Abstract
Helicobacter pylori (H. pylori) is a type of pathogenic bacteria that can live in the host’s stomach for a long time. H. pylori infection affects 50% of the world’s population. Outer membrane vesicles (OMVs) secreted by H. pylori can enter the host blood circulation and participate in mediating systemic diseases. However, the effects of OMVs exposure during pregnancy on the inflammatory response and hormone metabolism remain unclear. H. pylori was cultured and its outer membrane vesicles were isolated. The structure and morphology of OMVs were observed by transmission electron microscopy and characterized by LC–MS. E18-19 pregnant mice were injected with OMVs via the tail vein to establish the exposure model and IL-6, IL-1β, TNF-α in the serum of pregnant mice after intragastric administration of Resveratrol (Res) were determined. Targeted metabolomics was used to detect the metabolic changes of related hormones in vivo after Res intervention. In vivo injection of Res can significantly reduce the serum level of IL-6, IL-1β and TNF-α attributable to H. pylori OMVs, improve the imbalance of androgens and steroids caused by H. pylori OMVs and regulate the level of estrogen. Res can inhibit the systemic inflammatory response caused by H. pylori OMVs and regulate the imbalance of androgen and steroid hormones caused by H. pylori OMVs.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-17933-y.
Keywords: Helicobacter pylori, Outer membrane vesicles, Resveratrol, Inflammation, Hormone metabolism
Subject terms: Bacterial host response, Bacterial pathogenesis, Clinical microbiology
Introduction
Helicobacter pylori (H. pylori) is a gram-negative pathogen capable of colonizing the host’s stomach for long periods of time. About half of the world’s population is infected with H. pylori1–4. H. pylori infection is closely related to the onset of chronic active gastritis, peptic ulcer, and gastric cancer5–7 and is also associated with the occurrence and development of iron-deficiency anemia, arteriosclerosis, diabetes, Alzheimer’s disease, and other medical problems8–11. Moreover, H. pylori infection is associated with a higher risk of nausea and vomiting of pregnancy (NVP), severe NVP, hyperemesis gravidity, functional dyspepsia, and spontaneous preterm birth12.
H. pylori can produce outer membrane vesicles (OMVs)13. OMVs are spherical nanoscale vesicles with a double-layered membrane structure and a diameter of 20–400 nm14. Recent studies have shown that OMVs can enter the host’s circulatory system and may cross the placental barrier15.The envelope of the vesicles is formed from the bacterial outer membrane, and the vesicle cavity contains bioactive components such as cytotoxin-associated gene A (CagA), vacuolating cytotoxin (Vac), and urease (Ure) derived from the parent bacterium14. H. pylori OMVs are considered as carriers of information and material exchange between the bacteria and the environment. This information and material can enter the blood circulation and affect the whole body. Studies have confirmed that the production and release of OMVs by H. pylori represent an important pathogenic mechanism16,17.
At present, the clinical treatment of H. pylori infection is still based on a triple or quadruple antibiotic combination18. This method can quickly remove H. pylori from the stomach, but it has many disadvantages such as extensive side effects, ease of relapse after drug withdrawal, and bacterial resistance and intestinal flora imbalance following repeated drug use19. Ways of inhibiting H. pylori reproduction and of releasing H. pylori OMVs, reducing the inflammatory response caused by H. pylori OMVs in the blood, and then regulating endocrine disorders and metabolic imbalance caused by H. pylori OMVs have become the focus of our research. Resveratrol (Res) is a polyphenolic compound that is abundant in plants such as Polygonum cuspidatum and grape vines20,21. It has effects on immune regulation, anti-inflammation, anti-oxidation, the regulation of glucose and lipid metabolism, and the inhibition of bacterial growth22,23 . In addition, it has been reported that Res can inhibit the growth of H. pylori in the stomach24,25, based on which we speculate that Res can reduce the release of H. pylori OMVs, thereby reducing the systemic inflammatory response caused by H. pylori OMVs into the blood. It may play a regulatory role in the imbalance of maternal hormone metabolism caused by H. pylori OMVs.
We have established a systemic exposure model by injecting H. pylori OMVs via the tail vein of pregnant mice, detecting any changes in serum inflammatory factor IL-6, IL-1β and TNF-α content after the intervention of Res, and analyzing the metabolic changes of related hormones in the body after the intervention of Res by targeted metabolomics, with the aim of observing the intervention effects of Res and their material bases.
Materials and methods
Bacterial strains and growth conditions
The H. pylori SS1 strain (BioVector NTCC Inc., cat. no. HpSS1) used in this study was grown in Columbia blood agar medium under micro-aerobic conditions (85% N2, 10% CO2, 5% O2) in an incubator at 37 ℃ for 72 h. Cultures of H. pylori were scraped off the culture plates and then incubated in Brain Heart Infusion Broth (BHI, Oxiod) containing 10 μg/ml vancomycin, 2.5 μg/ml amphotericin B, 5 μg/ml trimethoprim, and 2.5 IU/ml polymyxin B. The culture flasks were placed in a 140 rpm shaking incubator and incubated for 72 h at 37 °C under micro-aerobic conditions15.
Isolation and purification of H. pylori OMVs
To isolate and purify H. pylori OMVs from H. pylori liquid medium, appropriate modifications were made according to published protocols26. The cultured liquid medium was collected, and the bacteria were pelleted by centrifugation (12,000 × g, 15 min, 4 °C). The supernatant was then filtered through 0.45-μm and 0.22-μm pore sizes filters following which it was subjected to 100 kDa ultrafiltration to collect OMVs. The pure OMVs were then extracted through an exclusion column kit (Invitrogen exosome spin column MW3000, USA) according to the manufacturer’s instructions26. OMVs protein was extracted and quantified using the BCA Protein Assay kit (Pierce Thermo Scientificial, Rockford, IL, USA).
Characterization of H. pylori OMVs
A 20 µL aliquot of H. pylori OMVs was dropwise added onto a copper mesh and then stained with 2% uranyl acetate for 5 min. The purity and morphology of H. pylori OMVs were identified by transmission electron microscopy (TEM). Images were taken by means of a HITACHI HT7800 transmission electron microscope (available at Electron Microscopy Center, Hebei Medical University). OMV proteins were separated by 10% SDS-PAGE and then stained with Coomassie Brilliant blue (R-250). Finally, gel images were obtained with an Amersham Imager 600 (GE Healthcare).
Total proteins in the extracted OMVs samples were analyzed using liquid chromatography tandem mass spectrometry (LC–MS/MS). Peptides were extracted by elution from each sample by using an Easy nLC 1200 system (Thermo Scientific, USA). Peptide fragments were then identified using a Q-Exactive HF-X mass spectrometer (Thermo Scientific, USA). The identification information of target protein molecules was obtained by searching the non-redundant protein database of the National Center for Biotechnology Information (NCBI) through a MASCOT search engine (Matrixscience.com).
Animals experiments and sample collection
All animals were maintained at the SPF level and verified to be free from H.pylori infection. C57BL/6 mice from our own colony were housed in a standard animal room with access to food and water ad libitum and under controlled conditions of humidity, temperature (21 ± 2 °C), and luminosity (200 lx) and a 12 h light/dark lighting schedule (lights on at 7:00 h). For mating, a single male mouse was mated with two nulliparous females of the same strain. Time of pregnancy was determined by visual inspection of the vaginal plug, which was defined as Day 0 of pregnancy. All animal experiments were conducted in compliance with the guidelines of the Animal Welfare Act and related regulations of the First Hospital of Hebei Medical University, No.20220460. All methods are in accordance with ARRIVE guidelines.
E19 pregnant mice (vaginal plug dates were designed as E1) were randomly divided into four groups (Control, OMVs, Res, Res + OMVs). In this experiment, each group consisted of six pregnant mice. Control group pregnant mice received an intravenous injection of 0.2 mL sterile phosphate-buffered saline (PBS) under the same experimental conditions. OMVs group: mice received a tail-vein injection of H. pylori OMVs (0.2 ml, 20 μg/ml) and an oral gavage of 0.2 mL (PBS). Res group: mice were administered resveratrol (Res, 3 mg/kg, dissolved in 0.2 mL PBS, MCE, USA) via oral gavage and received a tail vein injection of 0.2 mL sterile PBS. Res + OMVs group: mice received Res (3 mg/kg) administered by gavage in 0.2 mL PBS at 8:00 and were injected with H.pylori OMVs (0.2 ml, 20 μg/ml) via the tail vein at 13:00. All groups were further examined on Day 20 of pregnancy at 13:00: blood samples were collected by means of cardiac blood sampling, and serum samples were subsequently obtained by centrifugation. The samples were frozen at -80 °C until use. Only pregnant mice with 6 fetuses were included in this study.
Targeted metabolomics analysis
Targeted metabolomics analysis used serum samples from pregnant mice, collected via retroorbital venous plexus blood collection. Blood was centrifuged to separate serum, which was stored at -80℃ until use. We performed targeted metabolomics studies with minor modifications27. A total of 100μL of each sample was added to a correspondingly numbered 2 mL centrifuge tube, and 400μL of methanol extract was added. The serum samples were vortexed for 10 min, left on ice for 10 min, and centrifuged at 12,000 r/min for 5 min at 4 °C. Aliquots of 400μL of the supernatant was collected in a corresponding 1.5 mL centrifuge tube and allowed to dry out completely at 20 °C. The serum samples were redissolved in 100μL pure methanol, vortexed for 5 min, and centrifuged at 12,000 r/min for 3 min at 4 °C. Samples of 80μL of the supernatant were removed for LC–MS/MS upper machine analysis. The instrument system for data acquisition mainly included Ultra Performance Liquid Chromatography (UPLC) and Tandem Mass Spectrometry (MS/MS). Phenomenex Kinetex C18 (1.7 µm, 100 mm × 2.1 mm i.d.); Phase A, acetonitrile/water (30/70, 0.04% acetic acid); Phase B, acetonitrile/isopropanol (50/50, containing 0.04% acetic acid); 0 min A/B 95:5 (V/V), 1 min A/B 95:5 (V/V), 10 min A/B 10:90 (V/V), 12.5 min A/B 10:90 (V/V), 12.6 min A/B 95:5 (V/V); The A/B ratio was 95:5 (V/V) at 15 min. The detection mode was positive mode, Electrospray Ionization (ESI) temperature 550 ℃, mass spectrometer voltage 5500 V, Curtain Gas (CUR) 35 psi. In the triple quadrupole, each ion pair was scanned and detected according to the optimized Declustering Potential (DP) and Collision Energy (CE). The Multiple Reaction Monitoring (MRM) mode was used for monitoring.
Statistical analysis
The software GraphPad Prism 9.0.0 was used for statistical analysis. Data results are presented as mean ± SD. The Shapiro—Wilk test was employed to assess the data distribution. The results indicated that the data followed a normal distribution (P > 0.05). Student’s t test was used for paired pairs, the two-tailed t test was used for unpaired pairs, and a two-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were used for multiple group comparisons. And statistical analyses were performed using R4.2.2 (http:// www.R-project.org, The R Foundation) and Free Statistics software version 2.2. Statistical results are expressed as *P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns: no significance.
Results
Characterization of H. pylori OMVs.
We obtained OMVs from a liquid culture of H. pylori strain SS1(BioVector NTCC Inc., cat.no. Hp SS1) (Fig. 1A). The structural integrity of H. pylori OMVs was confirmed by transmission electron microscopy (Fig. 1B). When we compared the total protein profile of H. pylori OMVs with the whole extract of the H. pylori parent strain, we found that the protein profile of OMVs was not exactly the same as that of the extract from the parent strain (Fig. 1B). However, we identified, by LC–MS, that OMVs and H. pylori cells exhibit high overlap in core outer membrane-associated proteins.
Fig. 1.
Culture of H. pylori and isolation and identification of its outer membrane vesicles. A, overall protocol for H. pylori OMVs isolation, concentration, and purification protocols. B, typical spherical vesicle-like morphological structure observed by transmission electron microscopy. Results of Coomassie blue staining after H. pylori OMVs electrophoresis. St indicates the molecular marker standard.
Res can reduce the damage of H. pylori OMVs to fetal mice
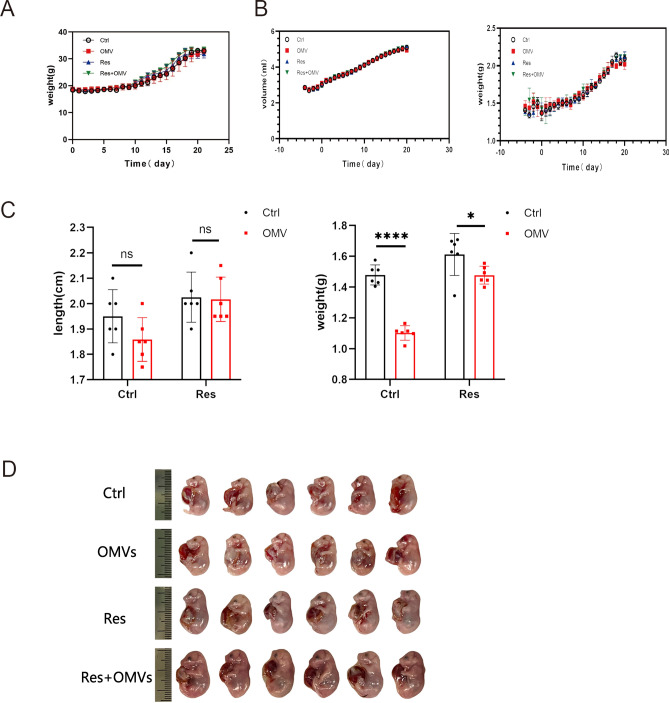
Body weight (Fig. 2A), water and diet (Fig. 2B) intake of pregnant mice did not change significantly during Res administration. These results suggest that Res has no significant effect on the health of pregnant mice. In addition, Res treatment significantly alleviated the adverse effects of H. pylori OMVs on fetal mice weight and body length(Fig. 2C). The placental and umbilical tissues were removed before weighing (Fig. 2C-D). All these data suggest that Res is safe and has a significant therapeutic effect on H. pylori OMVs.
Fig. 2.
Res can reduce the damage of H. pylori OMVs to fetal mice. A, changes in body weight of maternal mice during pregnancy. B, water intake and food intake of pregnant mice. C-D, body length and weight of fetal mice between different groups. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, ns: no significance.
Res can reduce the changes of inflammatory factors caused by H. pylori OMVs in vivo
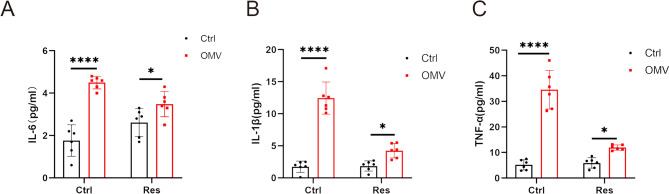
The levels of IL-6, IL-1β and TNF-α in the peripheral blood of pregnant mice in the OMVs group was significantly higher than that of the control group (P < 0.0001). The IL-6, IL-1β and TNF-α level of the Res group was slightly higher than that of the control group (P > 0.05) but was significantly lower than the OMVs group (P < 0.05). The IL-6 level of the Res + OMVs group was significantly higher than that of the control group (P < 0.01) and significantly lower than that of the OMVs group (P < 0.05), indicating that Res had anti-inflammatory effects. After Res treatment, the elevation of IL-6, IL-1β, TNF-α caused by H. pylori OMVs was significantly improved (Fig. 3A-C). All the above data indicate that Res can safely treat pregnant mice stimulated by H. pylori OMVs and reduce the inflammatory factors caused by H. pylori OMVs.
Fig. 3.
Res can reduce the changes of inflammatory factors caused by H. pylori OMVs in vivo. A-C, serum levels of IL-6, IL-1β and TNF-α in peripheral blood of pregnant mice in different groups were detected by ELISA. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, ns: no significance.
Targeted detection of metabolites in peripheral blood during pregnancy.
To investigate the hormonal changes in pregnant mice under various stimulation conditions, we performed targeted metabolomics of maternal serum samples by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS) and identified 26 metabolites. These included four estrogens, four progestins, five androgens, one vitamin, four corticosteroids, and eight sterols (Table 1).
Table 1.
Identification of targeted serum metabolite by UPLC-QTOF-MS/MS.
| N | Compounds | Class | Q1 (Da) |
Q3 (Da) |
Molecular Weight | Formula |
|---|---|---|---|---|---|---|
| 1 | Pregnenolone | Progestogen | 299.2 | 299.2 | 316.2402303 | C21H32O2 |
| 2 | 17α-Hydroxypregnenolone | Progestogen | 297.3 | 297.3 | 332.2351449 | C21H32O3 |
| 3 | 5α-Pregnane-3,20-dione | Progestogen | 317.3 | 281.3 | 316.2402303 | C21H28O4 |
| 4 | Progesterone | Progestogen | 315.2 | 97.1 | 314.2245802 | C21H30O2 |
| 5 | Vitamin-D3 | Vitamin | 385.3 | 367.3 | 384.339216 | C27H44O |
| 6 | Androstenedione | Androgen | 287.2 | 97.0 | 286.19328 | C19H26O2 |
| 7 | Testosterone | Androgen | 289.2 | 97.2 | 288.20893 | C19H28O2 |
| 8 | Androstanedione | Androgen | 289.2 | 271.2 | 288.20893 | C19H28O2 |
| 9 | 11-Ketoetiocholanolone | Androgen | 287.2 | 287.2 | 304.203845 | C19H28O3 |
| 10 | 5β-Androsterone | Androgen | 273.2 | 273.2 | 290.22458 | C19H30O2 |
| 11 | Deoxycorticosterone | Cortical hormon | 331.3 | 97.0 | 330.219495 | C21H30O3 |
| 12 | Corticosterone | Cortical hormon | 347.2 | 329.3 | 346.214409 | C21H30O4 |
| 13 | 11-Dehydrocorticosterone | Cortical hormon | 345.3 | 121.1 | 344.1987594 | C21H28O4 |
| 14 | Cortisone | Cortical hormon | 361.3 | 163.2 | 360.193674 | C21H28O5 |
| 15 | 4-Methoxy-Estrone | Estrogen | 301.4 | 283.4 | 300.172545 | C19H24O3 |
| 16 | 2-Methoxy-Estrone | Estrogen | 301.4 | 283.4 | 300.172545 | C19H24O3 |
| 17 | Estrone | Estrogen | 271.3 | 271.3 | 270.1619799 | C18H22O2 |
| 18 | 17β-Estradiol | Estrogen | 255.3 | 255.3 | 272.17763 | C18H24O2 |
| 19 | β-Sitosterol | Sterols | 397.3 | 397.3 | 414.386166 | C29H50O |
| 20 | 7α,25-Dihydroxycholesterol | Sterols | 401.3 | 401.3 | 418.3446953 | C27H46O3 |
| 21 | 7-Ketocholesterol | Sterols | 401.2 | 383.3 | 400.334131 | C27H44O2 |
| 22 | 7-Hydroxy-cholesten-3-one | Sterols | 383.3 | 383.3 | 400.334131 | C27H44O2 |
| 23 | Lathosterol | Sterols | 369.3 | 369.3 | 386.354866 | C27H46O |
| 24 | Desmosterol | Sterols | 367.3 | 367.3 | 384.339216 | C27H44O |
| 25 | 24-Hydroxycholesterol | Sterols | 385.3 | 385.3 | 402.34978 | C27H46O2 |
| 26 | Cholesterol | Sterols | 369.4 | 369.4 | 386.3548661 | C27H46O |
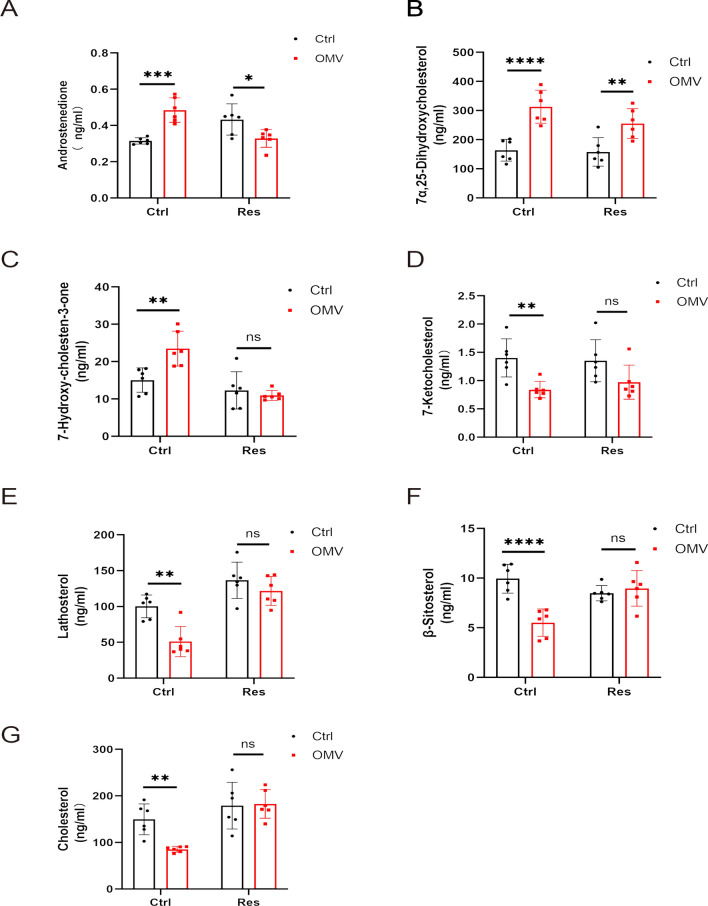
Further statistical analysis of the identified metabolites showed that the levels of maternal androstenedione, 7α, 25-dihydroxycholesterol, and 7-hydroxy-cholestene-3-one in the OMVs group were higher than those in the control group, and that the difference was statistically significant (P < 0.05). Early intervention with Res reduced the levels of androstenedione, 7α, 25-dihydroxycholesterol, and 7-hydroxy-cholestene-3-one, among which androstenedione and 7-hydroxy-cholestene-3-one were significantly reduced (P < 0.05). Maternal 7-ketocholesterol, lathosterol, β-sitosterol, and cholesterol in the OMVs group were significantly lower than those in the control group (P < 0.05). Early intervention with Res might have increased the levels of these four metabolites in the mother, among which lathosterol, β-sitosterol, and cholesterol were significantly increased (P < 0.05), suggesting that Res was capable of regulating the imbalance of metabolism caused by H. pylori OMVs (Fig. 4A-G).
Fig. 4.
Data analysis of peripheral blood metabolites in pregnancy under various conditions. A-G, targeted detection of metabolites (androstenedione, 7α, 25-dihydroxycholesterol, 7-hydroxy-cholestene-3-one, 7-ketocholesterol, lathosterol, β-sitosterol, and cholesterol) in peripheral blood during pregnancy. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, ns: no significance.
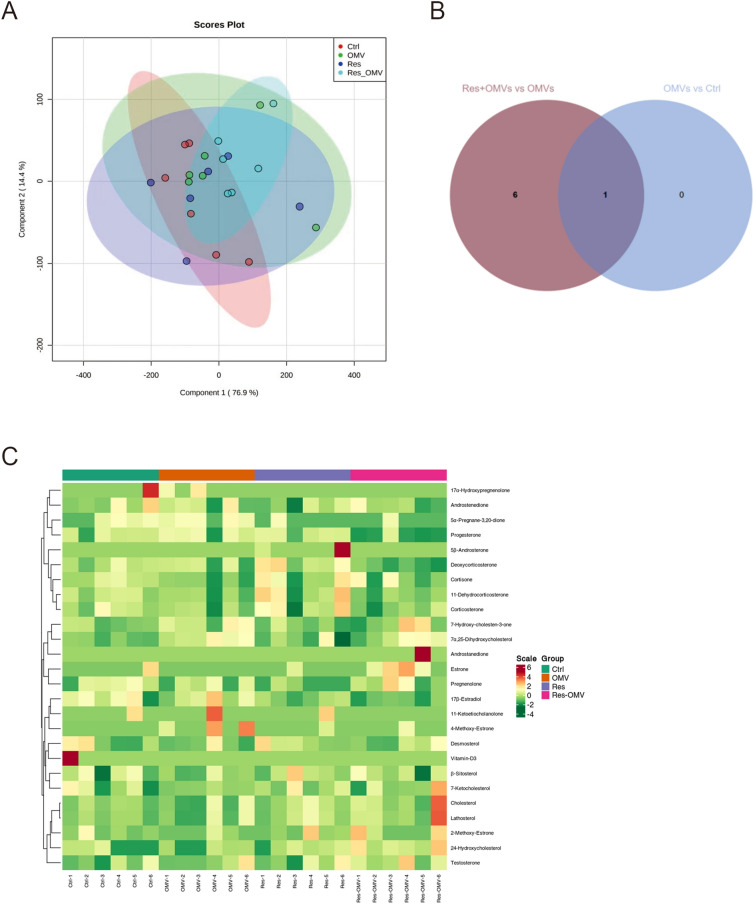
Principal component analysis of peripheral blood metabolites
Principal component analysis (PCA) was performed on the samples to allow a preliminary understanding of the overall metabolic differences among the samples of each group and the degree of variation among the samples within the group. The PCA score plot is shown in Fig. 5A. Each point in the figure represents the projection of the sample on the two-dimensional plane, and the projection position of each point represents the metabolite data set contained in each sample. Samples with similar physiological or pathological status have close locations in the PCA score plot. However, a more distant location means that the states are more different. The results showed that the data sets within each group had an obvious aggregation phenomenon, indicating that each biological sample was relatively stable during the analysis process, and that the experimental data had good accuracy and reliability.
Fig. 5.
Screening, identification, and overall heat map analysis of differential metabolites. Samples of each group were set as n = 6. A, PCA score plot of sample quality spectrum data for each group. Red represents the control group, green represents the OMV group, blue represents the Res group and cyan represents the Res + OMV group. B, venn graph of differential metabolites. C, cluster heatmap of the sample population. The horizontal axis shows the sample name, the vertical axis shows the metabolite information, the different colors indicate the different values obtained after the standardization of different contents (red represents high content, green represents low content), and Class is the classification of substances.
Screening, identification, and overall heat map analysis of differential metabolites
One differential metabolite was identified between the OMVs group and the control group: 4-Methoxy-Estrone (Table 2). Two differential metabolites were identified between the Res group and the control group: pregnenolone and 5α-Pregnane-3,20-dione (Table 3). Six differential metabolites were identified between the Res + OMVs group and the control group: 5α-Pregnane-3,20-dione, Progesterone, Deoxycorticosterone, 2-Methoxy-Estrone, Estrone, and 24-Hydroxycholesterol (Table 4). A total of 7 differential metabolites were identified between the Res + OMVs group and the OMVs group: 5α-Pregnane-3,20-dione, Progesterone, Deoxycorticosterone, 4-Methoxy-Estrone, 2-Methoxy-Estrone, Estrone, and 24-Hydroxycholesterol (Table 5). The differential metabolite shared between the OMVs group and the control group and between the Res + OMVs group and the OMVs group was 4-Methoxy-Estrone (Fig. 5B), which was in an elevated state during H. pylori OMVs exposure (Table 2) and in a decreased state after an advance intervention of Res (Table 5).
Table 2.
List of differential metabolites between the control and OMVs groups.
| N | Compounds | Class | control group vs OMVs group |
P |
|---|---|---|---|---|
| 1 | 4-Methoxy-Estrone | estrogen | ↑ | 0.109 |
Table 3.
List of differential metabolites between the control and Res groups.
| N | Compounds | Class | control group vs Res group |
P |
|---|---|---|---|---|
| 1 | Pregnenolone | Progestogen | ↓ | 0.019 |
| 2 | 5α-Pregnane-3,20-dione | Progestogen | ↓ | 0.110 |
Table 4.
List of differential metabolites between the control and Res + OMVs groups.
| N | Compounds | Class | control group vs Res + OMVs group |
P |
|---|---|---|---|---|
| 1 | 5α-Pregnane-3,20-dione | Progestogen | ↓ | 0.098 |
| 2 | Progesterone | Progestogen | ↓ | 0.022 |
| 3 | Deoxycorticosterone | Cortical hormon | ↓ | 0.005 |
| 4 | 2-Methoxy-Estrone | estrogen | ↑ | 0.392 |
| 5 | Estrone | Estrogen | ↑ | 0.157 |
| 6 | 24-Hydroxycholesterol | Sterols | ↑ | 0.034 |
Table 5.
List of differential metabolites between the OMVs groups and Res + OMVs groups.
| N | Compounds | Class | OMVs group vs Res + OMVs group |
P |
|---|---|---|---|---|
| 1 | 5α-Pregnane-3,20-dione | Progestogen | ↓ | 0.016 |
| 2 | Progesterone | Progestogen | ↓ | 0.018 |
| 3 | Deoxycorticosterone | Cortical hormon | ↓ | 0.077 |
| 4 | 4-Methoxy-Estrone | estrogen | ↓ | 0.185 |
| 5 | 2-Methoxy-Estrone | Estrogen | ↑ | 0.229 |
| 6 | Estrone | Estrogen | ↑ | 0.042 |
| 7 | 24-Hydroxycholesterol | Sterols | ↑ | 0.026 |
In addition, we determined the changes of peripheral blood metabolites under various conditions during pregnancy by UV (unit variance scaling) processing of the data, by cluster heat map analysis of all samples, and by using R program script to draw the cluster heat map. The results are shown in Fig. 5C.
Discussion
Our study has shown, for the first time, that: (ⅰ) Res can inhibit the systemic inflammatory response caused by H. pylori OMVs, and (ⅰi) Res can regulate the imbalance of androgen and steroid hormones caused by H. pylori OMVs.
Bariani MV et al.28 injected E15 pregnant mice with bacterial endotoxin (LPS) at an amount that caused 85% of the mice to have premature delivery with prenatal hemorrhage and stillbirth. However, after the early administration of Res, 64% of the mice underwent normal full-term delivery. The authors proposed that Res was able to reduce the LPS-induced expression of pro-inflammatory factors such as prostaglandin E2, iNOS, and COX-2. A similar study indicates that Res can prevent preterm birth caused by LPS29. According to another report30, Res can inhibit the growth of H. pylori in vitro and can be used for the dietary prevention and treatment of H. pylori infection. The colonization of H. pylori in the stomach of mice is affected by many factors, including the genetic background of mice, gastrointestinal microbial community, etc. It is difficult to ensure the consistency of the number and status of H. pylori colonization among different mice, thus increasing the variability of experimental results. In addition, intact H. pylori infection may trigger a variety of complex immune responses and pathological processes, and it is difficult to simply distinguish the independent effects of OMVs. As a relatively simple membrane vesicle structure secreted by H. pylori, OMVs can be used to study the effects of bioactive substances carried by them on the body more focused, and provide clearer clues for further understanding the OMVs related links in the pathogenesis of H. pylori. In addition, the method of gavage is affected by many factors such as gastric digestion, emptying and intestinal absorption, so it is difficult to accurately control the actual dose of OMVs into the blood circulation. Intravenous injection of OMVs in the tail vein can ensure that the set dose of OMVs is delivered directly and accurately into the blood circulation of mice, and avoid the dose deviation caused by the route of administration, which is crucial for the study of the dose–response relationship of OMVs. Therefore, in this study, OMVs were injected into the tail vein of mice to establish an animal model. The results of our study show that the expression of IL-6, IL-1β and TNF-α in the peripheral blood of pregnant mice increases after the tail vein injection of H. pylori OMVs, and that Res intervention in advance can effectively reduce the serum level of IL-6, IL-1β and TNF-α. This result indicates that Res has an inhibitory effect on the systemic inflammation caused by H. pylori OMVs.
Recent investigations have found that steroid hormones can regulate the physiological functions of the mother and fetus and are essential for the maintenance of pregnancy31. The placenta secretes androgens throughout pregnancy. When impaired placental function leads to abnormal androgen levels, it can cause adverse pregnancy events such as fetal growth restriction and pre-eclampsia. Pecks U et al32. found that lipid and steroid levels were most affected in pregnancies with fetal growth restriction, whereas only minor changes in concentrations were observed in other pregnancy-related disorders. Their data suggest that placental rather than maternal liver function strongly determines lipid and steroid levels during pregnancy. In a multicenter study of predictors of steroid hormone concentrations in early pregnancy, hormone concentrations were associated with maternal age, body mass index (BMI), ethnicity, and parity. The levels of most hormones were significantly lower in advanced maternal age. Each one-year increase in maternal age was associated with a 1–2% decrease in estrone (E1), estradiol (E2), total testosterone (TT), and free testosterone (FT). In contrast, for each unit increase in maternal BMI, estrogen (E1, E2, E3) levels decreased by 1–2%, but androgen (TT, FT) concentrations increased by 1–2%. Maternal demographic factors predict sex steroid hormone concentrations during pregnancy, and there is growing evidence that the prenatal endocrine environment influences the risk of future chronic diseases in both mother and offspring33. Baardman ME et al.34 have found that cholesterol is essential for fetal development, and that, in early life, because endogenous synthesis is relatively low, the fetus mainly relies on the maternal cholesterol supply. Cholesterol has been reported to play an important role in the synthesis of related hormones during pregnancy, and a reduction of cholesterol secretion will affect the normal development of the fetus35. Our results show that the levels of Androstenedione, 7α,25-Dihydroxycholesterol, and 7-Hydroxy-cholesten-3-one are increased in the H. pylori OMVs exposed group; 7-Ketocholesterol, Lathosterol, β-Sitosterol, and Cholesterol are decreased, with these hormones exhibiting the opposite changes after intervention with Res. This result suggests that Res has an opsonizing effect on the imbalance of androgens and sterols induced by H. pylori OMVs. The common differential metabolite between the OMVs group and the normal group, and between the Res + OMVs group and the OMVs group was 4-Methoxy-Estrone. This hormone was in an elevated state when H. pylori OMVs were exposed to it, while it was in a decreased state after the advance intervention of Res. Our analysis indicates that Res regulates the level of estrogen by reducing 4-Methoxy-Estrone. Another report has shown that overweight pregnant women given Res can significantly improve their glucose and lipid metabolism indexes36. This experiment also determined that the levels of pregnenolone and 5α-Pregnane-3,20-dione in normal mice were changed after intragastric administration of Res, indicating that Res has a regulatory effect on the expression levels of related hormones. These results suggest that Res play a role in anti-inflammation, in anti-oxidation, and in enhancing immune function by regulating the hormone levels of the body.
Conclusions
The results of this study show that, after the intervention of Res, the over-expressed inflammatory factors in the mother are significantly decreased, and that the abnormal changes of hormones closely related to embryonic development are partially corrected. Therefore, we conclude that Res intervention has a protective effect on the embryo during maternal systemic inflammation and substance metabolism imbalance mediated by H. pylori OMVs. However, our study has some limitations. Because our experiments were performed only in mice, any extrapolation of the results to humans is limited.
Supplementary Information
Acknowledgements
We thank Chenming Zhou (Department of Electron Microscopy Laboratory Center, Hebei Medical University, Shijiazhuang, China) for technical assistance. We thank Jie Liu, PhD ( Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital & Physician-Scientist Center of China ) for his helpful review and comments regarding the manuscript.
Author contributions
Zhongli Shi and Dailun Hu designed and supervised the study. Yusen Wei and Lu Zhou performed and completed the experiments. Xiaofei Zhao and Lijun Mao analyzed the data. Ziyi Jia and Bowei Mu performed the experiments. Yusen Wei, Xiaofei Zhao and Dailun Hu wrote the manuscript. All authors have approved the final manuscript.
Funding
This study was supported by grants from Hebei Province Clinical Medicine Excellent Talents Training Program (No.LS201908).
Data availability
Data underlying the results presented in this paper may be obtained from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yusen Wei, Lu Zhou and Xiaofei Zhao These authors contributed equally.
Contributor Information
Zhongli Shi, Email: 16301050@hebmu.edu.cn.
Dailun Hu, Email: 17300574@hebmu.edu.cn.
References
- 1.Crowe, S. E. Helicobacter pylori Infection. N. Engl. J. Med.380(12), 1158–1165 (2019). [DOI] [PubMed] [Google Scholar]
- 2.ELSHENAWI Y, HU S, HATHROUBI S. 2023 Biofilm of helicobacter pylori: Life cycle, features, and treatment options [J]. Antibiotics (Basel, Switzerland) 12 (8) [DOI] [PMC free article] [PubMed]
- 3.Sousa, C. et al. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbio.l48(3), 376–396 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Hooi, J. K. Y. et al. Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology153(2), 420–429 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner, P. et al. Helicobacter pylori infection. Nat. Rev. Dis. Primers.9(1), 19 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu, L. et al. Helicobacter pylori promotes gastric cancer progression through the tumor microenvironment. Appl. Microbiol. Biotechnol.106(12), 4375–4385 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Nabavi-Rad, A. et al. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes14(1), 2108655 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nodoushan, S. A. H. & Nabavi, A. The interaction of helicobacter pylori infection and Type 2 diabetes mellitus. Adv. Biomed. Res.8(1), 15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie, J. et al. Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer’s disease pathogenesis via C3–C3aR signalling. J. extracell. vesicles12(2), e12306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, L., Zhao, M. & Fu, X. Gastric microbiota dysbiosis and Helicobacter pylori infection. Front. Microbiol.14, 1153269 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiang, L. et al. Extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles in atherosclerosis. Helicobacter27(2), e12877 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Feng, Y. et al. The association between Helicobacter pylori and gastrointestinal disorders during pregnancy: A Multicenter retrospective study. Helicobacter29(1), e13032 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Wei, S. et al. Outer membrane vesicles secreted by helicobacter pylori transmitting gastric pathogenic virulence factors. ACS Omega7(1), 240–258 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González, M. F. et al. Helicobacter pylori outer membrane vesicles and extracellular vesicles from helicobacter pylori-infected cells in gastric disease development. Int. J. Mol. Sci.22, 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chmiela, M., Walczak, N. & Rudnicka, K. Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases. J. Biomed. Sci.25(1), 78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolori, S. et al. The effects of helicobacter pylori-derived outer membrane vesicles on hepatic stellate cell activation and liver fibrosis in vitro. Biomed. Res. Int.2023, 4848643 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, F. et al. Outer membrane vesicles from bacteria: Role and potential value in the pathogenesis of chronic respiratory diseases. Front. Cell. Infect. Microbiol.12, 1093327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, J. & Lu, H. A novel dual targeted antibacterial agent for Helicobacter pylori. Lancet. Infect. Dis.24(6), 569–570 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Fallone, C. A., Moss, S. F. & Malfertheiner, P. Reconciliation of recent helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology157(1), 44–53 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Jo, W. S. et al. Resveratrol analogue, HS-1793, inhibits inflammatory mediator release from macrophages by interfering with the TLR4 mediated NF-κB activation. Food sci. Biotechnol.31(4), 433–441 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, X. et al. Resveratrol protects against helicobacter pylori-associated gastritis by combating oxidative stress. Int. J. Mol. Sci.16(11), 27757–27769 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breuss, J. M., Atanasov, A. G. & Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci.20(7), 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients11(5), 946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia, M., Chen, H. & Liu, S. The synergy of resveratrol and alcohol against Helicobacter pylori and underlying anti-Helicobacter pylori mechanism of resveratrol. J. Appl. Microbiol.128(4), 1179–1190 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Spósito, L. et al. Engineering resveratrol-loaded chitosan nanoparticles for potential use against Helicobacter pylori infection. Euro. J. Pharm. Biopharm.199, 114280 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Palacios, E. et al. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J. Neuroinflammation20(1), 66 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adnan, S. et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom.49(2), 96–104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bariani, M. V. et al. Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth. Mol. Hum. Reprod.23(8), 571–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuya, H. et al. Resveratrol protects against pathological preterm birth by suppression of macrophage-mediated inflammation. Reprod. Sci. (Thousand Oaks, Calif)22(12), 1561–1568 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Brown, J. C. & Jiang, X. Activities of muscadine grape skin and polyphenolic constituents against Helicobacter pylori. J. Appl. Microbiol.114(4), 982–991 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Parsons, A. M. & Bouma, G. J. A potential role and contribution of androgens in placental development and pregnancy. Life11(7), 644 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pecks, U. et al. Maternal serum lipid, estradiol, and progesterone levels in pregnancy, and the impact of placental and hepatic pathologies. Geburtshilfe Frauenheilkd.76(7), 799–808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett, E. S. et al. Predictors of steroid hormone concentrations in early pregnancy: Results from a multi-center cohort. Matern. Child. Health J.23(3), 397–407 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baardman, M. E. et al. The origin of fetal sterols in second-trimester amniotic fluid: Endogenous synthesis or maternal-fetal transport?. Am. J. Obstet. Gynecol.207(3), 202.e19–25 (2012). [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira, A. A., Elder, E. & Spaans, F. Excessive hypercholesterolemia in pregnancy impairs rat uterine artery function via activation of Toll-like receptor 4. Clin. Sci.138(4), 137–151 (2024). [DOI] [PubMed] [Google Scholar]
- 36.Malvasi, A. et al. Can trans resveratrol plus d-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients?. Clin. Ter.168(4), e240–e247 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the results presented in this paper may be obtained from the corresponding author upon reasonable request.