Abstract
The increasing occurrence of pharmaceutical compounds in aquatic environments poses significant ecological and public health challenges due to the persistence and bioaccumulation potential. While Aspergillus flavus and Cunninghamella elegans have demonstrated efficacy in removing heavy metals and dyes, their potential for pharmaceutical bioremediation remains unexplored. This study investigated these fungi capacity to degrade three persistent fluorinated pharmaceutical–atorvastatin (ATO), ciprofloxacin (CIP), and fluoxetine (FLX), through an innovative biofilm-based approach. Using polyurethane foam (PUF) as a carrier in two different configurations (fixed foam (PUF-F) and moving foam (PUF-M)), the performance of both fungal species was evaluated. C. elegans biofilms on PUF-F demonstrated high removal efficiencies of 97.3% for ATO and 97.7% for CIP, while A. flavus achieved 92.4% FLX reduction in the same system. Notably, the biofilm-based systems consistently outperformed carrier-free cultures, confirming the advantage of immobilized fungal growth. Kinetic analysis indicated pseudo-first-order degradation with remarkably short half-lives (1.0–1.7 days), surpassing reported values for white-rot fungi. Although adsorption contributed minimally (< 10%) to overall removal, species-specific biofilm characteristics emerged as key factors: C. elegans exhibited superior surface hydrophobicity (0.76) and stress resistance, whereas A. flavus developed denser extracellular matrices. These findings highlight the potential of tailored fungal biofilm systems for efficient removal of recalcitrant pharmaceutical, presenting a promising biological solution for wastewater treatment applications. The study provides critical insights into species-specific degradation mechanisms and operational parameters that could guide the development of scalable fungal bioremediation technologies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10532-025-10182-w.
Keywords: Fungal biofilm, Fungal biodegradation, Bioremediation, Extracellular Enzymes, Polyurethane Foam
Introduction
Pharmaceutically active compounds (PhAC) have been identified as emerging pollutants, garnering international attention on their environmental fate and impact. These compounds have been detected in wastewater (WW) effluents and receiving rivers, as conventional WW treatments are not optimized for PhAC removal, leading to their persistence in effluents (Bijlsma et al. 2021; Gupta et al. 2024). To address this, alternative technologies such as membrane bioreactor (Werkneh 2022) and activated carbon adsorption (Rueda-Márquez et al. 2021) have been used. Also, chemical techniques like ozonation (Tang et al. 2020) and oxidation (Rayaroth et al. 2016) have been applied. Alternatively, biological methods have been employed either independently or in conjunction with other processes such as ozonation. Membrane bioreactors have a higher operational cost than integrating biological treatment (Lee et al. 2012). Furthermore, biological treatments are more energy-efficient and generate less waste than membrane bioreactors, improving their cost-efficiency and sustainability (Liang et al. 2019; Carneiro et al. 2020). Fungi possess significant potential to break down PhAC and mitigate their environmental risks through various mechanisms, including adsorption, intracellular uptake, and enzymatic breakdown by both intracellular and extracellular enzymes (Deshmukh et al. 2016). White-rot fungi (WRF) like Trametes versicolor, Phanerochaete chrysosporium, and Ganoderma lucidum have been investigated for PhAC removal (Cruz del Álamo et al. 2020).
Fluorinated PhAC pose unique challenges for biodegradation due to the exceptional stability of the carbon–fluorine (C–F) bond, one of the strongest covalent bonds (Han et al. 2021). The electronegativity of fluorine and the bond’s low polarizability render these compounds resistant to removal by the conventional WW treatment processes, leading to environmental persistence (Zhang and Liang 2023). WRF have been widely studied for PhAC degradation, their efficiency varies significantly for different fluorinated compounds, likely due to limited enzymatic specificity toward C–F bonds (Kózka et al. 2020; Cruz del Álamo et al. 2020). Among the fluorinated PhAC, three commonly prescribed representatives include fluoxetine (FLX), ciprofloxacin (CIP), and atorvastatin (ATO), which represent an antidepressant, an antibiotic, and an antilipidemic agent, respectively. These three PhAC have been detected in WW effluent worldwide (Halling-Sorensen et al. 2000; Kwon and Armbrust 2006; Ottmar et al. 2012). Several WRFs exhibit species-dependent degradation efficiencies, with demonstrated removal efficiencies of 70–95% for CIP by T. versicolor (Prieto et al. 2011) and Pleurotus ostreatus (Singh et al. 2017), 22–70% ATO by P. ostreatus and T. versicolor (Rodríguez-Rodríguez et al. 2012; Kózka et al. 2023), and 23–84.9% for FLX by P. ostreatus, P. chrysosporium, and Bjerkandera Adusta (Rodarte-Morales et al. 2011; Kózka et al. 2023) Notably, G. lucidum showed negligible CIP degradation (Chakraborty and Abraham 2017).
C. elegans has been used as an in vitro model of PhAC biotransformation (Palmer-Brown et al. 2019), and high biodegradation capacity for heavy metal, chemical and dyes was shown (Soboń et al. 2016). Meanwhile, A. flavus, with its versatile enzymatic potential (Chang and Ehrlich 2010), has a wide range of biotechnology applications. For example, non-aflatoxigenic strains have been extensively used to reduce aflatoxin production in crops (Dorner 2004), and enzyme production (Chellapandi 2010). Also, A. flavus demonstrated promising ability to remove pollutants like reactive blue dye (Mousavi et al. 2016), acid brown dye (Ghosh et al. 2018) and malachite green (Ali et al. 2009) as well as other heavy metals and harmful substances (Kapoor et al. 1999; Adegunlola et al. 2010; Sangeetha Devi et al. 2015; Mukherjee 2016; Ghosh et al. 2016; Kumar and Dwivedi 2019a, b). Polyurethane foam (PUF) is considered as an ideal carrier for biofilm formation due to its open structure, high porosity, low density, large specific area, non-biodegradability, and physical strength (Zhao et al. 2019). Several studies have confirmed its suitability as a support matrix for biofilm development, along with associated enzymatic activities (Gutiérrez-Acosta et al. 2012; Chu et al. 2014; Beneš et al. 2020).
While lignin-dependent WRF like T. versicolor have been extensively studied for PhAC degradation, their reliance on ligninolytic enzymes limits efficacy in lignin-deficient environments such as municipal WW. In contrast, A. flavus and C. elegans utilize versatile enzymatic capacity that enable efficient breakdown of PhAC independent of lignin, making them adaptable to diverse environments and further scalability. Their metabolic flexibility, combined with biofilm formation ability, addresses two critical gaps in fungal bioremediation: (1) the need for systems operational in low-lignin environments, and (2) enhanced substrate-enzyme contact via immobilization. This study is the first to compare the biofilm-based and planktonic modes of A. flavus and C. elegans for the removal of fluorinated PhAC (ATO, CIP, FLX). This work hypothesizes that immobilized biofilms enhance the degradation of fluorinated PhAC via prolonged enzymatic activity and adsorption, offering a viable, lignin-independent alternative to traditional WRF-based systems and expanding the applicability of non-ligninolytic fungi in targeted PhAC remediation.
Materials and methods
Materials
PhAC standards (> 98% purity) of atorvastatin, ciprofloxacin, and fluoxetine were obtained from Sigma-Aldrich (Darmstadt, Germany). All culture media (potato dextrose agar/broth, Sabouraud dextrose agar/broth) and solvents (acetonitrile, methanol, ethyl acetate, trifluoroacetic acid) were also obtained from Sigma-Aldrich. Deionized water was prepared using a MilliQ® purification system. C. elegans DSM 1908 was maintained on Sabouraud dextrose agar at 28 ℃ for 5 days, spores from 5-day-old cultures were harvested using 100 mL of 0.85% sterile saline solution. A. flavus link S44-1, obtained from the Department of Bioprocess Technology (Universiti Putra Malaysia), was cultured on potato dextrose agar under identical conditions, with spore suspension prepared in 0.1% Tween 80 solution. ATO and FLX stock solutions were prepared separately by adding 100 mg of each drug to 10 mL of N, N dimethyl formamide yielding a final concentration of 10 mg/mL. CIP was prepared by adding 100 mg of CIP to 10 mL of HCl solution (0.1 N) yielding the same concentration.
Culture conditions and optimization
The fungal cultures were grown in 250-mL Erlenmeyer flasks containing 100 mL of sterile broth (Sabouraud dextrose for C. elegans; potato dextrose for A. flavus) inoculated with 106 spores/mL. PUF carriers were sterilized by overnight immersion in 70% ethanol, followed by thorough washing with deionized water and drying to constant weight. Two PUF configurations were employed: 1 cm3 cubes (PUF-M) with ten pieces per flask, and rectangular strips (1 × 1 × 18.5 cm, PUF-F) placed along the flask base. Fungal-carrier attachment was evaluated after 7 days of incubation at 28 ℃ with 150 rpm agitation. Optimal conditions were determined by testing agitation speeds (100–250 rpm) and inoculum densities (104–10⁷ spores/mL). Three biological replicates were performed in all experiments to ensure statistical reliability.
PhAC removal
In PhAC removal experiments, each fungal strain was inoculated at a concentration of 10⁶ spores/mL (10% v/v) in 90 mL of appropriate medium within 250-mL Erlenmeyer flasks. Three experimental conditions were established: (1) carrier-free control, (2) PUF-M (1 cm3 cubes), and (3) PUF-F (1 × 1 × 18.5 cm strips). Following 48 h of pre-culture at 28 ℃ with 150 rpm agitation, 100 µL of sterile drug stock solutions containing ATO, CIP, and FLX were added (10 µg/mL final concentration). PhAC concentrations were quantified by HPLC at 24, 72, 120, and 168 h to determine degradation kinetics. To differentiate adsorption from biodegradation, fungal cultures were grown for 48 h, heat-inactivated by autoclaving (20 min at 121 ℃), and subsequently supplemented with PhAC stock solution. Three separate biological replicates were operated for each shaking flask setting. Parallel biotic controls (without PhAC) were maintained under identical conditions to assess analytical interference (matrix effect). A separate biomass-free experiment was conducted under the same conditions (7 days, 28 ℃ and 150 rpm) to serve as abiotic control and PhAC concentrations were normalized against abiotic controls to isolate biological degradation.
Removal kinetics and initial concentration effect
To evaluate the initial drug concentration impact on biodegradation performance, an experiment with varying PhAC loadings was conducted. After establishing 48 h fungal cultures, the cultures were spiked with ATO, CIP, and FLX to achieve three distinct initial concentrations (5, 30, and 60 µg/mL) while maintaining identical incubation conditions (28 ℃, 150 rpm agitation). The degradation data were fitted to multiple kinetic models to determine the most appropriate removal mechanism. Among these, the pseudo-first-order model provided the best fit (highest R2 value) for all tested PhAC, as described by the equation below:
| 1 |
where Cₜ is PhAC concentration at time t (µg/mL), C₀ is the initial concentration (µg/mL), k is pseudo-first-order rate constant (h⁻1), and t is incubation time (h). The corresponding half-life (t₁/₂) was calculated as
| 2 |
Fungal biofilm characterization
The surface characteristics and morphology of PUF were analysed using a microscope at 40 × magnification, with the pore diameter was calculated as the average of 10 measurements using Motic® Images Plus software (Version 2.0). Fungal cell hydrophobicity was determined through the microbial adhesion to hydrocarbons (MATH) method to calculate hydrophobicity percentage using the equation below:
| 3 |
The PUF-biofilm attachment was assessed at 12 h intervals, and after a 48 h period, adhesion strength was examined under challenging conditions involving sonication (30 W/L) and mechanical stirring (1500 rpm) for 30 min in 4 M NaOH solution at 30℃. To ensure reliability, the experiment was performed in triplicate. The extracellular matrix (ECM) from 48 h old biofilms composition was analysed for carbohydrates (phenol–sulfuric acid assay) and proteins (Bradford assay) contents. Biofilm morphology was examined using scanning electron microscopy (SEM) after glutaraldehyde fixation and gold coating under a JEOL IT 100 SEM.
HPLC method
Centrifugation was performed at 2500 × g for 15 min at 37 ℃, then the supernatant was subjected to extraction using 50 mL ethyl acetate. The organic extract was dried using sodium sulphate, and evaporated to dryness at 72 ℃. The vaporization residue was dissolved in 3 mL of methanol and transferred into 2-mL test vial. PhAC were quantified using reversed-phase high-performance liquid chromatography (RP-HPLC) (Sykum®, S 500, Germany) equipped with a C18 analytical column (250 mm × 4.6 mm, 5 μm particle size) and ultraviolet detector, and data were acquired and processed using Clarity software. The mobile phase consisted of 0.1% trifluoroacetic acid (solution A) and acetonitrile (solution B) with a gradient elution program: initial 10% B (0 min), increased to 30% B (12.5 min), held at 30% B (50 min), raised to 60% B (60 min), then returned to 10% B (62.5 min) and maintained until 80 min. The flow rate was maintained at 0.24 mL/min with the column temperature set at 25 ℃. Compound identification was achieved by comparing retention times with standards, while quantification was performed using peak area measurements against calibration curves with linearity range of 1–15 μg/mL. The LOD, LOQ, and other statistical data were presented in Supplementary Material S1.
Statistical analysis
All statistical analyses were conducted using OriginPro® software (v10.0.5.157, OriginLab Corporation). PhAC removal efficiencies were expressed as mean ± standard deviation. Significant differences between treatment groups were determined using t-test (p < 0.05), with all experiments performed in triplicate to ensure analytical reliability.
Result and discussion
PhAC removal
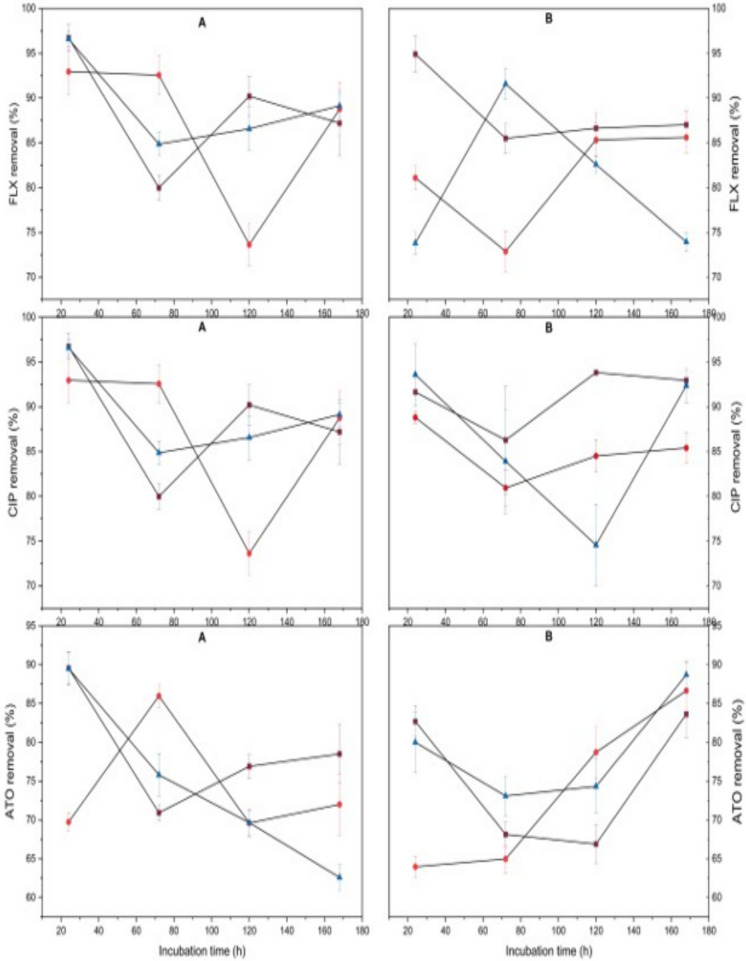
Both fungal species demonstrated effective removal of all three target compounds, albeit with distinct patterns. A. flavus achieved 91.6% FLX removal in PUF-F systems within 24 h, while C. elegans showed comparable performance (89.1%) in carrier-free conditions. CIP removal was exceptionally efficient, with both fungi achieving more than 95% removal within the first 24 h in carrier-free systems. For ATO, C. elegans in PUF-M configuration showed progressive removal, reaching 96.3% by 168 h, compared to A. flavus, which attained 89.5% in PUF-F at 24 h as shown in Fig. 1.
Fig. 1.
The removal of FLX, CIP and ATO using A. flavus (A) and C. elegans (B) in carrier-free ( ), PUF-M (
), PUF-M ( )and PUF-F (
)and PUF-F ( ) culture within 168 h incubation
) culture within 168 h incubation
Statistical analysis of PhAC removal revealed distinct patterns. For FLX, ANOVA analysis showed no significant differences between A. flavus and C. elegans (p > 0.05) or across carrier configurations (p > 0.05). In contrast, CIP and ATO removal differed significantly between species (p < 0.05), with C. elegans demonstrating superior degradation. Carrier configurations showed no significant effect (p > 0.05).
The observed removal efficiencies surpassed those reported in the literature for fungal treatment of PhAC. Previous studies with WRF showed notably lower efficiencies: P. ostreatus removed only 24% FLX after 96 h (Kózka et al. 2020), while T. versicolor required 7 days for 90% CIP removal (Prieto et al. 2011). In this study, ATO removal efficiency (85–96%) were particularly superior to the 60% reported for P. chrysosporium (Kózka et al. 2023) and 48–70% for T. versicolor in sewage sludge (Cruz-Morató et al. 2013).
The removal process followed a characteristic triphasic pattern: rapid initial removal (0–24 h), followed by a plateau/decline phase (24–72 h), and subsequent recovery. This suggests an initial adsorption phase followed by gradual biodegradation, consistent with similar patterns reported for FLX in aerobic granular sludge (Moreira et al. 2015) and CIP in agricultural soils (Rodríguez-López et al. 2022). In the current study, adsorption accounted for < 10% of total removal, following the order: CIP (5–9.7%) > FLX (1.8–8.6%) > ATO (2.2–4.9%), consistent with their respective lipophilicities (Wunder et al. 2011).
The observed differential degradation performance between the two fungi can be mechanistically explained by their distinct enzymatic capabilities (Khan 2025). While specific enzyme activities were not quantified in this study, previous genomic and proteomic analyses have established that C. elegans expresses a robust cytochrome P450 system capable of oxidative processes, along with phase II conjugation enzymes that facilitate xenobiotic detoxification (Palmer-Brown et al. 2019). Complementary studies (unpublished data) confirmed minimal laccase activity in both species under similar conditions, suggesting alternative enzymatic pathways dominate fluorinated PhAC degradation. For A. flavus, its well-characterized range of hydrolytic enzymes, including proteases and esterases (Cleveland et al. 2009), contribute to its superior performance against the ATO’s lactone ring structure. These enzymatic differences combined with species-specific biofilm architecture (later-quantified biofilm architectural traits Sect. 3.3) explain the observed variations in the removal of PhAC.
In the current study, the adsorption of PhAC onto fungal biofilms was governed by both biofilm surface hydrophobicity and compound lipophilicity, which collectively mediate electrostatic interactions at the biofilm-PhAC interface. Comparative analysis revealed that the C. elegans exhibited a greater adsorption capacity than A. flavus, correlating with its higher measured hydrophobicity (0.76 vs 0.60). Among the tested PhAC, CIP demonstrated the strongest adsorption affinity (5–9.7%) as shown in Fig. 2, due to its pronounced lipophilic character, consistent with prior reports of its superior adsorption to microbial biofilms (Wunder et al. 2011). FLX showed intermediate adsorption (1.8–6.1% for A. flavus; 6.3–8.6% for C. elegans, while ATO displayed the lowest adsorption (2.2–4.9% for both species), in agreement with its hydrophilic nature. However, adsorption contributions remained minor (< 10% of total removal) across all systems, aligning with previous studies emphasizing biodegradation dominance in fungal treatment (Manasfi et al. 2020). These findings demonstrate that while physicochemical properties influence initial PhAC removal, enzymatic degradation is the primary removal mechanism in fungal systems (Velázquez and Nacheva 2017).
Fig. 2.
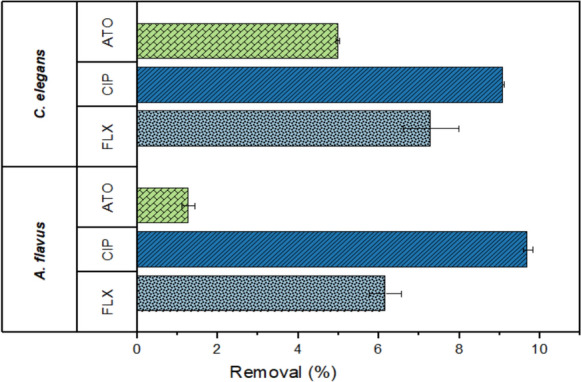
The adsorption of FLX, CIP and ATO on A. flavus and C. elegans biofilms after 168 h incubation in Erlenmeyer flask
The three PhAC exhibited distinct effects on fungal growth and biofilm development as illustrated in Fig. 3, with A. flavus showing greater sensitivity than C. elegans. While the biomass of A. flavus declined by 27.4% (PUF-F) and 41.1% (PUF-M), it increased by 20.3% in carrier-free conditions. C. elegans demonstrated more resilience, with biomass reductions of 4.9% (PUF-F), 14.1% (carrier-free), and 28.8% (PUF-M). ANOVA revealed no significant difference in adsorption between the fungi and the culture setting (p > 0.05). However, there was a significant difference between the three PhAC adsorptions (p < 0.05).
Fig. 3.
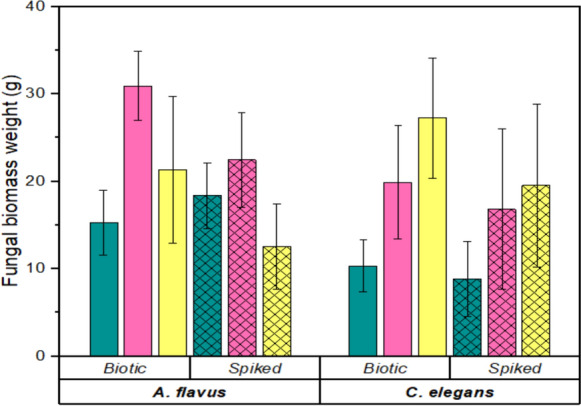
The impacts of FLX, CIP and ATO on A. flavus and C. elegans biomass in carrier-free ( ), PUF-M (
), PUF-M ( ) and PUF-F (
) and PUF-F ( ) after 168 h incubation
) after 168 h incubation
A. flavus biofilm showed greater disruption than C. elegans as shown in Fig. 3. Some PhAC have antifungal activity, for example FLX had activity against A. flavus (Lass-Flörl et al. 2001). C. elegans demonstrated relative resistance, potentially due to its natural production of statin-like compounds (Balraj et al. 2018). These PhAC affect biofilm formation by interfering with quorum sensing mechanisms, as evidenced by the promotion of planktonic growth in carrier-free systems — a similar phenomenon observed with CIP in A. fumigatus cultures (Sass et al. 2022). These results highlight the importance of species-specific responses when considering fungal bioremediation applications, particularly for systems targeting multiple PhAC contaminants.
PhAC initial concentration effect and removal kinetics
The initial concentration of PhAC significantly influenced removal efficiency—higher concentrations led to reduced degradation efficiency. In A. flavus cultures, increasing the concentration from 5 to 60 µg/L decreased removal efficiencies from 81 to 57% (FLX), 87% to 73% (CIP), and 78% to 64% (ATO) after 168 h. A similar trend was observed for C. elegans, with efficiencies dropping from 98 to 84% (CIP) and 93% to 79% (ATO) at higher concentrations as illustrated in Fig. 4.
Fig. 4.

The impact of initial concentration 5, 30 and 60 µg/mL of FLX ( ), CIP (
), CIP ( ) and ATO (
) and ATO ( ) removal in A. flavus and C. elegans cultures
) removal in A. flavus and C. elegans cultures
The observed concentration-dependent degradation patterns align with established enzymatic saturation kinetics in microbial systems (Chakraborty and Abraham 2017), mirroring reports of laccase inhibition in T. versicolor at CIP > 20 µg/L (Carneiro et al. 2020). At higher PhAC concentrations, the reduction in removal efficiency reflects the competitive nature of enzymes active sites (Palmer-Brown et al. 2019), and the potential substrate toxicity effects on fungi at supra-environmental levels (≥ 50 µg/L). The retained efficiency at lower concentrations (5–10 µg/L) matches typical environmental detections (Bijlsma et al. 2021). The plateau effect suggests these fungal systems are particularly suited for polishing treatments, where influent PhAC concentrations have been pre-reduced by conventional processes.
The degradation of FLX, CIP, and ATO by A. flavus and C. elegans adhered to pseudo-first-order kinetics (Supplementary Material S3–S5), as evidenced by high linear regression coefficients (R2 > 0.95) (Table 1).
Table 1.
Comparative kinetic parameters for FLX, CIP and ATO removals
| Microorganism | Drug | Removal (%) (time in days) | Linear regression (R2) | Rate constant (k, day⁻1) | Half-life (t₁/₂, days) | Reference |
|---|---|---|---|---|---|---|
| P. ostreatus | FLX | 24% (4) | 0.92 | 0.03 | 23.1 | Kózka et al. (2020) |
| P. ostreatus | FLX | > 80% (4) | 0.95 | 0.12 | 5.8 | Kózka et al. (2023) |
| P. chrysosporium | ATO | 22% (1 h) | 0.89 | 0.08 | 8.7 | Kózka et al. (2023) |
| T. versicolor | CIP | 90% (7) | 0.97 | 0.33 | 2.1 | Prieto et al. (2011) |
| T. harzianum | CIP | 81% (7) | 0.91 | 0.25 | 2.8 | Manasfi et al. (2020) |
| B. adusta | FLX | 23–46% (14) | 0.88 | 0.04 | 17.3 | Rodarte-Morales et al. (2011) |
| A. flavus | FLX | 92.4 ± 4.3% | 0.99 | 0.41 ± 0.02 | 1.7 ± 0.1 | Current study |
| C. elegans | CIP | 99.1 ± 0.8% | 0.99 | 0.66 ± 0.03 | 1.0 ± 0.1 | Current study |
| C. elegans | ATO | 96.3 ± 2.9% | 0.998 | 0.43 ± 0.02 | 1.6 ± 0.1 | Current study |
Pseudo first-order is commonly observed in microbial systems when substrate concentrations are negligible relative to biomass (Marco-Urrea et al. 2009). This model assumes a constant enzyme concentration. Alternative models (e.g., zero-order or second order) were also evaluated but were rejected due to poorer fit (R2 < 0.85), reinforcing the dominance of first-order degradation mechanisms under these conditions. Similar kinetics have been reported for CIP removal (Carneiro et al. 2020), and FLX degradation (Ericson 2010), suggesting fungal degradation often follows this pattern at environmentally relevant concentrations. The short t₁/₂ values (1.0–1.7 days) indicate these fungi could achieve > 90% PhAC removal within 5 days, This high efficiency, particularly at low concentrations (5–10 µg/L), positions A. flavus and C. elegans as possible model for polishing treatments in WW plants (Bijlsma et al. 2021). The rate constants for A. flavus (0.41 day⁻1 for FLX) and C. elegans (0.66 day⁻1 for CIP) surpassed those of other fungi (e.g., B. adusta: 0.04 day⁻1 for FLX) by an order of magnitude (Table 1). This disparity is attributable to differences in strain-specific enzymatic efficiencies.
Fungal biofilm characteristics
Both fungal species showed distinct biofilm formation characteristics in different carrier systems. A. flavus achieved higher biomass yields (14.6 ± 2 g in PUF-F vs. 11.7 ± 3.1 g for C. elegans), while C. elegans demonstrated superior surface adhesion (hydrophobicity score 0.76 ± 0.1 vs. 0.6 ± 0.2 for A. flavus). PUF-F configuration promoted better biofilm formation than PUF-M for both species (Fig. 5–1), due to reduced mechanical disturbance and enhanced surface area for attachment, consistent with previous reports (Amadio et al. 2013). A similar advantage was reported for the fixed carrier in C. elegans culture (Amadio et al. 2013), where an optimal biofilm formation was obtained in a wall-attached stainless-steel coil. Moreover, (de Melo Souza et al. 2016) utilized the same technique to develop fungal biofilm in a bioreactor setting.
Fig. 5:
5–1: The fungal biomass growth range within 7 days of incubation. A. flavus ( ), C. elegans (
), C. elegans ( ). 5–2: Biomass weight (g) variation with different inoculum concentrations. A. flavus and C. elegans in PUF-F and PUF-M. 5–3: Different shaking speed effect on biofilm formation for A. flavus and C. elegans in PUF-F and PUF-M configuration. 5–4: The change in biomass weight at every 12 h interval for 48 h using 106 spore/mL, A: C. elegans in PUF-M. B: C. elegans in PUF-F. C: A. flavus in PUF-M. D: A. flavus in PUF-F. 5–5: The (
). 5–2: Biomass weight (g) variation with different inoculum concentrations. A. flavus and C. elegans in PUF-F and PUF-M. 5–3: Different shaking speed effect on biofilm formation for A. flavus and C. elegans in PUF-F and PUF-M configuration. 5–4: The change in biomass weight at every 12 h interval for 48 h using 106 spore/mL, A: C. elegans in PUF-M. B: C. elegans in PUF-F. C: A. flavus in PUF-M. D: A. flavus in PUF-F. 5–5: The ( ) associated and (
) associated and ( ) dissociated biomass percentage for A. flavus and C. elegans after 30 min under stressful condition in 4 M sodium hydroxide solution. A: C. elegans after sonication. B: C. elegans after mechanical shaking. C. A. flavus after sonication. D: A. flavus after mechanical shaking
) dissociated biomass percentage for A. flavus and C. elegans after 30 min under stressful condition in 4 M sodium hydroxide solution. A: C. elegans after sonication. B: C. elegans after mechanical shaking. C. A. flavus after sonication. D: A. flavus after mechanical shaking
Inoculum concentration significantly affected biofilm development, with 106 spores/mL yielding optimal results for both species (Fig. 5–2). Lower concentrations (104–105 spores/mL) produced sparse biofilms, this was evident by the presence of small, isolated clusters of mycelium nodes, due to low initial mycelium nodulation. However, a higher spore concentration (107 spore/mL), resulted in a low biomass growth rate, due to limited oxygenation and carbon sources during excessive mycelium nodulation. (Talukdar et al. 2020) attributed the decline in A. flavus efficiency in dense inoculum to the covering of binding sites caused by excessive interlinking in the fungal mycelium. Similar findings have also been reported (Selim et al. 2021).
Optimal growth conditions were established at 150 rpm agitation, balancing oxygenation, and shear stress (Fig. 5–3). At low shaking speed, inadequate mixing and oxygen transfer may occur, leading to slower growth and lower productivity. Meanwhile, high shaking speed could lead to excessive shear stress on the fungal cells, which can negatively impact their viability and biofilm formation capacity. (Elhussiny et al. 2023) reported a 56% increase in A. flavus biomass, when the speed was increased from 100 to 200 rpm. The fluctuation of C. elegans biomass was more intense, due to the vulnerability and limited adaptability of the strain under stress conditions. Previous studies found that the optimum shaking speed for C. elegans was 150–200 rpm (Amadio et al. 2013; Sponchiado et al. 2019; Song et al. 2023). Species-specific optimization is vital, where adhesion properties, carrier design, and cultivation parameters collectively determine system performance.
Biofilm growth dynamics revealed significant differences between species and carrier types. A. flavus showed faster colonization rates (0.24 g/12 h in PUF-F) compared to C. elegans (0.184 g/12 h), with both species exhibiting approximately 50% growth reduction in PUF-M systems (Fig. 5–4). Specific factors that could affect the attachment including the carrier’s characteristics and fungi affinity, as well as general factors like temperature, pH, and the nutrient availability (Meletiadis et al. 2001). In the present study, albeit with lower hydrophobicity, A. flavus demonstrated a higher adhesion rate. This is consistent with the previous findings that stated, hydrophobicity only affects the initial fungi-carrier interaction, but the main determinant is fungi genetic makeup. Stress tests demonstrated that C. elegans exhibited superior carrier adhesion, retaining 40% biomass under harsh conditions, compared to 15% for A. flavus (Fig. 5–5). This highlights C. elegans potential for bioreactor applications despite having lower growth rates.
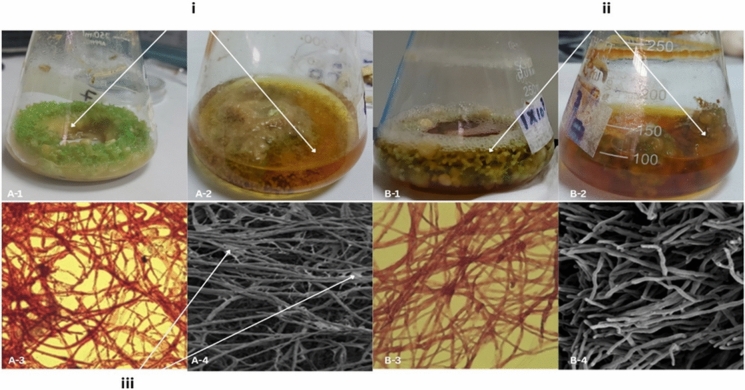
ECM composition analysis showed that, A. flavus biofilms contained threefold more carbohydrates and 1.5-fold more proteins than C. elegans (Supplementary Material S2). The hydrophilic carbohydrates are responsible for ECM hydration and lubrication, along with their role as binding sites for signalling molecules, contributing to ECM structural integrity. Meanwhile, proteins are hydrophobic with more diverse chemical properties. In the present study, carbohydrates predominated over protein in both biofilms. Other studies reported carbohydrates as the main component in C. elegans ECM (Amadio and Murphy 2010; Singh et al. 2011). In addition, A. flavus has a lower protein expression in biofilm-based cultivation (Francis et al. 2020). The SEM analysis confirmed the structural difference between the two fungal biofilms as illustrated in Fig. 6. A. flavus biofilm formed denser hyphal network observed by SEM (22.41 ± 0.52 µm average hyphae diameter and 8.29 ± 2.62 µm interstitial space vs. 3.93 ± 0.41 µm average hyphae diameter and 12.67 ± 4.23 µm average interstitial space for C. elegans). A. flavus formed compact, ECM-rich biofilms, C. elegans produced more uniform structures with minimal ECM, suggesting different strategies for surface colonization (Bajoul Kakahi et al. 2019) These structural differences align with their distinct adhesion properties and might influence their performance.
Fig. 6.
A-1: A. flavus in PUF-F. A-2: A. flavus in PUF-M. A-3: A. flavus hyphae under 40 × microscope (106 spore/mL). A-4: A. flavus 48 h old biofilm hyphae under SEM (500 ×). B-1: C. elegans in PUF-F. B-2: C. elegans in PUF-M. B-3 C. elegans hyphae under 40 × microscope (106 spore/mL). B-4: C. elegans 48 h old biofilm hyphae under SEM (500 ×). (i): The detached fungal biomass. (ii): The foam-attached fungal biomass. (iii): The ECM inside the fungal hyphae
Conclusion
This study demonstrates that C. elegans and A. flavus can form stable biofilms on PUF carriers, offering an effective biological solution for removing persistent fluorinated PhAC. The distinct degradation profiles of the two fungi, with A. flavus excelling in FLX removal (91.6%) and C. elegans showing superior performance for CIP (99%) and ATO (96.3%), suggest their potential for tailored applications in treatment systems. The predominance of biodegradation over adsorption (< 10% contribution) confirms the role of fungal enzymatic activity as the primary removal mechanism, supporting their use in long-term treatment scenarios. However, the observed inhibition of biofilm formation by PhAC highlights the need for gradual acclimation in real-world implementations. These findings have practical implications for wastewater treatment: (1) PUF-based biofilm system could enhance removal by targeting recalcitrant PhAC, (2) fungal combinations could be optimized for specific contaminant profiles, and (3) carrier design should balance biomass retention and mass transfer. The demonstrated removal efficiencies (85–99%) at environmentally relevant concentrations (5–60 μg/L) position these fungal systems as promising tertiary treatment options, particularly for facilities receiving PhAC industry effluents or hospital waste. Future research should focus on scaling up these systems and evaluating their performance in continuous-flow reactors with non-sterile WW settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
I.A. contributed to the conceptualization, experiment execution, data analysis, and manuscript drafting. F.W.F.W. provided intellectual guidance, contributed to study design, and critically revised the manuscript. M.H. and M.S.M. provided methodological insights and reviewed the manuscript. All authors reviewed and approved the final manuscript.
Funding
Open access funding provided by The Ministry of Higher Education Malaysia and Universiti Putra Malaysia.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adegunlola GA, Oloke JK, Majolagbe ON et al (2010) Microbial desulphurization of crude oil using immobilized spores of Aspergillus flavus. Adv Environ Biol 4:155–158 [Google Scholar]
- Ali H, Ahmad W, Haq T (2009) Decolorization and degradation of malachite green by Aspergillus flavus and Alternaria solani. Afr J Biotechnol 8:1574–1576 [Google Scholar]
- Amadio J, Murphy CD (2010) Biotransformation of fluorobiphenyl by Cunninghamella elegans. Appl Microbiol Biotechnol. 10.1007/s00253-009-2346-4 [DOI] [PubMed] [Google Scholar]
- Amadio J, Casey E, Murphy CD (2013) Filamentous fungal biofilm for production of human drug metabolites. Appl Microbiol Biotechnol 97:5955–5963. 10.1007/s00253-013-4833-x [DOI] [PubMed] [Google Scholar]
- Bajoul Kakahi F, Ly S, Tarayre C et al (2019) Modulation of fungal biofilm physiology and secondary product formation based on physico-chemical surface properties. Bioprocess Biosyst Eng. 10.1007/s00449-019-02187-6 [DOI] [PubMed] [Google Scholar]
- Balraj J, Jairaman K, Kalieswaran V, Jayaraman A (2018) Bioprospecting lovastatin production from a novel producer Cunninghamella blakesleeana. 3 Biotech 8:1–19. 10.1007/S13205-018-1384-Y. (/METRICS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneš H, Vlčková V, Paruzel A et al (2020) Multifunctional and fully aliphatic biodegradable polyurethane foam as porous biomass carrier for biofiltration. Polym Degrad Stab 176:109156. 10.1016/J.POLYMDEGRADSTAB.2020.109156 [Google Scholar]
- Bijlsma L, Pitarch E, Fonseca E et al (2021) Investigation of pharmaceuticals in a conventional wastewater treatment plant: removal efficiency, seasonal variation and impact of a nearby hospital. J Environ Chem Eng 9:105548. 10.1016/J.JECE.2021.105548 [Google Scholar]
- Carneiro RB, Mukaeda CM, Sabatini CA et al (2020) Influence of organic loading rate on ciprofloxacin and sulfamethoxazole biodegradation in anaerobic fixed bed biofilm reactors. J Environ Manage 273:111170. 10.1016/J.JENVMAN.2020.111170 [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Abraham J (2017) Comparative study on degradation of norfloxacin and ciprofloxacin by Ganoderma lucidum JAPC1. Korean J Chem Eng 34:1122–1128. 10.1007/S11814-016-0345-6 [Google Scholar]
- Chang P-K, Ehrlich KC (2010) What does genetic diversity of Aspergillus flavus tell us about Aspergillus oryzae? Int J Food Microbiol 138:189–199. 10.1016/j.ijfoodmicro.2010.01.033 [DOI] [PubMed] [Google Scholar]
- Chellapandi P (2010) Production and preliminary characterization of alkaline protease from Aspergillus flavus and Aspergillus terreus. J Chem 7:479–482. 10.1155/2010/502583 [Google Scholar]
- Chu L, Wang J, Quan F et al (2014) Modification of polyurethane foam carriers and application in a moving bed biofilm reactor. Process Biochem 49:1979–1982. 10.1016/J.PROCBIO.2014.07.018 [Google Scholar]
- Cleveland TE, Yu J, Fedorova N et al (2009) Potential of Aspergillus flavus genomics for applications in biotechnology. Trends Biotechnol 27:151–157. 10.1016/J.TIBTECH.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Cruz del Álamo A, Pariente MI, Molina R, Martínez F (2020) Trametes versicolor immobilized on rotating biological contactors as alternative biological treatment for the removal of emerging concern micropollutants. Water Res 170:115313. 10.1016/j.watres.2019.115313 [DOI] [PubMed] [Google Scholar]
- Cruz-Morató C, Ferrando-Climent L, Rodriguez-Mozaz S et al (2013) Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res 47:5200–5210. 10.1016/j.watres.2013.06.007 [DOI] [PubMed] [Google Scholar]
- de Melo Souza PL, Arruda EL, Pazini F et al (2016) One step N -glycosylation by filamentous fungi biofilm in bioreactor of a new phosphodiesterase-3 inhibitor tetrazole. Bioorg Med Chem Lett 26:3177–3181. 10.1016/j.bmcl.2016.04.085 [DOI] [PubMed] [Google Scholar]
- Deshmukh R, Khardenavis AA, Purohit HJ (2016) Diverse metabolic capacities of fungi for bioremediation. Ind J Microbiol 56(3):247–264. 10.1007/S12088-016-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner JW (2004) Biological control of aflatoxin contamination of crops. J Toxicol Toxin Rev 23:425–450. 10.1081/TXR-200027877 [Google Scholar]
- Elhussiny NI, El-Refai HA, Mohamed SS et al (2023) Aspergillus flavus biomass catalytic lipid modification: optimization of cultivation conditions. Biomass Convers Biorefin 1:1–13. 10.1007/S13399-023-04396-2/TABLES/12 [Google Scholar]
- Ericson JF (2010) Evaluation of the OECD 314B activated sludge die-away test for assessing the biodegradation of pharmaceuticals. Environ Sci Technol 44:375–381. 10.1021/ES902205R/SUPPL_FILE/ES902205R_SI_001.PDF [DOI] [PubMed] [Google Scholar]
- Francis F, Druart F, Di Mavungu JD et al (2020) Biofilm mode of cultivation leads to an improvement of the entomotoxic patterns of two aspergillus species. Microorganisms 8:705. 10.3390/MICROORGANISMS8050705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Dastidar MG, Sreekrishnan TR (2016) Bioremediation of a chromium complex dye using Aspergillus flavus and Aspergillus tamarii. Chem Eng Technol 39:1636–1644. 10.1002/CEAT.201500515 [Google Scholar]
- Ghosh A, Dastidar MG, Sreekrishnan TR (2018) Bioremediation of Chromium Complex Dye by Growing Aspergillus flavus 10.1007/978-981-10-5795-3_8
- Gupta A, Kumar S, Bajpai Y et al (2024) Pharmaceutically active micropollutants: origin, hazards and removal. Front Microbiol 15:1339469. 10.3389/fmicb.2024.1339469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Acosta OB, Arriaga S, Escobar-Barrios VA et al (2012) Performance of innovative PU-foam and natural fiber-based composites for the biofiltration of a mixture of volatile organic compounds by a fungal biofilm. J Hazard Mater 201:202–208. 10.1016/J.JHAZMAT.2011.11.068 [DOI] [PubMed] [Google Scholar]
- Halling-Sorensen B, Holten Lutzhoft HC, Andersen HR, Ingerslev F (2000) Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim and ciprofloxacin. J Antimicrob Chemother 46:53–58. 10.1093/JAC/46.SUPPL_1.53 [PubMed] [Google Scholar]
- Han J, Kiss L, Mei H et al (2021) Chemical aspects of human and environmental overload with fluorine. Chem Rev 121:4678–4742. 10.1021/acs.chemrev.0c01263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104. 10.1016/S0960-8524(98)00192-8 [Google Scholar]
- Khan MF (2025) Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes 2025, Vol 13, Page 1034 13:1034. 10.3390/PR13041034
- Kózka B, Nałęcz-Jawecki G, Turło J, Giebułtowicz J (2020) Application of Pleurotus ostreatus to efficient removal of selected antidepressants and immunosuppressant. J Environ Manage 273:111131. 10.1016/j.jenvman.2020.111131 [DOI] [PubMed] [Google Scholar]
- Kózka B, Sośnicka A, Nałęcz-Jawecki G et al (2023) Various species of Basidiomycota fungi reveal different abilities to degrade pharmaceuticals and also different pathways of degradation. Chemosphere 338:139481. 10.1016/J.CHEMOSPHERE.2023.139481 [DOI] [PubMed] [Google Scholar]
- Kumar V, Dwivedi SK (2019a) Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 237:124567. 10.1016/J.CHEMOSPHERE.2019.124567 [DOI] [PubMed] [Google Scholar]
- Kwon JW, Armbrust KL (2006) Laboratory persistence and fate of fluoxetine in aquatic environments. Environ Toxicol Chem 25:2561–2568. 10.1897/05-613R.1 [DOI] [PubMed] [Google Scholar]
- Lass-Flörl C, Dierich MP, Fuchs D et al (2001) Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J Antimicrob Chemother 48:775–779. 10.1093/JAC/48.6.775 [DOI] [PubMed] [Google Scholar]
- Lee CO, Howe KJ, Thomson BM (2012) Ozone and biofiltration as an alternative to reverse osmosis for removing PPCPs and micropollutants from treated wastewater. Water Res 46:1005–1014. 10.1016/J.WATRES.2011.11.069 [DOI] [PubMed] [Google Scholar]
- Liang C, Zhang L, Nord NB et al (2019) Dose-dependent effects of acetate on the biodegradation of pharmaceuticals in moving bed biofilm reactors. Water Res 159:302–312. 10.1016/j.watres.2019.04.026 [DOI] [PubMed] [Google Scholar]
- Manasfi R, Chiron S, Montemurro N et al (2020) Biodegradation of fluoroquinolone antibiotics and the climbazole fungicide by Trichoderma species. Environ Sci Pollut Res Int 27:23331–23341. 10.1007/S11356-020-08442-8. (/METRICS) [DOI] [PubMed] [Google Scholar]
- Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74:765–772. 10.1016/J.CHEMOSPHERE.2008.10.040 [DOI] [PubMed] [Google Scholar]
- Meletiadis J, Meis JFGM, Mouton JW, Verweij PE (2001) Analysis of growth characteristics of filamentous fungi in different nutrient media. J Clin Microbiol 39:478–484. 10.1128/JCM.39.2.478-484.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira IS, Amorim CL, Ribeiro AR et al (2015) Removal of fluoxetine and its effects in the performance of an aerobic granular sludge sequential batch reactor. J Hazard Mater 287:93–101. 10.1016/J.JHAZMAT.2015.01.020 [DOI] [PubMed] [Google Scholar]
- Mousavi B, Hedayati MT, Hedayati N et al (2016) Aspergillus species in indoor environments and their possible occupational and public health hazards. Curr Med Mycol 2:36. 10.18869/ACADPUB.CMM.2.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A (2016) Role of Aspergillus in Bioremediation Process. Elsevier B.V.
- Ottmar KJ, Colosi LM, Smith JA (2012) Fate and transport of atorvastatin and simvastatin drugs during conventional wastewater treatment. Chemosphere 88:1184–1189. 10.1016/J.CHEMOSPHERE.2012.03.066 [DOI] [PubMed] [Google Scholar]
- Palmer-Brown W, Miranda-CasoLuengo R, Wolfe KH et al (2019) The CYPome of the model xenobiotic-biotransforming fungus Cunninghamella elegans. Sci Rep 9:9240. 10.1038/s41598-019-45706-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A, Möder M, Rodil R et al (2011) Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour Technol 102:10987–10995. 10.1016/J.BIORTECH.2011.08.055 [DOI] [PubMed] [Google Scholar]
- Rayaroth MP, Aravind UK, Aravindakumar CT (2016) Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ Chem Lett 14:259–290. 10.1007/s10311-016-0568-0 [Google Scholar]
- Rodarte-Morales AI, Feijoo G, Moreira MT, Lema JM (2011) Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J Microbiol Biotechnol 27:1839–1846. 10.1007/s11274-010-0642-x [Google Scholar]
- Rodríguez-López L, Santás-Miguel V, Cela-Dablanca R et al (2022) Ciprofloxacin and Trimethoprim Adsorption/Desorption in Agricultural Soils. Int J Environ Res Public Health 19:8426. 10.3390/IJERPH19148426/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez CE, Jelic A, Pereira MA, Sousa DZ, Petrovic M, Alves MM, Barceló D, Caminal G, Vicent T, (2012) Bioaugmentation of sewage sludge with trametes versicolor in solid-phase biopiles produces degradation of pharmaceuticals and affects microbial communities. Environ Sci Technol. 10.1021/es301788n [DOI] [PubMed] [Google Scholar]
- Rueda-Márquez JJ, Moreno-Andrés J, Rey A et al (2021) Post-treatment of real municipal wastewater effluents by means of granular activated carbon (GAC) based catalytic processes: a focus on abatement of pharmaceutically active compounds. Water Res 192:116833. 10.1016/j.watres.2021.116833 [DOI] [PubMed] [Google Scholar]
- Sangeetha Devi R, Rajesh Kannan V, Nivas D et al (2015) Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar Pollut Bull 96:32–40. 10.1016/J.MARPOLBUL.2015.05.050 [DOI] [PubMed] [Google Scholar]
- Sass G, Scherpe L, Martinez M et al (2022) Metrics of antifungal effects of ciprofloxacin on Aspergillus fumigatus planktonic growth and biofilm metabolism; effects of iron and siderophores. J Fungi. 10.3390/JOF8030240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim MT, Salem SS, Mohamed AA et al (2021) Biological treatment of real textile effluent using aspergillus flavus and fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J Fungi 7:193. 10.3390/JOF7030193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Shivaprakash MR, Chakrabarti A (2011) Biofilm formation by zygomycetes: quantification, structure, and matrix composition. Microbiology (n y) 157:2611–2618. 10.1099/mic.0.048504-0 [DOI] [PubMed] [Google Scholar]
- Singh SK, Khajuria R, Kaur L (2017) Biodegradation of ciprofloxacin by white rot fungus Pleurotus ostreatus. 3 Biotech 7:69. 10.1007/s13205-017-0684-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboń A, Szewczyk R, Długoński J (2016) Tributyltin (TBT) biodegradation induces oxidative stress of Cunninghamella echinulata. Int Biodeterior Biodegrad 107:92–101. 10.1016/j.ibiod.2015.11.013 [Google Scholar]
- Song M, Yu Q, Liu Y et al (2023) Microbial transformation of pimavanserin by cunninghamella blakesleeana AS 3.970. Catalysts 13:1220. 10.3390/CATAL13081220/S1 [Google Scholar]
- Sponchiado R, Sorrentino JM, Olegário N et al (2019) Microbial transformation of ambrisentan to its glycosides by Cunninghamella elegans. Biomed Chromatogr 33:e4496. 10.1002/BMC.4496 [DOI] [PubMed] [Google Scholar]
- Talukdar D, Jasrotia T, Sharma R et al (2020) Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ Technol Innov 20:101050. 10.1016/j.eti.2020.101050 [Google Scholar]
- Tang K, Ooi GTH, Spiliotopoulou A et al (2020) Removal of pharmaceuticals, toxicity and natural fluorescence by ozonation in biologically pre-treated municipal wastewater, in comparison to subsequent polishing biofilm reactors. Water 12:1059. 10.3390/w12041059 [Google Scholar]
- Velázquez YF, Nacheva PM (2017) Biodegradability of fluoxetine, mefenamic acid, and metoprolol using different microbial consortiums. Environ Sci Pollut Res Int 24:6779–6793. 10.1007/S11356-017-8413-Y. (/METRICS) [DOI] [PubMed] [Google Scholar]
- Werkneh AA (2022) Application of membrane-aerated biofilm reactor in removing water and wastewater pollutants: current advances, knowledge gaps and research needs - a review. Environmental Challenges 8:100529. 10.1016/J.ENVC.2022.100529 [Google Scholar]
- Wunder DB, Bosscher VA, Cok RC, Hozalski RM (2011) Sorption of antibiotics to biofilm. Water Res 45:2270–2280. 10.1016/J.WATRES.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Zhang W, Liang Y (2023) The wide presence of fluorinated compounds in common chemical products and the environment: a review. Environ Sci Poll Res 30(50):108393–108410. 10.1007/S11356-023-30033-6 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu D, Huang W et al (2019) Insights into biofilm carriers for biological wastewater treatment processes: current state-of-the-art, challenges, and opportunities. Bioresour Technol 288:121619. 10.1016/j.biortech.2019.121619 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.