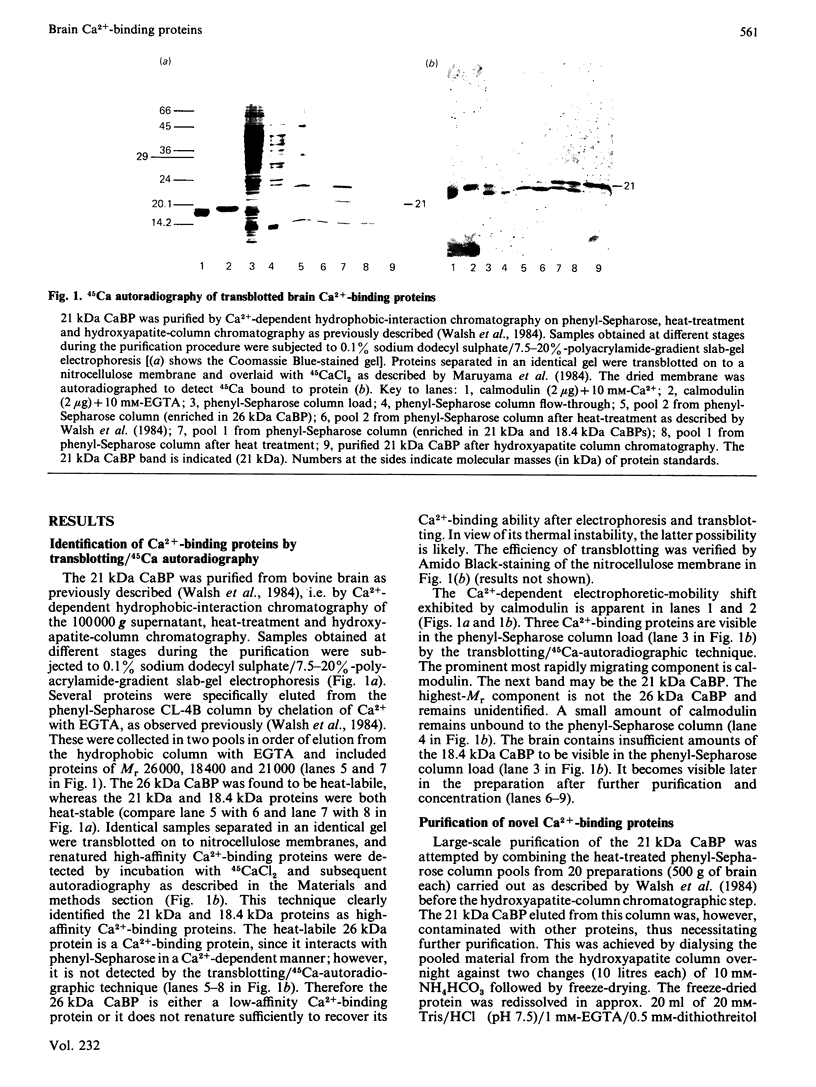
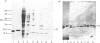Abstract
We have previously described the use of Ca2+-dependent hydrophobic-interaction chromatography to isolate the Ca2+ + phospholipid-dependent protein kinase (protein kinase C) and a novel heat-stable 21 000-Mr Ca2+-binding protein from bovine brain [Walsh, Valentine, Ngai, Carruthers & Hollenberg (1984) Biochem. J. 224, 117-127]. The procedure described for purification of the 21 000-Mr calciprotein to electrophoretic homogeneity has been modified to permit the large-scale isolation of this Ca2+-binding protein, enabling further structural and functional characterization. The 21 000-Mr calciprotein was shown by equilibrium dialysis to bind approx. 1 mol of Ca2+/mol, with apparent Kd approx. 1 microM. The modified large-scale purification procedure revealed three additional, previously unidentified, Ca2+-binding proteins of Mr 17 000, 18 400 and 26 000. The 17 000-Mr and 18 400-Mr Ca2+-binding proteins are heat-stable, whereas the 26 000-Mr Ca2+-binding protein is heat-labile. Use of the transblot/45CaCl2 overlay technique [Maruyama, Mikawa & Ebashi (1984) J. Biochem. (Tokyo) 95, 511-519] suggests that the 18 400-Mr and 21 000-Mr Ca2+-binding proteins are high-affinity Ca2+-binding proteins, whereas the 17 000-Mr Ca2+-binding protein has a relatively low affinity for Ca2+. Consistent with this observation, the 18 400-Mr and 21 000-Mr Ca2+-binding proteins exhibit a Ca2+-dependent mobility shift on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, whereas the 17 000-Mr Ca2+-binding protein does not. The amino acid compositions of the 17 000-Mr, 18 400-Mr and 21 000-Mr Ca2+-binding proteins show some similarities to each other and to calmodulin and other members of the calmodulin superfamily; however, they are clearly distinct and novel calciproteins. In functional terms, none of the 17 000-Mr, 18 400-Mr or 21 000-Mr Ca2+-binding proteins activates either cyclic nucleotide phosphodiesterase or myosin light-chain kinase, both calmodulin-activated enzymes. However, the 17 000-Mr Ca2+-binding protein is a potent inhibitor of protein kinase C. It may therefore serve to regulate the activity of this important enzyme at elevated cytosolic Ca2+ concentrations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., Lee F. T., DePaoli-Roach A., Roach P. J. Phosphorylation of glycogen synthase by the Ca2+- and phospholipid-activated protein kinase (protein kinase C). J Biol Chem. 1984 Jul 25;259(14):8743–8747. [PubMed] [Google Scholar]
- Aitken A., Klee C. B., Cohen P. The structure of the B subunit of calcineurin. Eur J Biochem. 1984 Mar 15;139(3):663–671. doi: 10.1111/j.1432-1033.1984.tb08055.x. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Wu W. C., Nairn A. C., Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold M. W., Wilson K. J., Heizmann C. W. Isolation of neuronal parvalbumin by high-performance liquid chromatography. Characterization and comparison with muscle parvalbumin. Biochemistry. 1982 Dec 7;21(25):6552–6557. doi: 10.1021/bi00268a035. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Endo T., Naka M., Hidaka H. Ca2+-phospholipid dependent phosphorylation of smooth muscle myosin. Biochem Biophys Res Commun. 1982 Apr 14;105(3):942–948. doi: 10.1016/0006-291x(82)91061-0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Sellers J. R., Eisenberg E., Adelstein R. S. Binding of gizzard smooth muscle myosin subfragment 1 to actin in the presence and absence of adenosine 5'-triphosphate. Biochemistry. 1983 Feb 1;22(3):530–535. doi: 10.1021/bi00272a002. [DOI] [PubMed] [Google Scholar]
- Ieyasu H., Takai Y., Kaibuchi K., Sawamura M., Nishizuka Y. A role of calcium-activated, phospholipid-dependent protein kinase in platelet-activating factor-induced serotonin release from rabbit platelets. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1701–1708. doi: 10.1016/s0006-291x(82)80107-1. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Isobe T., Ishioka N., Okuyama T. Structural relation of two S-100 proteins in bovine brain; subunit composition of S-100a protein. Eur J Biochem. 1981 Apr;115(3):469–474. doi: 10.1111/j.1432-1033.1981.tb06225.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Okuyama T. The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem. 1978 Sep 1;89(2):379–388. doi: 10.1111/j.1432-1033.1978.tb12539.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Okuyama T. The amino-acid sequence of the alpha subunit in bovine brain S-100a protein. Eur J Biochem. 1981 May;116(1):79–86. doi: 10.1111/j.1432-1033.1981.tb05303.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Tsugita A., Okuyama T. The amino acid sequence and the subunit structure of bovine brain S-100 protein (PAP I-b). J Neurochem. 1978 Apr;30(4):921–923. doi: 10.1111/j.1471-4159.1978.tb10805.x. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Hosey M. M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J Biol Chem. 1984 Jan 10;259(1):534–540. [PubMed] [Google Scholar]
- Katoh N., Wise B. C., Kuo J. F. Phosphorylation of cardiac troponin inhibitory subunit (troponin I) and tropomyosin-binding subunit (troponin T) by cardiac phospholipid-sensitive Ca2+-dependent protein kinase. Biochem J. 1983 Jan 1;209(1):189–195. doi: 10.1042/bj2090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. Ca2+-activated, phospholipid-dependent protein kinase catalyzes the phosphorylation of actin-binding proteins. Biochem Biophys Res Commun. 1984 Feb 14;118(3):736–742. doi: 10.1016/0006-291x(84)91456-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Ballester R., Rosen O. M. Purified rat brain calcium- and phospholipid-dependent protein kinase phosphorylates ribosomal protein S6. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6858–6862. doi: 10.1073/pnas.80.22.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S., Nishida E., Ohta Y., Sakai H. Isolation of low molecular weight actin-binding proteins from porcine brain. J Biochem. 1984 Feb;95(2):377–385. doi: 10.1093/oxfordjournals.jbchem.a134618. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Purification and characterization of a novel Ca2+-binding protein (CBP-18) from bovine brain. J Biol Chem. 1984 Feb 25;259(4):2047–2050. [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Mazzei G. J., Kuo J. F. Phosphorylation of skeletal-muscle troponin I and troponin T by phospholipid-sensitive Ca2+-dependent protein kinase and its inhibition by troponin C and tropomyosin. Biochem J. 1984 Mar 1;218(2):361–369. doi: 10.1042/bj2180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. R., Walsh M. P. Inhibition of the Ca2+- and phospholipid-dependent protein kinase by a novel Mr 17,000 Ca2+-binding protein. Biochem Biophys Res Commun. 1985 Jun 14;129(2):603–610. doi: 10.1016/0006-291x(85)90194-9. [DOI] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E., Maekawa S., Sakai H. Cofilin, a protein in porcine brain that binds to actin filaments and inhibits their interactions with myosin and tropomyosin. Biochemistry. 1984 Oct 23;23(22):5307–5313. doi: 10.1021/bi00317a032. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Phosphorylation of smooth muscle myosin: evidence for cooperativity between the myosin heads. Science. 1981 Sep 18;213(4514):1383–1385. doi: 10.1126/science.6455737. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Strang-Brown P., Walker P. L., Iida S. Ca2+ binding to calmodulin. Methods Enzymol. 1983;102:135–143. doi: 10.1016/s0076-6879(83)02014-5. [DOI] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Schwantke N., Le Peuch C. J. A protein kinase C inhibitory activity is present in rat brain homogenate. FEBS Lett. 1984 Nov 5;177(1):36–40. doi: 10.1016/0014-5793(84)80976-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Muranyi J., Ahmed M. Identification of a novel calcium binding protein from bovine brain. FEBS Lett. 1983 Nov 28;164(1):80–84. doi: 10.1016/0014-5793(83)80023-4. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Teo T. S., Wang T. H. Hysteretic substrate activation of bovine heart c-AMP phosphodiestrase. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1306–1311. doi: 10.1016/s0006-291x(72)80117-7. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. Vinculin, a cytoskeletal substrate of protein kinase C. J Biol Chem. 1983 Oct 10;258(19):11423–11426. [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]
- Zot H. G., Potter J. D. Purification of actin from cardiac muscle. Prep Biochem. 1981;11(4):381–395. doi: 10.1080/00327488108065530. [DOI] [PubMed] [Google Scholar]