Abstract
Aims
Past research suggested respiratory syncytial virus (RSV) specific serum immunoglobulin A (IgA) antibodies as a biomarker of previous RSV infections in young infants accounting for maternal immunity. This study aimed to confirm this association and to establish a serological threshold for discriminating RSV-naïve infants from those who have experienced RSV.
Material and methods
This study involves 135 infants from the LoewenKIDS study with nasal swabs collected at each acute respiratory infection, and serum samples collected at ages 1 and 2 years. RSV presence in swabs was ascertained by reverse transcriptase PCR; RSV-specific IgG and IgA antibody levels against five different structural proteins and RSV neutralising antibodies were measured in sera. Robust Mixture Discriminant Analysis was used to determine the cut-off values and account for false negatives.
Results
Of 135 included infants, 131 had available data at year 1 (Y1) and 95 at year 2 (Y2). Pre-F IgA concentrations were higher in infants with PCR-confirmed RSV infections. There was a further increase in IgA, IgG and neutralising antibody titre concentrations from Y1 to Y2 consistent with re-infections. Based on robust mixture discriminant analysis, the cut-off values of pre-F IgA level indicative of past RSV infection were 0.23 AU·mL−1 at Y1 and 0.22 AU·mL−1 at Y2.
Conclusion
This study shows that in children aged <2 years, a previous RSV infection is accompanied by serum pre-F IgA antibody levels above 0.22 AU·mL−1, a value close to a previously proposed cut-off (0.19 AU·mL−1) based on seroresponse data only. The confirmed threshold can be of use in studies assessing vaccination strategies.
Shareable abstract
This study shows that in children aged <2 years, a previous RSV infection is accompanied by serum pre-F IgA antibody levels above 0.22 AU·mL−1, a value close to a previously proposed cut-off (0.19 AU·mL−1) based on seroresponse data only https://bit.ly/4gTp06v
Introduction
Human respiratory syncytial virus (RSV), a highly transmissible and seasonal virus, is the leading cause of lower respiratory tract infections (LRTIs) in children [1]. About 60–70% of children get infected with RSV in their first year of life and almost all children within 2 years of life [2]. According to a recent global estimate, RSV was associated with 33 million LRTI episodes, 3.6 million hospital admissions and 26 300 in-hospital deaths in children younger than 5 years [3].
RSV infections can present as a variety of clinical syndromes, ranging from mild upper respiratory illness to life-threatening bronchiolitis and pneumonia. Over time, several risk factors have been identified for severe RSV including preterm birth (<32 weeks’ gestational age), haemodynamically significant congenital heart disease, chronic lung disease, severe immunodeficiency and lack of breastfeeding [4]. However, studies have shown that the vast majority of severe RSV infections occur in otherwise healthy infants who have no identifiable risk factors [5, 6]. Strong epidemiological evidence indicates that primary RSV infection occurring during early childhood (i.e., between 0 and 2 years of age) is associated with an increased risk of asthma and chronic wheeze development later in life [7, 8]. Over the past decades, and in particular since the structure of the pre-F protein has been solved [9], critical progress has been made in the development of RSV prophylactics. In 2023, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) approved two immunisation solutions to protect infants against RSV – the monoclonal antibody (mAb) nirsevimab (Beyfortus) [10] and the recombinant vaccine Abrysvo [11, 12].
Traditionally, RSV neutralising antibody titres (NT) have been used as a major serological hallmark of previous exposure to RSV [13, 14]. However, in young infants, these readouts do not allow differentiation between the serological legacy of a prior RSV infection and passive immunisation from maternally derived antibodies or prophylactically administered antibodies. Maternal antibodies are present at birth and can confer temporary protection [15–17], as titres in the newborn wane postpartum. Given that monoclonal antibodies licensed or under development are directed against the fusion (F) protein [18], discrimination of infection may be feasible with an immunoassay detecting antibodies against other proteins (e.g. RSV nucleoprotein) or measuring other immunoglobin isotypes (e.g. IgA) [19]. The presence of those different types of antibodies may have implications for paediatric RSV vaccine development (e.g. interference) [20, 21]. Therefore, the development of a serological framework allowing dissection of infection-induced antibodies from maternally derived or prophylactically administered antibodies could support future RSV vaccine development and be a useful tool for serosurveillance studies.
IgA emerges as a promising candidate for detecting RSV infections in young children [22, 23]. Indeed, unlike IgG, IgA is not efficiently transferred across the placenta, allowing for a clearer distinction between maternal and infant antibodies and enabling detection of primary infection even in a context where IgGs, of maternal origin or acquired through passive immunisation, are still present in the infant's system. Possible limitations of IgA include reduced sensitivity and rapid clearance from the body after infection.
Prior research on a small set of samples suggested that a rise in RSV F and G IgA titres can be used to detect an RSV infection in young infants with lasting maternal antibodies [22]. A study based on serum samples from two nation-wide cross-sectional serosurveillance studies analysed IgG and IgA antibodies to RSV prefusion F (pre-F), postfusion F (post-F), nucleoprotein (N), Ga (G of RSV-A) and Gb (G of RSV-B) proteins. The study confirmed age-related trends in RSV-specific IgG and IgA serum titres at a population level and posited that in children 0–24 months of age, an IgA cut-off of 0.19 AU·mL−1 could discriminate between samples mainly composed of anti-pre-F IgG of maternal origin (pre-F IgA ≤0.19 AU·mL−1) and samples with evidence of a recent RSV infection (pre-F IgA >0.19 AU·mL−1) [23]. In another study, the authors considered an IgA cut-off of 0.2 AU·mL−1 for children younger than 500 days and an IgG cut-off of 1.0 AU·mL−1 for those older than 500 days, increasing specificity by requiring antibodies against at least two out of the three tested RSV proteins (pre-F, post-F, N) meet the threshold [24]. However, these prior results were based solely on cross-sectional testing of IgA and IgG and lacked direct laboratory or clinical evidence of an RSV infection in a longitudinal perspective.
The aim of our study was to provide direct evidence from a prospective birth cohort recording all infection episodes and, therefore, allowing differentiation between children who had an RSV infection and those who did not. The RSV-specific IgA threshold is further consolidated by additional clinical, serological and virological parameters that are supportive evidence of past RSV infection.
Methods
Description of the LoewenKIDS subcohort
The present study was carried out using data and blood samples from a nested subcohort of the LoewenKIDS study. A detailed description of the study design is provided elsewhere [25]. Briefly, the LoewenKIDS study is an ongoing, population-based, observational birth cohort study (Clinicaltrials.Gov Identifier: NCT02654210), which recruited 782 infants during pregnancy or up to 3 months postpartum from five study regions in Germany (Braunschweig, Hannover, Halle, Munich, Bremen) between November 2014 and February 2018. Infants born in the region of conduct of this study and their caregivers who were able to provide consent in the German language were included. Parents aged <18 years and those unable to provide informed consent in the German language, and infants suffering from any severe disease (parents were informed that this study did not provide any treatment) were excluded. Demographics and clinical characteristics such as age, sex, birthweight and height, weight and height as per gestational age, mode of birth, birth institution and need for hospital admission in the first 2 weeks were collected.
Participants are followed up until the age of 15 years. Within the first 6 years of life, all common respiratory symptoms were recorded in a daily symptom diary. Nasal swabs were collected at the time of an acute respiratory infection, which is defined as one or more symptoms of category A (fever, wheezing, cough with sputum, otitis media or pneumonia diagnosed by a physician), or two or more symptoms of category B (runny nose or nasal congestion, sore throat, cough, chills, loss of appetite, increased need to sleep, increased attachment). These definitions were adapted from Lambert et al. [26]. Asymptomatic samples were collected once per year.
A total of 285 children were enrolled in a nested subcohort with more frequent collection of asymptomatic samples (every 3 months in the first 2 years of life) and additional blood sampling at year 1 (Y1) and year 2 (Y2). Nasal swabs were collected in tubes with transport media (eNat or Amies Medium) and sent via regular mail to the laboratory within 48 h of collection. Specimens were stored at −80°C until further analysis. Individuals who did not have a blood serum sample at Y1 or Y2 were excluded from the analysis.
Study assessment
The main end-points of the study analysis were serum RSV pre-F IgA concentration at Y1 and Y2, and occurrence of previous RSV disease, as confirmed by positive reverse transcriptase PCR (RT-PCR) (Allplex Respiratory Panel Assays, Seegene) testing of nasal swabs collected during the first 2 years of life. Infants were categorised into the following groups: 1) those with confirmed RSV in Y1; 2) those without RSV in Y1; 3) those with confirmed RSV in Y1 or Y2; and 4) those without RSV in Y1 or Y2. Infants with confirmed RSV at Y2 were further categorised into three groups: those with confirmed RSV during Y1 only, those with confirmed RSV during Y2 only, and those with confirmed RSV during Y1 and Y2. Secondary end-points assessing RSV-specific serum IgG concentrations and NTs at Y1 and Y2 were included in the analysis as supportive data.
RSV multiplex assay
A recently developed high-throughput multiplex immunoassay was used to measure RSV-specific IgG and IgA antibody levels against five different structural proteins, three of which were used in this study to determine RSV-specific seroconversion, i.e. pre-F and post-F proteins, and N protein. Antibody levels for IgA and IgG were expressed in (arbitrary) concentration units (AU·mL−1), set against a reference serum (NIBSC Antiserum to Respiratory Syncytial Virus 16/284) as previously defined [19, 27].
RSV microneutralisation assay
A microneutralisation assay was performed to determine the level of neutralising antibodies against RSV in serum samples collected from infants. Serial two-fold dilutions of heat-inactivated serum samples were incubated with a constant challenge dose of RSV (A2 strain (ATCC; catalog# VR-1540)) and guinea pig complement at 37°C with 5% CO2 for 1 h. The serum–virus mixtures were inoculated into wells of a 96-well microplate with preformed HEp-2 (ATCC catalog# CCL-23) cell monolayers and the microplates centrifuged for 15 min at 700×g. Additional assay media was added to all wells without removing the existing inoculum and incubated at 37°C with 5% CO2 for 2 days. After washing and fixation of the HEp-2 cell monolayers, RSV antigen production in cells was detected by successive incubations with an anti-RSV fusion protein mouse monoclonal antibody (Envigo, clone # CCC), HRP IgG conjugate (Jackson ImmunoResearch Laboratories) and a chromogenic substrate. The resulting optical density (OD) was measured using a microplate reader. The reduction in RSV infectivity, as compared to that in the virus control wells, constituted a positive neutralisation reaction indicating the presence of neutralising antibodies in the serum sample. The 50% neutralisation titre (MN50) was defined as the reciprocal of the serum dilution for which the virus infectivity was reduced by 50% relative to the virus control on each plate. The MN50 for each sample was interpolated by calculating the slope and intercept using the last dilution with an OD below the 50% neutralisation point and the first dilution with an OD above the 50% neutralisation point: MN50 titre = (OD of 50% neutralisation point-intercept)/slope.
Statistical analysis
The geometric mean concentrations of IgA and IgG and geometric mean titres for NT were calculated and presented with 95% confidence interval (CI). Distribution data of the different antibody markers were also presented by age and history of detected RSV infection status. A receiver operating characteristic (ROC) curve was generated for each biomarker based on a simple logistic regression model. The best cut-off value was determined based on Youden index, J = sensitivity + specificity – 1. Principal component analysis (PCA) was conducted, and PCA plots of PC1 and PC2 were generated to determine the relation between the whole set of biomarkers and to detect potential outliers. To assess potential confounders, we first identified variables that could influence both the exposure and outcome based on prior literature and clinical relevance. Multivariable logistic regression models were then used to adjust for these confounders. To consider the potential false-negative classification of infection status (such as asymptomatic cases, non-reported symptoms or absence of swab), a semi-supervised learning method – robust mixture discriminant analysis (RMDA) model – was also used to determine the cut-off values (supplementary material). It considered two structures in the data: an unsupervised modelling based on mixture models and a supervised modelling relying on the label information. If some learning data had wrong labels, the comparison of the supervised information with an unsupervised modelling of the data allowed the detection of inconsistent labels. The cut-off value for each biomarker was then determined by the posterior probability obtained from the RMDA model. All statistical analyses were conducted on log10-transformed data. Statistical analyses were performed using R (version 4.3.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
LoewenKIDS nested subcohort
A total of 135 infants from the nested subcohort of the LoewendKIDS cohort had available blood samples and were included in this study. Among these, 131 infants had available data (blood sample and nasal swabs valid results) at Y1, and 95 infants at Y2 (table 1).
TABLE 1.
Characteristics of study population
| Characteristics | Year 1 | Year 2 |
|---|---|---|
| Subjects, n | 131 | 95 |
| Age at blood sampling months, median (min, max) | 11.70 (10, 18) | 25.17 (21, 33) |
| Sex, female, n (%) | 63 (48.1) | 51 (53.7) |
| Birth mode – caesarean, n (%) | 32 (24.4) | 24 (25.3) |
| Weight at birth g, median (min, max) | 3492.50 (1100, 4670) | 3500.00 (1920, 4670) |
| Parental socioeconomic status score, median (min, max)# | 10.00 (6, 10) | 10.00 (6, 10) |
| Number of ARDs by year, n | 973 | 957¶ |
| Number of ARDs by subject by year, median (min, max) | 7.00 (0, 20) | 8.00¶ (0, 19) |
| Number of nasal swabs by year, n | 586 | 491¶ |
| Number of nasal swabs by subject by year, median (min, max) | 4.00 (0, 13) | 5.00+ (0, 18) |
ARDs: acute respiratory diseases. #: scores were calculated for each parent separately (1–3 for low, middle and high education and 1 for not employed and 2 for employed) and added up; maximum score is 10 [30]. ¶: numbers refer to year 2 only.
Out of 131 infants evaluable at Y1, RSV infection was detected in 41 (31.3%) during the first year as confirmed by PCR in at least one of the collected nasal swabs. For the remaining 90 infants (68.7%), there was no detection of RSV infection. Among 95 infants evaluable at Y2, 53 (55.8%) had at least one positive test by the age of 2 years, 21 (22.1%) of which tested positive in Y2 only and 14 (14.7%) in both Y1 and Y2 (figure 1).
FIGURE 1.
Study population and detection of respiratory syncytial virus (RSV) infection. Y1: year 1; Y2: year 2.
Among infants with several RSV infections documented during the same year, all but two of them had RSV-positive nasal swabs fewer than 28 days apart, suggesting they corresponded to the same episode.
Characterisation of immunological markers
Overall, there was an increase in all measured biomarker concentrations from Y1 to Y2 across the four groups. Among RSV cases, the distribution of biomarkers was consistent both at Y1 and Y2 (supplementary table S1).
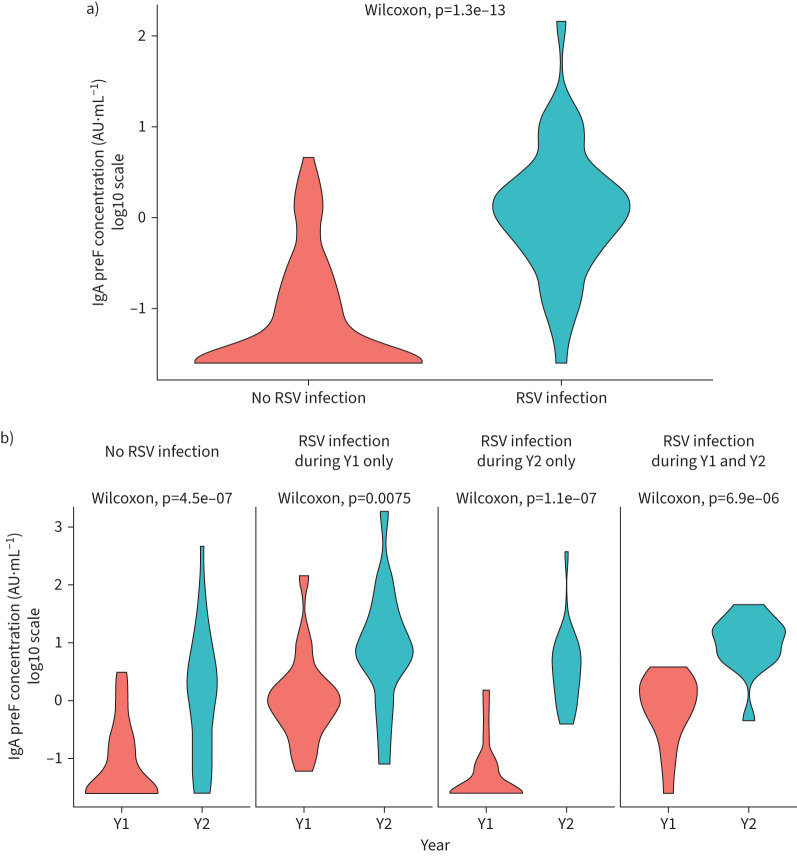
Looking specifically at pre-F IgA, concentrations were higher in infants who had an RSV infection detected in their first year of life than in those who did not (figure 2a). Pre-F IgA concentrations were higher at Y2 than at Y1, independent of their RSV infection status (figure 2b). However, there was an overlap between the detected RSV infections and no RSV infection group titres at both Y1 and to a greater extent at Y2, indicating a lack of detection of RSV infections by PCR between Y1 and Y2.
FIGURE 2.
Violin plot illustrating the kernel density distribution of the pre-F IgA (log10) concentration at a) year 1 (Y1) and b) year 2 (Y2) according to the detection of previous respiratory syncytial virus (RSV) infection.
Among the potential confounders assessed, namely the time elapsed since last infection, delivery mode, sex, presence of an older sibling, birthweight and length, none were found to be significant.
RSV infection detection in relation to immunological markers
As part of previous work, a pre-F IgA concentration above the threshold of 0.19 AU·mL−1 was suggested to be indicative of a previous RSV infection. The agreement between RSV infection detection with RT-PCR and with serological biomarkers is summarised in table 2. Overall, infants who had an RSV infection detected by RT-PCR before blood sampling had an IgA antibody concentration above 0.19 AU·mL−1 after the infection (35 out of 41 (85.4%) at Y1; 52 out of 53 (98.1%) at Y2). The mean age at first RSV infection of these infants was 6.8 months (range 1.3–13.2 months). The remaining six infants who did not have a pre-F IgA concentration above the threshold of 0.19 AU·mL−1, despite an RSV infection detected by RT-PCR, had similar mean age at first infection (6.8 months, range 3.7–9.4 months). A fraction of infants without any RSV infection detected by RT-PCR had anti-RSV IgA levels above the 0.19 AU·mL−1 cut-off at Y1 and/or Y2 (19 out of 90 (21.1%) at Y1; 29 out of 42 (69.04%) at Y2). This may be due to asymptomatic infections for which no swabs were taken, symptomatic infections without swabs or pathogen detection problems.
TABLE 2.
RSV infections and anti-Pre-F serum IgA concentration during the first 2 years of life
| Based on pre-F IgA threshold (0.19 AU·mL−1) | Based on pre-F IgG threshold (2 AU·mL−1) | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Year 1 | ||||
| Based on RT-PCR confirmed infection | ||||
| Yes | 35 | 6 | 37 | 4 |
| No | 19 | 71 | 37 | 53 |
| Year 2 | ||||
| Based on RT-PCR confirmed infection | ||||
| Yes | 52 | 1 | 53 | 0 |
| No | 29 | 13 | 33 | 9 |
RT-PCR: reverse transcriptase PCR.
The fraction of infants with positive PCR and with pre-F IgG concentrations above the threshold was marginally higher compared to pre-F IgA. However, this was also the case among those without positive PCR, particularly in the first year, suggesting a portion of the measured pre-F IgGs is of maternal origin.
Diagnostic potential of Pre-F IgA using reported values
PCA was used to illustrate the relation between the whole set of biomarkers and the detection of RSV infections. Two distinct clusters by RSV status at Y1 were clearly identified as well as clear outliers, shown as red dots in figure 3a. At Y2, looking at the RSV infection detected overall (figure 3b), there is a lack of clear separation between detected RSV cases and the others. Considering the RSV infections detected during Y1 and Y2 (figure 3c), there appears to be a separation between groups: infants with an RSV infection detected during Y2 only (blue dots in the figure) are characterised by a lower Y1 antibody profile and a higher Y2 antibody profile; infants with an RSV infection detected during Y1 and during Y2 (purple) are mixed with infants with an RSV infection during Y1 only (green) and are characterised by a higher Y1 antibody profile and a lower antibody profile at Y2. This suggests both potential missed RSV infections during Y2 and a limited increase in antibody levels after a second infection.
FIGURE 3.
Principal component analysis (PCA) biplot with subjects coloured according to respiratory syncytial virus (RSV) detection at a) year 1 (Y1), at b) year 2 (Y2) and c) during Y1 and Y2. In each biplot, the points represent the subjects, and the colour of the points represent the RSV status. The arrows show the correlation between variables, the length of the arrow corresponding to magnitude of influence of the variable, and the distance between arrows shows the correlation. Positively correlated variables are grouped together, while negatively correlated variables are positioned on the opposite side of the origin.
Based on the logistic regression analysis, the Youden-optimised cut-off value of pre-F IgA for RSV infection was 0.315 AU·mL−1 at Y1 and 3.52 AU·mL−1 at Y2 (supplementary figure S1). The accuracy of pre-F IgA to confirm RSV infection using the Y1 cut-off value was 84.7%, and it was 73.3% using the Y2 cut-off value.
Diagnostic potential of pre-F IgA using semi-supervised analysis
The PCA plots and the density plots showed that Y2 data may contain some false-negative cases for RSV infection.
The semi-supervised analysis revealed the emergence of two distinct clusters at Y2 by RMDA-predicted RSV status (figure 4). This analysis indicated that a subset of individuals (15 at Y1 and 30 at Y2) were likely misclassified as negative for RSV infection. Moreover, the cut-off values of pre-F IgA level for RSV exposure based on the RMDA method was 0.23 AU·mL−1 at Y1 and 0.22 AU·mL−1 at Y2, indicating stability across time. Furthermore, re-infections with RSV at later timepoints were associated with a further rise in IgG concentration against all measured antigens for children who already tested positive for RSV-specific IgG and IgA antibody at Y1 (supplementary table S2).
FIGURE 4.
Principal component analysis (PCA) plots labeled by observed (left) and robust mixture discriminant analysis (RMDA) predicted (right) respiratory syncytial virus (RSV) infection. PCA plot using all readouts at year 1 (Y1) (top) and year 2 (Y2) (bottom). Each point represents a subject and the colour represents the RSV status from the observed data (left) and predicted status from RMDA (right).
Discussion
Our study showed a clear association between elevated IgA levels and a history of RSV infection in infants. Infants with documented RSV infections during their first year displayed antibody levels above 0.23 AU·mL−1. When adjusting for missed infections, the second-year data not only confirmed but reinforced this association. These results are consistent with those observed in a previous population survey, in which a pre-F IgA antibody threshold concentration had been established based on sero-modelling [23]. The present study further strengthens the validity of these findings by incorporating complementary clinical information relying on parental monitoring of acute respiratory illness symptoms, collection of nasal swab samples and laboratory diagnosis of RSV infection during the first 2 years of life.
Furthermore, the findings from this study highlight the challenge of detecting RSV infections from a routine clinical and diagnostic perspective, especially in the first year of life. Despite a robust study design, detection of RSV infection remains imperfect, which could be due to asymptomatic [28] or mildly symptomatic infections missed by the parents, lack of or delayed sampling, or incomplete reporting, particularly in the second year. Additional sample collection challenges and assay limitations may also have contributed to missed infection cases. Serology at year 1 provides complementary information allowing missed RSV infections to be captured. To address potential errors in case identification, an RMDA post hoc analysis was performed. The cut-off values and the predicted classification are derived simultaneously based on the posterior probability from RMDA. This approach effectively avoids overfitting, as outcomes are not used twice (i.e., to establish the new classification and then to determine the cut-off values).
Our study has several limitations. First, due to the small sample size, we were not able to follow the conventional practice of splitting data into training and testing sets. This aspect warrants consideration in future work. This limitation was compounded by a reduction in participants from year 1 to year 2, which may have introduced attrition bias and impacted the reliability and comparability of our longitudinal results. Future studies should implement strategies to minimise attrition and analyse characteristics of dropouts. Second, getting blood samples at birth and shortly after the disease episodes would allow a more accurate insight into antibody titre trends over time and information on maternal antibody kinetics (IgA versus IgG) to better define their association with detected RSV infections. Third, the cohort used in this research was exclusively from a German population, which may not fully represent the global diversity of RSV infections and immune responses. Furthermore, population heterogeneity, including genetic factors, IgA production deficits, co-infections, environmental exposures and variations in healthcare systems, might affect the applicability of our proposed thresholds in broader contexts. These unexamined variables may have affected the interpretation of IgA levels and the proposed threshold, highlighting the need for more comprehensive analyses in future research. Finally, external factors were not explored in this analysis, and the impact of repeat RSV infections on antibody levels represents a promising avenue for further exploration.
In conclusion, this work confirms that pre-F IgA measured in the first or second year of life can be used as a surrogate marker of previous RSV infection with a threshold of 0.22 AU·mL−1 proposed as indicative of a past RSV infection. While this threshold is assay specific, an international reference standard was used to allow bridging to other assays. The NIBSC 16/284 international RSV standard diluted to 2000 IU·ML−1 corresponded to 474 AU·mL−1 and 65.5 AU·mL−1 in our Pre-F IgG and Pre-F IgA assays, respectively. Pre-F IgA antibody determinations are expected to be useful in future studies assessing the efficacy of candidate vaccines against RSV. Further testing with a larger, more diverse cohort and more reliable separation between RSV-positive and RSV-negative groups can confirm the generalisability of these results. Indeed, while several RSV prophylactics indicated for the paediatric population have been approved to date, they all rely on passive immunisation, whether through the direct administration of monoclonal antibodies (i.e., palivizumab/Synagis for high-risk infants and young children; nirsevimab/Beyfortus, new-generation extended half-life mAb indicated for all infants in their first RSV season, and in children up to 24 months of age [10]) or through vaccine administration to pregnant individuals to protect infants from birth to 6 months of age (i.e., Abrysvo, bivalent RSV A and B pre-F recombinant vaccine) [11, 12]. Multiple vaccine candidates, including particle-based, subunit, live-attenuated, and mRNA standalone or combination vaccines, as well as monoclonal antibodies, are currently in various stages of clinical trials [18, 29] and may benefit from a serological framework allowing to discriminate between RSV-naïve children and those with a history of RSV infection. Potential uses include the characterisation of the baseline serostatus of trial subjects to assess the impact of prior RSV infections on vaccine efficacy, the investigation of pre-F IgA contribution to immune protection or as a serum-based alternative diagnostic marker of RSV infection.
Acknowledgments
The authors would like to thank Haritha Adhikarla, Sanofi, for coordinating testing activities at Sanofi labs; Katherine Fries, Rebekah Baldwin and Ping Luo, Sanofi, for their role in developing and qualifying the RSV microneutralisation assay; Mosquera Luis and Vipra Dhir, from Sanofi, for developing and qualifying the RSV-F IgA ELISA; and Sanie Sesay, Sanofi, for contributing to the study concept. The authors also thank Manojkumar Patel and Anwesha Mandal, Sanofi, for their medical writing assistance.
Footnotes
PROMISE investigators: Jeroen Aerssens, Benoit Callendret and Gabriela Ispas (Janssen, Beerse, Belgium); Bahar Ahani (AstraZeneca, Gaithersburg, MD, USA); Jessica Atwell, Elizabeth Begier, Monica Turiga and Tin Tin Htar (Pfizer, Paris, France); Mathieu Bangert, Rolf Kramer and Charlotte Vernhes (Sanofi Pasteur, Lyon, France); Philippe Beutels (University of Antwerp, Antwerpen, Belgium); Louis Bont (University Medical Centre Utrecht, Utrecht, the Netherlands); Harry Campbell, Harish Nair, You Li, Sebastien Kenmoe, Richard Osei-Yeboah and Xin Wang (University of Edinburgh, Edinburgh, UK); Rachel Cohen, Gael Dos Santos, Philip Joosten and Theo Last (GSK, Wavre, Belgium); Veena Kumar (Novavax, Gaithersburg, MD, USA); Nuria Machin (Teamit Research, Barcelona, Spain); Hanna Nohynek (Finnish National Institute for Health and Welfare, Helsinki, Finland); Peter Openshaw (Imperial College London, London, UK); John Paget (Netherlands Institute for Health Services Research, Utrecht, the Netherlands); Andrew Pollard (University of Oxford, Oxford, UK); Anne Teirlinck (National Institute for Public Health and the Environment, Bilthoven, the Netherlands); Arantxa Urchueguía-Fornes, Ainara Mira-Iglesias, Alejandro Orrico-Sánchez and Javier Díez-Domingo (Vaccine Research Department, FISABIO-Public Health and CIBER de Epidemiología y Salud Pública, Instituto de Salud Carlos III, Valencia, Spain); Johannesen Caroline Klint (Nordsjællands Hospital, Denmark); Mark Miller (School of Public Health and Community Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden); Rafael Mikolajczyk (Institute for Medical Epidemiology, Biometry, and Informatics, Medical Faculty, Martin Luther University of Halle-Wittenberg, Halle, Germany); and Terho Heikkinen (Department of Pediatrics, University of Turku and Turku University Hospital, Turku, Finland).
Provenance: Submitted article, peer reviewed.
Author contributions: C. Vernhes, A. Moureau, R. Mikolajczyk and R. van Binnendijk contributed to the conception and design of the study. C. Vernhes, A. Moureau, Y. Jia, R.S. Thwaites and D. Zhang contributed to the methodology. The investigation was led by R. Schepp and R. van Binnendijk. Y. Jia was responsible for formal data analysis. R. Schepp and R. van Binnendijk performed data validation. R. Schepp contributed to the resourcing. Data curation was managed by B. Klee, C. Gottschick, R. Mikolajczyk and R. van Binnendijk. Visualisation was executed by Y. Jia. C. Vernhes was responsible for overall supervision. Project administration was jointly handled by C. Vernhes and A. Moureau, and funding acquisition was managed by C. Vernhes and R. Mikolajczyk.
Conflict of interest: C. Gottschick, B. Klee, R. Schepp and R.S. Thwaites are members of the PROMISE consortium. Y. Jia, A. Moureau and D. Zhang are employees of Sanofi and may hold shares in the company. C. Vernhes was a Sanofi employee at the time of the work and holds shares in the company; she is currently employed by Vaccines Europe. R. Mikolajczyk and R. van Binnendijk have no conflicts of interest to declare.
Support statement: The PROMISE project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement 101034339. This Joint Undertaking receives support from the European Union Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations. The funders had no role in the design of the study; the collection, analysis or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. LoewenKIDS study was funded by the internal funding of the participating institutions. Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
Collaborators: Jeroen Aerssens, Benoit Callendret, Gabriela Ispas, Bahar Ahani, Jessica Atwell, Elizabeth Begier, Monica Turiga, Tin Tin Htar, Mathieu Bangert, Rolf Kramer, Charlotte Vernhes, Philippe Beutels, Louis Bont, Harry Campbell, Harish Nair, You Li, Sebastien Kenmoe, Richard Osei-Yeboah, Xin Wang, Rachel Cohen, Gael Dos Santos, Philip Joosten, Theo Last, Veena Kumar, Nuria Machin, Hanna Nohynek, Peter Openshaw, John Paget, Andrew Pollard, Anne Teirlinck, Arantxa Urchueguía-Fornes, Ainara Mira-Iglesias, Alejandro Orrico-Sánchez, Javier Díez-Domingo, Johannesen Caroline Klint, Mark Miller, Rafael Mikolajczyk, and Terho Heikkinen
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
01288-2024.SUPPLEMENT
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1.Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. doi: 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 2018; 217: 1356–1364. doi: 10.1093/infdis/jiy056 [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399: 2047–2064. doi: 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown PM, Schneeberger DL, Piedimonte G. Biomarkers of respiratory syncytial virus (RSV) infection: specific neutrophil and cytokine levels provide increased accuracy in predicting disease severity. Paediatr Respir Rev 2015; 16: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics 2020; 146: e20193611. doi: 10.1542/peds.2019-3611 [DOI] [PubMed] [Google Scholar]
- 6.Córdova-Dávalos LE, Hernández-Mercado A, Barrón-García CB, et al. Impact of genetic polymorphisms related to innate immune response on respiratory syncytial virus infection in children. Virus Genes 2022; 58: 501–514. doi: 10.1007/s11262-022-01932-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas-Salazar C, Chirkova T, Gebretsadik T, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. The Lancet 2023; 401: 1669–1680. doi: 10.1016/S0140-6736(23)00811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang Z, Tan S, Ma D. Respiratory syncytial virus: from pathogenesis to potential therapeutic strategies. Int J Biol Sci 2021; 17: 4073–4091. doi: 10.7150/ijbs.64762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340: 1113–1117. doi: 10.1126/science.1234914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keam SJ. Nirsevimab: first approval. Drugs 2023; 83: 181–187. doi: 10.1007/s40265-022-01829-6 [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency . Abrysvo . Date last updated: 26 September 2023. Date last accessed: 10 October 2023. www.ema.europa.eu/en/medicines/human/EPAR/abrysvo
- 12.U.S. Food and Drug Administration. Abrysvo . Date last updated: 14 September 2023. Date last accessed: 10 October 2023. www.fda.gov/vaccines-blood-biologics/abrysvo
- 13.Karron RA, Luongo C, Mateo JS, et al. Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-seronegative children. J Infect Dis 2020; 222: 82–91. doi: 10.1093/infdis/jiz408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piedra PA, Hause AM, Aideyan L. Respiratory syncytial virus (RSV): neutralizing antibody, a correlate of immune protection. In: Tripp RA, Jorquera PA, eds. Human Respiratory Syncytial Virus: Methods and Protocols. New York, Springer New York, 2016; pp. 77–91. [DOI] [PubMed] [Google Scholar]
- 15.Glezen WP, Paredes A, Allison JE, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98: 708–715. doi: 10.1016/S0022-3476(81)80829-3 [DOI] [PubMed] [Google Scholar]
- 16.Ochola R, Sande C, Fegan G, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 2009; 4: e8088. doi: 10.1371/journal.pone.0008088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jans J, Wicht O, Widjaja I, et al. Characteristics of RSV-specific maternal antibodies in plasma of hospitalized, acute RSV patients under three months of age. PLoS One 2017; 12: e0170877. doi: 10.1371/journal.pone.0170877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PATH . RSV vaccine and mAb snapshot. Date last updated: 21 September 2023. Date last accessed: 10 October 2023. www.path.org/resources/rsv-vaccine-and-mab-snapshot/
- 19.Schepp RM, de Haan CAM, Wilkins D, et al. Development and standardization of a high-throughput multiplex immunoassay for the simultaneous quantification of specific antibodies to five respiratory syncytial virus proteins. mSphere 2019; 4: e00236-19. doi: 10.1128/mSphere.00236-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 2014; 5: 446. doi: 10.3389/fimmu.2014.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkins D, Yuan Y, Chang Y, et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat Med 2023; 29: 1172–1179. doi: 10.1038/s41591-023-02316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy BR, Alling DW, Snyder MH, et al. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol 1986; 24: 894–898. doi: 10.1128/jcm.24.5.894-898.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berbers G, Mollema L, van der Klis F, et al. Antibody responses to respiratory syncytial virus: a cross-sectional serosurveillance study in the Dutch population focusing on infants younger than 2 years. J Infect Dis 2021; 224: 269–278. doi: 10.1093/infdis/jiaa483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andeweg SP, Schepp RM, van de Kassteele J, et al. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci Rep 2021; 11: 8953. doi: 10.1038/s41598-021-88524-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschick C, Raupach-Rosin H, Langer S, et al. Cohort profile: The LoewenKIDS Study – life-course perspective on infections, the microbiome and the development of the immune system in early childhood. Int J Epidemiol 2019; 48: 1382–1383. doi: 10.1093/ije/dyz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert SB, O'Grady KF, Gabriel SH, et al. Respiratory illness during winter: a cohort study of urban children from temperate Australia. J Paediatr Child Health 2005; 41: 125–129. doi: 10.1111/j.1440-1754.2005.00561.x [DOI] [PubMed] [Google Scholar]
- 27.Schepp RM, Kaczorowska J, van Gageldonk PGM, et al. Effect of palivizumab prophylaxis on respiratory syncytial virus infection in very preterm infants in the first year of life in The Netherlands. Vaccines (Basel) 2023; 11: 1807. doi: 10.3390/vaccines11121807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teoh Z, Conrey S, McNeal M, et al. Burden of respiratory viruses in children less than 2 years old in a community-based longitudinal US Birth cohort. Clin Infect Dis 2023; 77: 901–909. doi: 10.1093/cid/ciad289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verwey C, Madhi SA. Review and update of active and passive immunization against respiratory syncytial virus. BioDrugs 2023; 37: 295–309. doi: 10.1007/s40259-023-00596-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhm A, Ellsässer G, Lüdecke K. [The Brandenburg social index: a tool for health and social reporting at regional and communal levels in the analysis of data of school beginners]. Gesundheitswesen 2007; 69: 555–559. doi: 10.1055/s-2007-992772 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
01288-2024.SUPPLEMENT
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.