n) | OR (95% CI) | p* |
|---|---|---|---|---|---|
| PCV7 | 6B | 0.51 (1) | 0.00 (0) | 0.05 (0.002–1.274) | 0.07 |
| 19F | 0.00 (0) | 1.18 (15) | 4.89 (0.292–82.09) | 0.2699 | |
| PCV13 | 1 | 0.51 (1) | 0.00 (0) | 0.05 (0.002–1.274) | 0.07 |
| 3 | 3.05 (6) | 1.50 (19) | 0.49 (0.191–1.230) | 0.1275 | |
| 5 | 1.02 (2) | 0.00 (0) | 0.03 (0.002–0.646) | 0.0249 | |
| 6A | 2.03 (4) | 0.24 (3) | 0.11 (0.026–0.516) | 0.0048 | |
| 7F | 1.02 (2) | 0.16 (2) | 0.15 (0.022–1.102) | 0.0624 | |
| 19A | 3.05 (6) | 1.18 (15) | 0.38 (0.146–0.996) | 0.049 | |
| PCV20 | 8 | 0.00 (0) | 0.63 (8) | 2.67 (0.153–46.406) | 0.5007 |
| 10A | 3.55 (7) | 6.48 (82) | 1.88 (0.856–4.129) | 0.1159 | |
| 11A | 9.64 (19) | 10.43 (132) | 1.09 (0.657–1.809) | 0.7373 | |
| 12F | 0.51 (1) | 0.24 (3) | 0.47 (0.048–4.498) | 0.5088 | |
| 15B/C | 9.14 (18) | 12.56 (159) | 1.43 (0.856–2.384) | 0.1726 | |
| 22F | 2.54 (5) | 3.63 (46) | 4.60 (1.860–11.352) | 0.0009 | |
| 33F | 5.08 (10) | 3.48 (44) | 0.67 (0.333–1.361) | 0.2706 | |
| NVT | 6C | 7.61 (15) | 1.50 (19) | 0.18 (0.092–0.370) | < 0.0001 |
| 7C | 0.00 (0) | 1.90 (24) | 7.79 (0.472–128.599) | 0.1513 | |
| 9N | 0.00 (0) | 1.42 (18) | 5.85 (0.351–97.518) | 0.2183 | |
| 10B | 0.00 (0) | 0.24 (3) | 1.09 (0.056–21.264) | 0.9526 | |
| 15A | 8.63 (17) | 4.42 (56) | 0.49 (0.279–0.862) | 0.0133 | |
| 16F | 2.54 (5) | 3.08 (39) | 1.22 (0.475–3.135) | 0.6788 | |
| 17F | 0.51 (1) | 1.66 (21) | 3.03 (0.442–24.718) | 0.244 | |
| 20 | 0.51 (1) | 0.16 (2) | 0.31 (0.028–3.436) | 0.3401 | |
| 21 | 8.12 (16) | 7.42 (94) | 0.91 (0.552–1.577) | 0.7302 | |
| 21A | 0.00 (0) | 0.08 (1) | 0.47 (0.019–11.534) | 0.6425 | |
| 23A | 5.58 (11) | 4.03 (51) | 0.71 (0.363–1.387) | 0.3156 | |
| 23B | 8.63 (17) | 3.32 (42) | 0.36 (0.203–0.652) | 0.0007 | |
| 23B1 | 0.51 (1) | 7.03 (89) | 14.82 (2.053–106.997) | 0.0075 | |
| 24B | 0.00 (0) | 0.24 (3) | 1.09 (0.056–21.265) | 0.9526 | |
| 24B/F | 0.00 (0) | 0.63 (8) | 2.67 (0.153–46.406)) | 0.5007 | |
| 24F | 0.51 (1) | 1.34 (17) | 2.67 (0.353–20.159) | 0.3416 | |
| Serogroup 24 | 0.00 (0) | 1.26 (16) | 5.21 (0.311–87.225) | 0.2508 | |
| 25A | 0.00 (0) | 0.08 (1) | 0.47 (0.019–11.534) | 0.6425 | |
| 31 | 2.54 (5) | 1.74 (22) | 0.68 (0.254–1.915) | 0.4403 | |
| 34 | 1.02 (2) | 0.39 (5) | 0.39 (0.075–2.007) | 0.258 | |
| 35A | 0.00 (0) | 0.16 (2) | 0.78 (0.037–16.328) | 0.8733 | |
| 35B | 4.06 (8) | 5.85 (74) | 1.47 (0.696–3.091) | 0.3139 | |
| 35F | 6.60 (13) | 6.48 (82) | 0.98 (0.535–1.796) | 0.9485 | |
| 37 | 0.00 (0) | 0.47 (6) | 2.04 (0.114–36.003) | 0.6283 | |
| 38 | 1.02 (2) | 1.26 (16) | 1.25 (0.285–5.470) | 0.7689 | |
| NT | Alternative aliB NT | 0.00 (0) | 0.63 (8) | 2.67 (0.153–46.406) | 0.5007 |
| NT | 0.00 (0) | 0.95 (12) | 3.94 (0.232–66.743) | 0.3428 | |
| Swiss NT | 0.00 (0) | 0.55 (7) | 2.35 (0.134–41.347) | 0.5587 |
§ Early PCV period: 01/07/2010-30/06/2012 inclusive; Late PCV period 01/07/2012 to the end of the study
Table 1
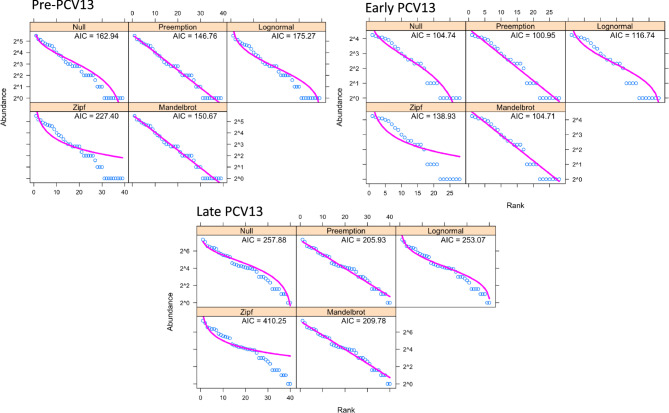
Serotype diversity, as measured using ecological statistics which describe the composition of a community (in this case, serotypes), between pre-PCV13, early and late PCV13 periods remained unchanged, with Simpsons indexes of 0.944, 0.937 and 0.940, respectively. There was also no significant difference in the total number of serotypes observed (serotype richness), or in Shannon diversity, a measure of evenness. There was a significant difference in serotype composition between PCV13 eras (permanova, p < 0.001). This was driven by the removal of VT serotypes which were more prevalent in the pre-PCV13 era and their replacement with non-VTs, specifically 15A, 15B/C, 11A, 21, 35B, 10A, 23B/B1 as shown in Fig. 5. We next examined whether PCV13 introduction resulted in changes to serotype rank-abundance distributions (Fig. 6). Here we show that the niche preemption model (which fits a geometric series, or Motomura model) best described the rank abundance data for all periods examined and consequently there was no indication that serotype population structure in carriage was perturbed by PCV13 introduction.
Fig. 5.
Principal Components Analysis of serotype abundance between pre-PCV13, early- and late-PCV13 periods. PCV eras are shown as coloured points with serotypes that explain the variance as arrows with the amount of variance explained indicated by arrow length. Pre-PCV13 era composition is defined by the presence of VTs 6B, 6A, 19A, 19F, 23F and the non-VT 6C
Fig. 6.
Serotype Rank-abundance distributions models in pre-, early- and late-PCV13 periods. Five models were used to examine serotype abundance. Null is equivalent to the broken-stick model. In all three the Preemption model was the best fit with the lowest AIC and suggests no disruption to population structure has occurred due to PCV13 introduction
The prevalence of other respiratory pathobionts has been monitored in this cohort since 2008/09, the third year of the study and two years before the introduction of PCV13. Acknowledging that most data were collected after the introduction of PCV13 in 2010, significant increases in carriage of Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis and non-pneumococcal alpha-haemolytic Streptococci were observed in all age groups (Figure 7). However, these increases stabilised, and no significant differences were observed between the early- and late-PCV13 periods. Importantly, no increase in the proportion of methicillin-resistant S. aureus was observed with only 1.9% (n=1/52), 3.1% (n=1/32) and 0.7% (n=4/579) in the pre-, early- and late-PCV13 periods respectively.
Fig. 7.
Box and whisker plots showing carriage prevalence of Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis and non-pneumococcal Alpha-haemolytic Streptococci contrasted between pre-PCV13, early- and late-PCV13 periods. Error bars show 95% CI. Significant increases in all four species are apparent between the pre-PCV13 and early-PCV13 period. No significant differences are seen between early and late-PCV13 periods
Discussion
The introduction of PCVs has alleviated a significant burden of disease from pneumococcal infections both in the UK and worldwide [42, 43]. Despite this success, several factors drive the requirement to continue monitoring pneumococcal carriage epidemiology. These include responses to selective pressures driven by vaccine introductions, such as capsule switching and serotype replacement, as well as the continued evolution of PCVs as valency is increased, immunogenicity is lessened for higher valency vaccines, new vaccine constructs are developed, and schedules amended (for example the switch from 2 + 1 dosing to 1 + 1 in the UK [2]). In this context, the Southampton pneumococcal carriage study, a long-running, cross-sectional, annual survey of paediatric carriage in the UK, represents a useful marker for changes in serotype epidemiology.
As reported previously, and in line with similar studies [17, 35, 44], carriage of pneumococci has remained consistent in our study since the introduction of PCV7 in 2006 even with the almost complete removal of targeted serotypes from circulation. The low-level persistence of VT-serotypes 3, 19A and 19F in carriage mirrors that of similar UK data [17] and of other European countries [45–49].
As a recognised endpoint in vaccine studies [15], carriage enables comparisons between direct and indirect impacts of schedules, as well as the opportunity to model the potential impact of alternative strategies based on new formulations [50]. Whilst serotypes 8, 12F and 9N have been previously noted for increased disease burden in England and Wales, accounting for > 40% of all-age IPD in 2016/17 [12] these are infrequently carried in the UK paediatric population, accounting for less than 3% of carriage isolates in the present study. More recently, 8 and 12F have almost completely disappeared from IPD cases in children [2]. In fact, post-pandemic (2022/23) analysis of childhood IPD in England and Wales has shown the dominance of serotypes 10A, 23B, 15B/C and 22F, which accounted for 37% of cases [2]. Except for 23B, which accounted for about a quarter, this burden is caused by serotypes that are included in PCV20. Considering the potential impact on carriage, those same serotypes, again excluding 23B, represented ~ 20% of carriage isolates in the final year of this study. The potential impact of PCV20 on both disease and carriage is therefore clear. Carriage also gives an indication of invasive potential. For example, 23B accounted for ~ 10% of IPD in 2022/23 and 7% of carriage in this study, a symmetry of prevalence observed for both 15B/C and 22F. In keeping with other studies these serotypes may therefore be considered of average invasiveness. In contrast, and similar to the highly invasive serotypes 8 and 12F, 10 A was relatively infrequent in our carriage (3.5%) in comparison to IPD incidence where it was the most common cause of invasive disease in England and Wales in 2022/23 [2], a feature that has been noted in other European countries [51].
Recent increases in serotype 7C-associated disease [52] was suggested to mirror an increase in carriage as shown in a 2017–2020 follow-up paediatric carriage study also conducted in the South of England, UK [17]. Whilst our analysis of early versus late PCV13 periods did not reveal a similar significant increase, we note that most isolates (n = 23/24) were from after 2016/17 (year 11), which is the year preceding the noted increase in disease. Whether this dynamic persists is an important question. As is the case with the reduction of 8 and 12F in disease, secular trends in serotype prevalence are difficult to interpret, particularly with reference to modelling the impact of higher valency vaccines [53]. Similar trends were seen with serotype 22F for example which expanded in both disease and carriage following PCV7 introduction in the UK [19, 53], before a contraction that remains unexplained. These examples only serve to underline the importance of pneumococcal carriage epidemiology for modelling disease and vaccine impacts.
Of interest is the increased carriage of other URT bacterial species shortly after the introduction of PCV13. There was concern that niche disruption or reduced fitness of replacement pneumococcal serotypes may result in the increased carriage of competitor species, particularly S. aureus [54]. This phenomenon was observed in post-PCV7 studies for S. aureus and H. influenzae [55] and with PCV13 [29, 56]. Whether new PCVs have similar effects remains to be seen.
There are limitations to this study. The first is that the study was conducted in a single geographic area within the UK, and in a cross-sectional manner. Therefore, extrapolation to national trends should be done with caution. Additionally, inferences regarding serotype invasiveness using carriage prevalence are made based on IPD data derived from national surveillance as no Southampton-specific IPD data with serotype information was available. Finally, carriage is being inferred from a single point-in-time isolate and therefore we are unable to examine other important features of pneumococcal carriage such as density, carriage duration or multi-serotype carriage [57, 58]. Nevertheless, the considerable strength of this work remains the nearly two decades of surveillance which has highlighted serotype expansions [18], changing genomic epidemiology of VT serotypes [20] and the impact of the SARS-CoV2 pandemic [39].
In conclusion, we have shown PCV20 serotypes accounted for approximately 40% of carriage since 2012/13, with three serotypes (10A, 11A and 15B/C) being particularly common while 8 and 12F were rarely seen. By contrast, the serotypes only included in PCV15 (22F and 33F) represented just 7% in the same period. Given these prevalences, carriage epidemiology is likely to change significantly if either vaccine is introduced, although recent data suggest PCV15 may increase overall IPD burden as the direct effects are hindered by the NVTs [53]. We highlighted rare, residual VT carriage and showed continued fluctuations in individual non-VT serotypes, most notably 15A, 23B and the genetic subtype of 23B termed 23B1. Finally, we showed that PCV13 did not impact the pneumococcal population ecology despite clear reductions in targeted serotypes, but that an immediate increase in carriage of other respiratory pathobionts did occur although has since stabilised.
Supplementary Information
Acknowledgements
We are grateful to staff in both the Pathogen Genomics team and those associated with the Global Pneumococcal Sequencing Project at the WSI for their support in genome sequencing of collected isolates. We are indebted to staff at the NIHR Southampton Wellcome Trust Clinical Research Facility and the Hampshire and Isle of Wight Healthcare Foundation Trust for their assistance in the collection of samples and to staff at UKHSA for swab processing between 2006/07 and 2011/12. The authors also acknowledge the use of the IRIDIS High Performance Computing Facility, and associated support services at the University of Southampton, in the completion of this work. Finally, we acknowledge both the guardians and participants without whom this study would not have been possible.
Abbreviations
- BAC
Columbia Agar with Chocolated Horse Blood and Bacitracin
- CHOC
Columbia Agar with Chocolated Horse Blood
- CNA
Columbia Colistin Naladixic Acid agar
- IPD
Invasive pneumococcal disease
- NIP
National immunisation programme
- NVT
Non-vaccine type
- PCV
Pneumococcal conjugate vaccine
- URT
Upper respiratory tract
- STGG
Skim milk, tryptone, glucose, and glycerin
- VT
Vaccine type
Authors’ contributions
SCC and SNF conceived and secured funding for the study with assistance from JMJ. SCC and SNF were the site primary investigators. VTD, JJ, AJJL, SG, REH, EJD and RAG assisted with participant recruitment and laboratory microbiology. Whole genome sequencing was facilitated by SDB. DWC, JJ, RAG, VTD, SL and KLO analysed the whole genome sequencing data. DWC wrote the manuscript. All authors reviewed the manuscript.
Funding
This is a University Hospital Southampton NHS Foundation Trust sponsored study, led by investigators from the University of Southampton, and funded under a collaborative agreement by Pfizer Inc. Funding for whole genome sequencing was provided by the Wellcome Sanger Institute.
Data availability
All sequencing data (fastqs) has been deposited in the European Nucleotide Archive under study accession PRJEB2417 (Whole genome sequencing of carried Streptococcus pneumoniae during the implementation of pneumococcal conjugate vaccines in the UK).
Declarations
Ethics approval and consent to participate
The UK National Health Service (NHS) Research Ethics Service approved this study (06/Q1704/105 and 14/NS/1064). All methods and research practises outlined below were performed in accordance with relevant regulations and the Declaration of Helsinki. Written informed consent was provided by parents or legal guardians for each child.
Consent for publication
Not applicable.
Competing interests
DWC was a post-doctoral researcher on GSK funded projects in 2014/15, and currently receives grant support from Pfizer and the National Institute for Health via the NIHR Southampton Biomedical Research Centre. SNF receives support from the National Institute for Health Research funding via the NIHR Southampton Wellcome Trust Clinical Research Facility and the NIHR Southampton Biomedical Research Centre. SNF and SCC act as principal investigators for clinical trials and other studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers. No personal payments are received from them. SNF, JMJ and SCC have participated in advisory boards for vaccine manufacturers but receive no personal payments for this work. SNF, SCC and JMJ have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS Trusts or Universities, or to independent charities. RAG, VTD and JJ received PhD studentships via the University of Southampton from Pfizer. RAG received post-doctoral support in 2012 on a GSK funded research project via the University of Southampton. KLO received PhD studentship support from GSK, again via the University of Southampton. JC, ML, KH, JS and BG are employees of Pfizer Inc and, as such, may hold stocks. All other authors have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ikuta KS, Swetschinski LR, Robles Aguilar G, Sharara F, Mestrovic T, Gray AP, et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2022;400(10369):2221–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertran M, D’Aeth JC, Abdullahi F, Eletu S, Andrews NJ, Ramsay ME, et al. Invasive pneumococcal disease 3 years after introduction of a reduced 1 + 1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: a prospective national observational surveillance study. Lancet Infect Dis. 2024;24(5):546–56. 10.1016/S1473-3099(23)00706-5. Epub 2024 Feb 1. Erratum in: Lancet Infect Dis.2024;24(6):e356. 10.1016/S1473-3099(24)00224-X. PMID: 38310905. [DOI] [PubMed]
- 3.Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM, Parkhill J, et al. Population genomics of post-vaccine changes in Pneumococcal epidemiology. Nat Genet. 2013;45(6):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladstone RA, Jefferies JM, Tocheva AS, Beard KR, Garley D, Chong WW, et al. Five winters of Pneumococcal serotype replacement in UK carriage following PCV introduction. Vaccine. 2015;33(17):2015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigurdsson S, Erlendsdóttir H, Quirk SJ, Kristjánsson J, Hauksson K, Andrésdóttir BDI, et al. Pneumococcal vaccination: direct and herd effect on carriage of vaccine types and antibiotic resistance in Icelandic children. Vaccine. 2017;35(39):5242–8. [DOI] [PubMed] [Google Scholar]
- 6.Dunne EM, Satzke C, Ratu FT, Neal EFG, Boelsen LK, Matanitobua S, et al. Effect of ten-valent Pneumococcal conjugate vaccine introduction on Pneumococcal carriage in fiji: results from four annual cross-sectional carriage surveys. Lancet Global Health. 2018;6(12):e1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenzie GA, Hill PC, Jeffries DJ, Hossain I, Uchendu U, Ameh D, et al. Effect of the introduction of Pneumococcal conjugate vaccination on invasive Pneumococcal disease in the gambia: a population-based surveillance study. Lancet Infect Dis. 2017;17(9):965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tocheva AS, Jefferies JM, Rubery H, Bennett J, Afimeke G, Garland J, et al. Declining serotype coverage of new Pneumococcal conjugate vaccines relating to the carriage of Streptococcus pneumoniae in young children. Vaccine. 2011;29(26):4400–4. [DOI] [PubMed] [Google Scholar]
- 9.Ganaie F, Saad Jamil S, McGee L, van Tonder Andries J, Bentley Stephen D, Lo Stephanie W et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio. 2020;11(3):e00937–20. PMID: 32430472; PMCID: PMC7240158. 10.1128/mBio.00937-20 [DOI] [PMC free article] [PubMed]
- 10.Ganaie FA, Saad JS, Lo SW, McGee L, Bentley SD, van Tonder AJ, et al. Discovery and characterization of Pneumococcal serogroup 36 capsule subtypes, serotypes 36A and 36B. J Clin Microbiol. 2023;61(4):e0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent Pneumococcal conjugate vaccination in England and wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–8. [DOI] [PubMed] [Google Scholar]
- 12.Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive Pneumococcal disease in England and wales, 2000–17: a prospective National observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. [DOI] [PubMed] [Google Scholar]
- 13.Gierke R. Current epidemiology of pediatric pneumococcal disease, United States. Advisory Committee on Immunization Practices. 2023; CDC, Atlanta, GA. https://www.cdc.gov/acip/downloads/slides-2023-02-22-24/Pneumococcal-02-Gierke-508.pdf
- 14.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nzenze SA, Madhi SA, Shiri T, Klugman KP, de Gouveia L, Moore DP, et al. Imputing the direct and indirect effectiveness of childhood Pneumococcal conjugate vaccine against invasive Pneumococcal disease by surveying Temporal changes in nasopharyngeal Pneumococcal colonization. Am J Epidemiol. 2017;186(4):435–44. [DOI] [PubMed] [Google Scholar]
- 16.Southern J, Andrews N, Sandu P, Sheppard CL, Waight PA, Fry NK, et al. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent Pneumococcal conjugate vaccine in England. PLoS ONE. 2018;13(5):e0195799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiley KS, Ratcliffe H, Voysey M, Jefferies K, Sinclair G, Carr M, et al. Nasopharyngeal carriage of Pneumococcus in children in England up to 10 years after 13-Valent Pneumococcal conjugate vaccine introduction: persistence of serotypes 3 and 19A and emergence of 7 C. J Infect Dis. 2022;227(5):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loman NJ, Gladstone RA, Constantinidou C, Tocheva AS, Jefferies JMC, Faust SN, et al. Clonal expansion within Pneumococcal serotype 6 C after use of Seven-Valent vaccine. PLoS ONE. 2013;8(5):e64731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devine VT, Cleary DW, Jefferies JMC, Anderson R, Morris DE, Tuck AC, et al. The rise and fall of Pneumococcal serotypes carried in the PCV era. Vaccine. 2017;35(9):1293–8. [DOI] [PubMed] [Google Scholar]
- 20.Cleary DW, Lo SW, Kumar N, Bentley SD, Faust SN, Clarke SC. Comparative genomic epidemiology of serotype 3 IPD and carriage isolates from southampton, UK between 2005 and 2017. Microb Genom. 2023;9(3):mgen000945. 10.1099/mgen.0.000945. PMID: 36867094; PMCID: PMC10132069. [DOI] [PMC free article] [PubMed]
- 21.Cleary DW, Clarke SC. The nasopharyngeal Microbiome. Emerg Top Life Sci. 2017;1(4):297–312. [DOI] [PubMed] [Google Scholar]
- 22.Lewnard JA, Huppert A, Givon-Lavi N, Pettigrew MM, Regev-Yochay G, Dagan R, et al. Density, serotype diversity, and fitness of Streptococcus pneumoniae in upper respiratory tract cocolonization with nontypeable Haemophilus influenzae. J Infect Dis. 2016;214(9):1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shak JR, Vidal JE, Klugman KP. Influence of bacterial interactions on Pneumococcal colonization of the nasopharynx. Trends Microbiol. 2013;21(3):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bergh MR, Biesbroek G, Rossen JWA, de Steenhuijsen Piters WAA, Bosch AATM, van Gils EJM, et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and Bacteria. PLoS ONE. 2012;7(10):e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GM, Huang SS, Rifas-Shiman SL, Hinrichsen VL, Pelton SI, Kleinman K, et al. Epidemiology and risk factors for Staphylococcus aureuscolonization in children in the post-PCV7 era. BMC Infect Dis. 2009;9(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14(10):1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David MZ, Daum RS. Community-Associated Methicillin-Resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewnard JA, Givon-Lavi N, Huppert A, Pettigrew MM, Regev-Yochay G, Dagan R, et al. Epidemiological markers for interactions among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in upper respiratory tract carriage. J Infect Dis. 2016;213(10):1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan T, Das RS, Arya BK, Chaudhary A, Chatterjee J, Das Bhattacharya S. Impact of Pneumococcal conjugate vaccine on the carriage density of Streptococcus pneumoniae and Staphylococcus aureus in children living with HIV: a nested case-control study. Hum Vaccin Immunother. 2020;16(8):1918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gils EJM, Hak E, Veenhoven RH, Rodenburg GD, Bogaert D, Bruin JP, et al. Effect of Seven-Valent Pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS ONE. 2011;6(6):e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boelsen LK, Dunne EM, Mika M, Eggers S, Nguyen CD, Ratu FT, et al. The association between Pneumococcal vaccination, ethnicity, and the nasopharyngeal microbiota of children in Fiji. Microbiome. 2019;7(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olwagen CP, Adrian PV, Nunes MC, Madhi SA. Evaluation of the association of Pneumococcal conjugate vaccine immunization and density of nasopharyngeal bacterial colonization using a multiplex quantitative polymerase chain reaction assay. Vaccine. 2018;36(23):3278–85. [DOI] [PubMed] [Google Scholar]
- 33.Bosch AATM, van Houten MA, Bruin JP, Wijmenga-Monsuur AJ, Trzciński K, Bogaert D, et al. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the Pneumococcal conjugate vaccine in the Netherlands. Vaccine. 2016;34(4):531–9. [DOI] [PubMed] [Google Scholar]
- 34.Camilli R, Vescio MF, Giufrè M, Daprai L, Garlaschi ML, Cerquetti M, et al. Carriage of Haemophilus influenzae is associated with Pneumococcal vaccination in Italian children. Vaccine. 2015;33(36):4559–64. [DOI] [PubMed] [Google Scholar]
- 35.Cleary DW, Jones J, Gladstone RA, Osman KL, Devine VT, Jefferies JM, et al. Changes in serotype prevalence of Streptococcus pneumoniae in southampton, UK between 2006 and 2018. Sci Rep. 2022;12(1):13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epping L, van Tonder AJ, Gladstone RA, Consortium TGPS, Bentley SD, Page AJ et al. SeroBA: rapid high-throughput serotyping of Streptococcus pneumoniae from whole genome sequence data. Microb Genom. 2018;4(8):e000204. 10.1099/mgen.0.000204. Erratum for: Microb Genom. 2018;4(7). 10.1099/mgen.0.000186. PMID: 30175955; PMCID: PMC6159549. [DOI] [PMC free article] [PubMed]
- 37.R Core Team. R: A language and environment for statistical computing. [Available from: https://www.R-project.org/.
- 38.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-; 2016. [Google Scholar]
- 39.Cleary DW, Campling J, Lahuerta M, Hayford K, Southern J, Gessner BD, et al. Non-pharmaceutical interventions for COVID-19 transiently reduced Pneumococcal and < i > haemophilus influenzae carriage in a cross-sectional pediatric cohort in southampton, UK. Microbiol Spectr. 2024;12(8):e00224–24. [DOI] [PMC free article] [PubMed]
- 40.Cleary DW, Devine VT, Jefferies JM, Webb JS, Bentley SD, Gladstone RA, et al. Comparative genomics of carriage and disease isolates of Streptococcus pneumoniae serotype 22F reveals Lineage-Specific divergence and niche adaptation. Genome Biol Evol. 2016;8(4):1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapatai G, Sheppard CL, Troxler LJ, Litt DJ, Furrer J, Hilty M, et al. Pneumococcal 23B molecular subtype identified using whole genome sequencing. Genome Biol Evol. 2017;9(8):2122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savulescu C, Krizova P, Lepoutre A, Mereckiene J, Vestrheim DF, Ciruela P, et al. Effect of high-valency Pneumococcal conjugate vaccines on invasive Pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respiratory Med. 2017;5(8):648–56. [DOI] [PubMed] [Google Scholar]
- 43.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent Pneumococcal conjugate vaccine on invasive Pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43. [DOI] [PubMed] [Google Scholar]
- 44.Rybak A, Levy C, Ouldali N, Bonacorsi S, Béchet S, Delobbe JF et al. Dynamics of antibiotic resistance of Streptococcus pneumoniae in france: A pediatric prospective nasopharyngeal carriage study from 2001 to 2022. Antibiotics. 2023;12(6):1020. 10.3390/antibiotics12061020 [DOI] [PMC free article] [PubMed]
- 45.Levy C, Varon E, Ouldali N, Béchet S, Bonacorsi S, Cohen R. Changes in invasive Pneumococcal disease spectrum after 13-Valent Pneumococcal conjugate vaccine implementation. Clin Infect Dis. 2020;70(3):446–54. [DOI] [PubMed] [Google Scholar]
- 46.Uddén F, Filipe M, Slotved H-C, Yamba-Yamba L, Fuursted K, Pintar Kuatoko P, et al. Pneumococcal carriage among children aged 4–12 years in Angola 4 years after the introduction of a Pneumococcal conjugate vaccine. Vaccine. 2020;38(50):7928–37. [DOI] [PubMed] [Google Scholar]
- 47.Corcoran M, Mereckiene J, Cotter S, Murchan S, Lo SW, McGee L, et al. Using genomics to examine the persistence of Streptococcus pneumoniae serotype 19A in Ireland and the emergence of a sub-clade associated with vaccine failures. Vaccine. 2021;39(35):5064–73. [DOI] [PubMed] [Google Scholar]
- 48.Hanquet G, Krizova P, Dalby T, Ladhani SN, Nuorti JP, Danis K, et al. Serotype replacement after introduction of 10-Valent and 13-Valent Pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. 2022;28(1):137–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slotved HC, Dalby T, Harboe ZB, Valentiner-Branth P, Casadevante VF, Espenhain L, et al. The incidence of invasive Pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon. 2016;2(11):e00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughtrie AL, Jefferies JM, Cleary DW, Doncaster CP, Faust SN, Kraaijeveld AR, et al. Microbial epidemiology and carriage studies for the evaluation of vaccines. J Med Microbiol. 2019;68(10):1408–18. [DOI] [PubMed] [Google Scholar]
- 51.Vissers M, Wijmenga-Monsuur AJ, Knol MJ, Badoux P, van Houten MA, van der Ende A, et al. Increased carriage of non-vaccine serotypes with low invasive disease potential four years after switching to the 10-valent Pneumococcal conjugate vaccine in the Netherlands. PLoS ONE. 2018;13(3):e0194823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makwana A, Ladhani SN, Kapatai G, Campion E, Fry NK, Sheppard C. Rapid spread of Pneumococcal nonvaccine serotype 7 C previously associated with vaccine serotype 19F, England and Wales. Emerg Infect Dis. 2018;24(10):1919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YH, Bertran M, Litt DJ, Ladhani SN, Miller E. Potential impact of replacing the 13-valent Pneumococcal conjugate vaccine with 15-valent or 20-valent Pneumococcal conjugate vaccine in the 1 + 1 infant schedule in england: a modelling study. Lancet Public Health. 2024;9(9):e654–63. [DOI] [PubMed] [Google Scholar]
- 54.Spijkerman J, Prevaes SMPJ, van Gils EJM, Veenhoven RH, Bruin JP, Bogaert D, et al. Long-Term effects of Pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS ONE. 2012;7(6):e39730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biesbroek G, Wang X, Keijser B, Eijkemans JF, Trzciński RMJ, Rots K. Seven-Valent Pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Disease J. 2014;20(2):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wouters I, Desmet S, Van Heirstraeten L, Herzog SA, Beutels P, Verhaegen J, et al. How nasopharyngeal Pneumococcal carriage evolved during and after a PCV13-to-PCV10 vaccination programme switch in belgium, 2016 to 2018. Eurosurveillance. 2020;25(5):1900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaguza C, Senghore M, Bojang E, Lo SW, Ebruke C, Gladstone RA et al. Carriage dynamics of Pneumococcal serotypes in naturally colonized infants in a rural African setting during the first year of life. Front Pediatr. 2021;8:587730. 10.3389/fped.2020.587730. PMID: 33489998; PMCID: PMC7820366. [DOI] [PMC free article] [PubMed]
- 58.Wyllie AL, Wijmenga-Monsuur AJ, van Houten MA, Bosch AATM, Groot JA, van Engelsdorp Gastelaars J, et al. Molecular surveillance of nasopharyngeal carriage of Streptococcus pneumoniae in children vaccinated with conjugated polysaccharide Pneumococcal vaccines. Sci Rep. 2016;6(1):23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data (fastqs) has been deposited in the European Nucleotide Archive under study accession PRJEB2417 (Whole genome sequencing of carried Streptococcus pneumoniae during the implementation of pneumococcal conjugate vaccines in the UK).