Abstract
Objective
To determine the effectiveness of bilateral decompression combined with a unilateral transforaminal lumbar interbody fusion approach in centralizing a lordotic cage and preventing contralateral radiculopathy by ensuring equal foraminal elevation.
Methods
This is a retrospective cohort study based on clinical records and radiological data. Eighty-seven patients diagnosed with lumbar spinal stenosis at L3–S1 levels underwent bilateral decompression and transforaminal lumbar interbody fusion between 2017 and 2022. The procedures were performed through a posterior midline incision, followed by insertion of a lordotic cage to restore spinal alignment. Fluoroscopy and microscopy confirmed the precise placement of the cage. Clinical outcomes were assessed using visual analog scale and Oswestry disability index scores, with radiological evaluations through computed tomography and magnetic resonance imaging.
Results
Postoperative imaging demonstrated the centralization of the lordotic cage in both anteroposterior and lateral planes, ensuring equal foraminal elevation bilaterally. The visual analog scale and Oswestry disability index scores significantly improved at all follow-up intervals. The incidence of contralateral radiculopathy was minimized (1%) due to prophylactic decompression, and the foraminal area increased by more than 20%.
Conclusions
Bilateral decompression combined with a unilateral transforaminal lumbar interbody fusion approach enables effective stabilization and alignment of spinal segments. The central placement of the lordotic cage contributes to symmetrical foraminal elevation, reducing the risk of contralateral radiculopathy.
Keywords: Transforaminal lumbar interbody fusion, bilateral decompression, contralateral radiculopathy, lordotic cage, foraminal elevation, spinal stenosis
Introduction
Transforaminal lumbar interbody fusion (TLIF) is a surgical technique developed from posterior lumbar interbody fusion. Initially described by Blume and Rojas in the early 1980s, this method has become a widely used standard procedure in the treatment of lumbar degenerative diseases. 1 TLIF gained popularity in the late 1990s due to its biomechanical advantages. 2 The unilateral transforaminal approach in TLIF helps prevent excessive traction on the dural sac, nerve roots, and lumbar muscles. Moreover, it preserves the contralateral lamina and facet joints, thereby reducing the invasiveness of the surgery.
This technique minimizes mechanical alterations to the posterior column of the vertebra by preserving bony structures such as the pedicle and lamina. This preservation increases the stability of adjacent segments and helps prevent postoperative complications. 3 Long-term clinical follow-up assessments indicate that TLIF provides clinically satisfactory outcomes. However, TLIF is not entirely risk-free. Contralateral radiculopathy (symptom of nerve root compression) following unilateral TLIF is a common complication that can negatively impact the overall effectiveness of the surgery and patient satisfaction.4–6
The incidence of contralateral radiculopathy varies between 2% and 8.5%, depending on the TLIF technique used. 7 Several preoperative and postoperative factors contribute to the development of contralateral radiculopathy. Preoperative factors include contralateral intervertebral foramen stenosis and excessive segmental mobility. Postoperative factors such as screw malposition, newly developed nucleus pulposus herniation, hematoma formation, unilateral placement of the interbody fusion cage, and excessive segmental lordosis can lead to this complication.8–11
Although various studies have addressed contralateral radiculopathy following unilateral TLIF,9–11 the literature provides limited information regarding preventive measures for this complication. In this study, we aimed to evaluate the effectiveness of prophylactic contralateral foraminal and sublaminar decompression in preventing symptomatic contralateral radiculopathy when added to the classical TLIF technique.
Materials and methods
The study was approved by the institutional ethics committee (KSYLEAH-KAEK 2025/43) and conducted in accordance with the Declaration of Helsinki (1975), as revised in 2024. This retrospective observational study analyzed the radiological and clinical outcomes of 87 patients diagnosed with degenerative lumbar spinal stenosis who underwent bilateral decompression combined with unilateral TLIF between December 2019 and January 2023. All patient data were deidentified prior to analysis to ensure anonymity and confidentiality. As this was a retrospective study based on anonymized data, obtaining individual signed consent was not required according to institutional ethical guidelines. All patients underwent bilateral foraminotomy, decompression, and TLIF procedures. Follow-up assessments were conducted through clinical and radiological examinations. The absence of radiolucency around screws, evidence of bone bridging within the cage or between the anterior vertebral bodies on computed tomography (CT), and no movement between segments on flexion/extension radiographs were accepted as criteria for successful fusion. Patients were selected consecutively from institutional surgical records based on predefined inclusion and exclusion criteria.
The reporting of this study conforms to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines for observational research. 12
Patients were included in the study if they met the following criteria: (a) a confirmed diagnosis of degenerative lumbar spinal stenosis involving one or more of the L3–L4, L4–L5, or L5–S1 segments; (b) the presence of both axial back pain and radicular symptoms; (c) failure to respond to at least 6 months of conservative treatment, including physical therapy, medications, or epidural injections; and (d) availability of a minimum of 2 years of clinical and radiological follow-up data.
Patients were excluded from the study if they had a history of lumbar spine surgery or conservatively treated vertebral fractures, structural spinal deformities such as lumbar scoliosis, or a bone mineral density score below −3.5, indicating severe osteoporosis. These factors were considered to potentially confound surgical outcomes and compromise the homogeneity of the study population.
Surgical procedure
All surgical procedures were performed by the same surgeon. Posterior spinal elements were exposed through a midline longitudinal incision. Pedicle screws were inserted using the freehand technique. Before the final screws were placed, shorter and thinner temporary screws were used for preliminary stabilization. This allowed safe distraction during the insertion of the lordotic cage into the intervertebral space and maintained the stability of the final screws.
Decompression of the ipsilateral dural sac and nerve root was performed on the side of the major pathology, including ipsilateral facetectomy and partial hemilaminectomy. Osteophytes in the contralateral foramen and facet joint were removed, ensuring adequate decompression. In cases of bilateral radiculopathy or severe spinal stenosis (spinal canal cross-sectional area <50 mm2), the base of the spinous process was resected. Decompression was controlled under microscopy to confirm complete release of the nerve roots.
Meticulous discectomy was performed in the intervertebral disc space, ensuring complete removal of disc material beneath the contralateral nerve root. Endplates were cleared of disc residues and cartilage. Bone grafts harvested during laminectomy and facetectomy were cleaned of soft tissues and mixed with 1 g of powdered vancomycin. These grafts were compacted behind the anterior longitudinal ligament. Similarly prepared autografts were placed inside a fenestrated crescent-shaped titanium cage with a 5° lordotic angle. The cage was inserted perpendicular to the sagittal axis, ensuring balanced height in the coronal plane.
Fluoroscopy was used to confirm proper placement, and nerve roots were assessed via microscopy to ensure no compression. Neuromonitoring was performed in all cases to monitor changes in nerve conduction. Postoperative mobilization was initiated the day after surgery. Hemovac drainage was removed when the drainage was less than 25 cc (usually on the second or third day).
Patients were evaluated at preoperative, early postoperative, 3-month, 6-month, and 2-year intervals. Visual analog scale (VAS) and Oswestry disability index (ODI) scores were recorded. Additionally, surgical duration, blood loss, and length of hospital stay were documented.
Preoperative assessments included anteroposterior, lateral, and flexion–extension radiographs as well as magnetic resonance imaging and CT. Control CT scans were repeated at 6 and 24 months. Radiological assessments were conducted independently by two clinicians blinded to the clinical outcomes.
The degree of spondylolisthesis, disc height, and segmental lordosis were evaluated on lateral radiographs. The cross-sectional area of the spinal canal was measured on T2-weighted axial magnetic resonance images. Solid fusion was defined as the absence of more than 3° of movement on sagittal CT reconstructions and flexion–extension radiographs.
Statistical analysis
Descriptive statistics, including mean, standard deviation, minimum, maximum, frequency, and median values, were used. The Shapiro–Wilk test was employed to assess the normality of data distribution. As the data did not conform to normal distribution assumptions, nonparametric tests were preferred. The Mann–Whitney U and Kruskal–Wallis tests were used for independent quantitative variables, and the Wilcoxon signed-rank test for dependent quantitative data. Categorical data were analyzed using the chi-square and Fisher’s exact tests. A p-value of <0.05 was considered to indicate statistical significance. All analyses were conducted using Jamovi statistical software (version 2.5.4). No paired or unpaired Student’s t-tests were used in this analysis. The sample size of 87 patients was considered sufficient to provide stable descriptive results for observational comparison.
Results
Twelve patients were excluded due to insufficient postoperative follow-up data. The final analysis included 48 male and 39 female patients. The mean age was 47.8 ± 5.86 years for men and 52.9 ± 6.26 years for women. The mean follow-up duration was 41.83 ± 12 months. No significant differences were found between male and female patients in terms of demographic characteristics.
The mean angle difference on preoperative flexion–extension radiographs was 9.18° ± 1.26°. Thirteen patients (15%) had unilateral radiculopathy, 32 (37%) had bilateral radiculopathy, and 42 (48%) were diagnosed with severe stenosis. Regarding the levels of fusion, 20 patients (23%) underwent TLIF at the L3–L4 level, 41 (47%) at L4–L5 level, and 26 (30%) at L5–S1 level (Table 1).
Table 1.
Demographic and radiologic data.
| Variable | Mean ± SD |
|---|---|
| Number of patients (N) | 87 |
| Age (years) | 50.1 ± 6.52 |
| Follow-up duration (months) | 41.8 ± 12.6 |
| BMI (body mass index) | 25.3 ± 2.14 |
| Degree of spondylolisthesis (mm) | 3.8 ± 1.43 |
| Flexion–extension gap (degrees) | 9.45 ± 1.26 |
| Duration of operation (min) | 172.75 ± 19.8 |
| Estimated blood loss (mL) | 383.3 ± 41.6 |
| Hospital stay (days) | 4.25 ± 0.73 |
| Disc space height (preop/postop) (mm) | 6.75/11.12 |
| Segmental lordosis (preop/postop) (degrees) | 6.92/12.4 |
| Cross-sectional area (preop/postop) (mm²) | 56.3/88.1 |
| Foraminal area (preop/postop) (mm²) | 75.3/94.4 |
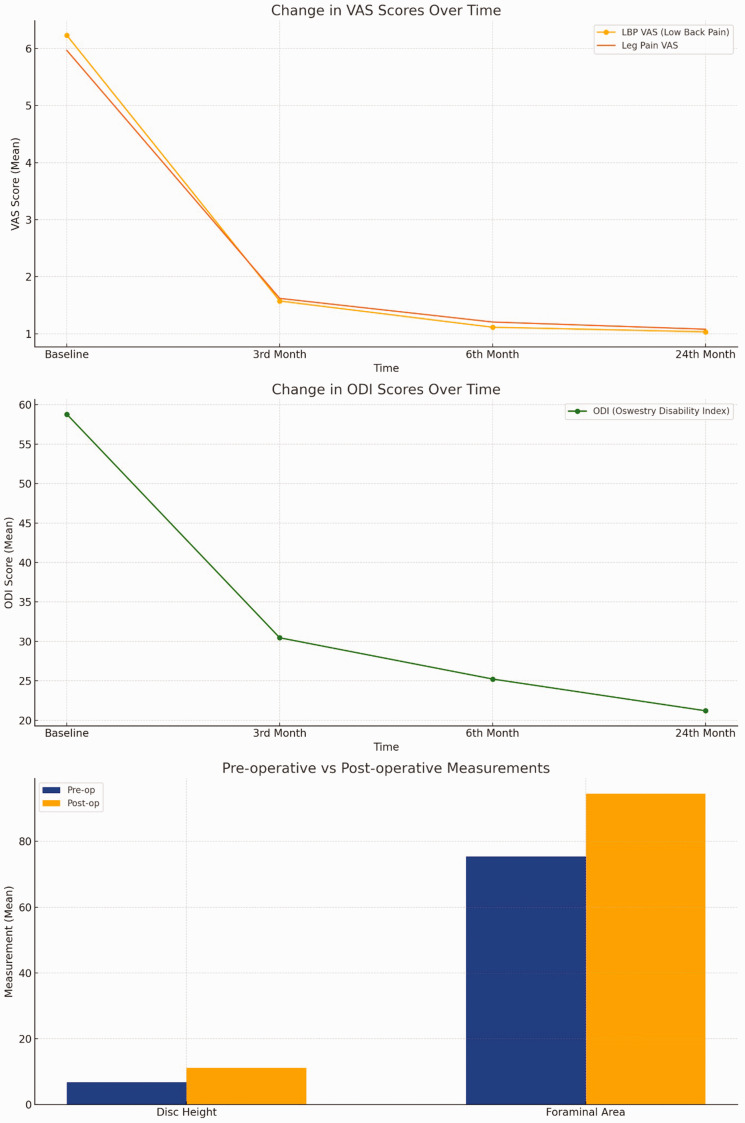
Analysis of VAS and ODI scores revealed significant improvements at 3 months, 6 months, and 2 years postoperatively compared with preoperative values (Table 1). The mean preoperative disc height was 6.76 ± 0.77 mm, which increased to 11.1 ± 1.08 mm postoperatively. Segmental lordosis improved from 6.92° ± 0.75° preoperatively to 12.4° ± 0.97° postoperatively. The cross-sectional area of the spinal canal increased from 56.3 ± 4.46 mm2 to 88.1 ± 4.17 mm2, and the foraminal area from 75.3 ± 2.29 mm2 to 94.4 ± 2.50 mm2 (Table 1, Figures 1 to 3).
Figure 1.
(a) Change in VAS scores over time: low back pain and leg pain VAS scores decreased over four time points, indicating pain reduction. (b) Change in ODI scores over time: ODI scores declined over time, reflecting improved patient function and (c) preoperative vs. postoperative measurements: disc height and foraminal area improved post-surgery, demonstrating treatment effectiveness. VAS: visual analog scale; ODI: Oswestry disability index.
Figure 3.
Centralization of the lordotic cage in both anteroposterior and lateral planes, ensuring equal elevation of both foramina. This positioning achieves optimal alignment and stabilization of the spinal segment.
Figure 2.
Pre- and postoperative intervertebral disc space. The postoperative image shows an increase in disc angle and distance, indicating successful decompression and stabilization of the spinal segment.
The mean operation duration was 173 ± 19.7 min, the estimated blood loss was 383.3 ± 41.6 mL, and the average length of hospital stay was 4.25 ± 0.73 days. The fusion rate was 95.4% (83 patients), the overall complication rate was 13.7% (12 patients), and the reoperation rate was 4.5% (4 patients). No revisions were required for the four patients with delayed or insufficient fusion.
Early revision surgery (within the first 3 days) was performed in three cases. The reasons for revision were postoperative hematoma in one patient, deep wound infection (treated with debridement and intravenous antibiotics) in another, and contralateral radiculopathy in the third patient. The patient with contralateral radiculopathy experienced dorsiflexion weakness in the contralateral big toe and severe pain and paresthesia in the lateral lower leg. Full recovery was achieved after revision surgery.
Adjacent segment disease developed in one patient, requiring instrumentation and fusion at the upper level 18 months postoperatively. Dural tears occurred during surgery in two patients but were repaired primarily, with no cerebrospinal fluid leakage reported during follow-up. A screw fracture was observed in one patient; however, as there were no symptoms and fusion was achieved, no additional intervention was required.
Two patients on anticoagulant therapy (aspirin) developed hematomas causing radiculopathy. One patient with severe pain underwent hematoma drainage surgery, with complete recovery achieved after all revision surgeries (Table 2).
Table 2.
Intraoperative and 2- year follow-up data.
| Complication/Outcome | Details |
|---|---|
| Duration of operation (min) | 172.75 |
| Estimated blood loss (mL) | 383.27 |
| Length of postoperative hospital stay (days) | 4.25 |
| Total complications (count/percentage) | 12/13.7% (revision rate, 3.4%) |
| Dural tear | 2 |
| Failed or displaced implants | 1 |
| Neurologic deficit | 1 (contralateral radiculopathy, revision required) |
| Postoperative hematoma | 2 (one case required revision surgery) |
| Deep wound infection | 1 (revision surgery performed) |
| Fusion failure | 4 |
| Adjacent segment disease | 1 (revision surgery at 18th month) |
The descriptive statistics of all cases demonstrated significant clinical and structural improvements following surgery. Notable improvements were observed in patients’ overall health status.
When analyzed by sex, both male and female patients benefited from pain relief, improved functional status, and structural recovery. Although sex appeared to influence specific outcomes such as blood loss, the overall recovery process was similar for both sexes.
Statistical analysis by surgical level showed that structural and clinical benefits were achieved across different levels, with similar recovery rates among them.
Analysis by stenosis severity revealed that patients with severe stenosis (anteroposterior diameter <10 mm) were older, had longer operation times, and experienced greater blood loss. Although these patients started with worse ODI and VAS scores, significant improvements were observed over time. Increases in disc height, segmental lordosis, and foraminal area further confirmed postoperative recovery. These results suggest that the severity of stenosis significantly impacts both clinical and structural outcomes.
The comparison of preoperative and postoperative measurements revealed significant differences in VAS and ODI scores (p < 0.001). Improvements in back and leg pain as well as functional status were noteworthy. Changes in disc height and lordosis further confirmed the effectiveness of the surgical outcomes.
Significant differences favoring male patients were found in terms of age, length of hospital stay, ODI scores, and 3-month VAS scores. Postoperative segmental lordosis was also significantly higher in men (p < 0.05). However, no significant differences were observed between sexes for operation time or blood loss.
The analysis by stenosis severity revealed significant differences in variables such as age, operation time, blood loss, back pain (VAS), and functional status (ODI) (p < 0.05). The severity of stenosis was found to affect the recovery process and treatment outcomes.
When comparing surgical levels, significant differences were found only in operation time and blood loss (p < 0.05), with higher values observed at the L5–S1 level. The follow-up duration showed marginal significance (p = 0.060). No other significant differences were detected among the groups.
No significant associations were found between sex, TLIF level, spondylolisthesis grade, unilateral radiculopathy, and complications (p > 0.05). However, severe stenosis (p = 0.009) and bilateral radiculopathy (p = 0.028) were significantly associated with complications.
Similarly, no significant associations were found between sex, TLIF level, spondylolisthesis grade, or radiculopathy (unilateral or bilateral) and the need for reoperation (p > 0.05). However, a significant relationship was found between severe stenosis and reoperation (p = 0.034).
Discussion
In the treatment of degenerative lumbar spinal stenosis, fusion combined with posterior instrumentation and decompression is considered a standard procedure. Successful fusion enhances functional recovery and spinal stability.13,14 Interbody fusion supports load transfer through the anterior column, increasing biomechanical stability postoperatively and providing a suitable foundation for successful fusion. 15 In our study, the fusion rate was 95.4% (83 patients), consistent with the literature.
TLIF is widely used due to its excellent surgical outcomes, lower complication rates, and high fusion rates compared with other fusion techniques.16,17 The unilateral approach in TLIF preserves anatomical structures on the contralateral side.18,19 However, complications such as ipsilateral nerve injury, dural tears, cerebrospinal fluid leakage, wound infection, fusion failure, and implant-related issues have been reported.4,20 In our study, patients with advanced spinal stenosis and bilateral radiculopathy were particularly prone to complications. The overall complication rate was 13.7% (12 patients), aligning with the literature, with a reoperation rate of 4.5% (4 patients). As reported in the literature, the presence of severe spinal stenosis and bilateral radiculopathy (indicating contralateral foraminal stenosis) was associated with higher complication and reoperation rates.
Contralateral radiculopathy is an important complication that spine surgeons must consider after TLIF, as its high incidence and unpredictability can negatively affect surgical outcomes. 10 The incidence of contralateral radiculopathy after minimally invasive surgery (MIS-TLIF) ranges from 1.9% to 6.9% for open TLIF and up to 8.5% for endoscopically assisted MIS-TLIF procedures.7,21 In our study, the incidence of contralateral radiculopathy was only 1%, owing to the implementation of prophylactic decompression.
Causes and prevention of contralateral radiculopathy
Contralateral radiculopathy can result from various factors, including contralateral foraminal stenosis, screw malposition, herniated nucleus pulposus, and hematoma. Among these, contralateral foraminal stenosis is reported as the most common cause.4,7,10,11,16 In our study, the mean contralateral foraminal area was 75.3 ± 2.29 mm2, which is close to the threshold value of 76 mm2 reported in the literature. 11 During revision surgery for the only case of contralateral radiculopathy, anterior osteophyte impingement was identified in the contralateral foramen. The low incidence of contralateral radiculopathy in our study is likely due to the more than 20% increase in foraminal area achieved through the procedure.
Compression applied to the pedicle screws may cause upward displacement of the superior facet, leading to foraminal narrowing. This, in turn, can result in nerve root impingement and symptom onset. 22 In our study, we routinely ensured facet joint release and adequate decompression during each procedure.
Impact of cage placement and segmental mobility
Proper placement of the interbody cage is critical in preventing contralateral nerve root impingement. If the contralateral disc material is not adequately removed, the cage may compress the nerve root during insertion.10,16 The placement of lordotic crescent and fenestrated cages perpendicular to the sagittal plane significantly increased the foraminal area, thereby reducing the risk of complications.
Curved cages have been reported to be more effective than flat cages in restoring lumbar segmental angles. 23 However, in cases where preoperative contralateral foramen narrowing is present, unilateral cage placement may be insufficient. Curved cages have been found to be superior to flat cages in restoring lumbar segmental angles. 23
Preoperative sagittal range of motion is particularly important in TLIF procedures at the L4–L5 level. Hwang et al. reported that symptomatic patients had a higher sagittal range of motion than asymptomatic patients. In our study, the mean preoperative flexion–extension angle was 9.18° ± 1.26°, exceeding the threshold value of 9.0° reported in the literature.7,24
Causes and management of foraminal narrowing
The superior articular process (SAP) should generally not extend beyond the inferior border of the vertebra. 22 Foraminal narrowing may occur due to superior or ventral subluxations of the inferior vertebra.25,26 The superior displacement of the SAP within the foramen may occur if the pedicle screws are excessively compressed or if excessive lordosis is achieved during cage placement. This issue is more pronounced in patients with preoperatively narrowed contralateral foramina. 10
Neutral and central positioning of the cage in the sagittal plane can prevent contralateral foraminal stenosis. 27 Excessive compression applied to the pedicle screws can cause upward movement of the SAP, leading to nerve root impingement. 22 In our study, we observed that controlled application of compression through posterior rods to increase lordosis helped reduce the risk of complications.
Limitations and future directions
This study has several limitations. First, the retrospective design inherently carries a risk of selection bias and limits the ability to establish causality. Second, the sample size, although sufficient for descriptive and comparative purposes, may not provide enough statistical power to detect subtle differences between subgroups. Third, the absence of a control group receiving unilateral decompression alone restricts our ability to directly compare surgical strategies. Furthermore, the follow-up duration, while adequate for fusion assessment, may not capture long-term complications such as adjacent segment disease or late-onset contralateral symptoms. Future studies with larger, multi-center cohorts and prospective, randomized controlled designs comparing different decompression strategies are needed to validate our findings.
Although bilateral decompression during unilateral TLIF may increase operative time and intraoperative blood loss, our experience suggests that this trade-off is justified by the reduced incidence of contralateral radiculopathy. We acknowledge that one patient in our series still developed symptoms due to severe contralateral foraminal stenosis, requiring revision surgery. This underscores the importance of careful preoperative imaging assessment. In our practice, prophylactic bilateral decompression is particularly considered in patients with bilateral foraminal narrowing, collapsed disc height, or severe facet hypertrophy, even if contralateral symptoms are not initially present.
To the best of our knowledge, this is one of the few retrospective studies focusing on the preventive role of bilateral decompression in TLIF procedures, specifically targeting contralateral foraminal stenosis. Our study highlights the importance of preoperative imaging and individualized decompression planning.
Conclusion
This study suggests that bilateral decompression combined with unilateral TLIF may offer effective neural decompression and favorable fusion rates, potentially comparable to more extensive procedures. The low incidence of contralateral radiculopathy (1%) observed in our cohort may be attributed to the prophylactic decompression strategy. However, the longer operative time and increased blood loss associated with this technique compared with those in minimally invasive alternatives remain important considerations. Further prospective studies are needed to confirm these findings.
Acknowledgments
None.
There is no conflict of interest in the study.
Funding: There was no funding for this study.
ORCID iDs: Bekir Eray Kilinc https://orcid.org/0000-0003-1229-9815
Ahmet Onur Akpolat https://orcid.org/0000-0001-7773-5476
Declaration
Authorship: All authors read and approved the final version of the manuscript.
Cem Sever: Methodology, investigation, writing–review & editing
Bekir Eray Kilinc: Data curation, investigation, visualization, writing–original draft review & editing, project administration
Oguzhan Cakir: Data curation, project administration
Hamit Caglayan Kahraman: Data analysis
Ahmet Onur Akpolat: Design, data collection
Ethical statement: The present study was approved by the institutional ethics committee (KSYLEAH-KAEK 2025/43).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Blume H, Rojas C. Unilateral lumbar interbody fusion (posterior approach) utilizing dowel graft. J Neurol Orthop Surg 1981; 2: 171–175. [Google Scholar]
- 2.Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 2004; 29: E65–E70. [DOI] [PubMed] [Google Scholar]
- 3.Wasinpongwanich K, Nopsopon T, Pongpirul K. Surgical treatments for lumbar spine diseases (TLIF vs. other surgical techniques): a systematic review and meta-analysis. Front Surg 2022; 9: 829469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt T, Shen FH, Shaffrey CI, et al. Contralateral radiculopathy after transforaminal lumbar interbody fusion. Eur Spine J 2007; 16: 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Kunder SL, Van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 2017; 17: 1712–1721. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Deng H, Long X, et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J 2016; 25: 1575–1580. [DOI] [PubMed] [Google Scholar]
- 7.Hwang SH, Park SW, Kim YB. Risk factors for symptomatic contralateral foraminal stenosis after unilateral transforaminal lumbar interbody fusion. World Neurosurg 2020; 133: e452–e458. [DOI] [PubMed] [Google Scholar]
- 8.Bai J, Zhang W, Zhang X, et al. A clinical investigation of contralateral neurological symptom after transforaminal lumbar interbody fusion (TLIF). Med Sci Monit 2015; 21: 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YL, Hu XD, Wang Y, et al. Contralateral radiculopathy after unilateral transforaminal lumbar interbody fusion: causes and prevention. J Int Med Res 2021; 49: 3000605211037475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang KM, Park SW, Kim YB, et al. Acute contralateral radiculopathy after unilateral transforaminal lumbar interbody fusion. J Korean Neurosurg Soc 2015; 58: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Liu ZY, Zhang LM, et al. Risk factor of contralateral radiculopathy following microendoscopy-assisted minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2018; 27: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M; STROBE Initiative et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Kepler CK, Vaccaro AR, Hilibrand AS, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976) 2014; 39: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 14.Resnick DK, Watters I, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014; 21: 54–61. [DOI] [PubMed] [Google Scholar]
- 15.Mummaneni PV, Dhall SS, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 2014; 21: 67–74. [DOI] [PubMed] [Google Scholar]
- 16.Hackenberg L, Halm H, Bullmann V, et al. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J 2005; 14: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Kim YB, Park SW, et al. Comparison of transforaminal lumbar interbody fusion with direct lumbar interbody fusion: clinical and radiological results. J Korean Neurosurg Soc 2014; 56: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houten JK, Post NH, Dryer JW, et al. Clinical and radiographically/neuroimaging documented outcome in transforaminal lumbar interbody fusion. Neurosurg Focus 2006; 20: E8. [DOI] [PubMed] [Google Scholar]
- 19.Potter BK, Freedman BA, Verwiebe EG, et al. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech 2005; 18: 337–346. [DOI] [PubMed] [Google Scholar]
- 20.Hosono N, Namekata M, Makino T, et al. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine 2008; 9: 403–407. [DOI] [PubMed] [Google Scholar]
- 21.Schwender JD, Holly LT, Rouben DP, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech 2005; 18: S1–S6. [DOI] [PubMed] [Google Scholar]
- 22.Hu HT, Ren L, Sun XZ, et al. Contralateral radiculopathy after transforaminal lumbar interbody fusion in the treatment of lumbar degenerative diseases: a case series. Medicine (Baltimore) 2018; 97: e0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JT, Shin MH, Lee HJ, et al. Restoration of lumbopelvic sagittal alignment and its maintenance following transforaminal lumbar interbody fusion (TLIF): comparison between straight type versus curvilinear type cage. Eur Spine J 2015; 24: 2588–2596. [DOI] [PubMed] [Google Scholar]
- 24.Wei F, Wang J, Zou J, et al. Effect of lumbar angular motion on central canal diameter: positional MRI study in 491 cases. Chin Med J (Engl) 2010; 123: 1422–1425. [PubMed] [Google Scholar]
- 25.Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am 1995; 77: 32–38. [PubMed] [Google Scholar]
- 26.Hey HW, Hee HT. Lumbar degenerative spinal deformity: surgical options of PLIF, TLIF and MI-TLIF. Indian J Orthop 2010; 44: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho PG, Park SH, Kim KN, et al. A morphometric analysis of contralateral neural foramen in TLIF. Eur Spine J 2015; 24: 783–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.