Abstract
Objective
This study aims to explore the correlation between Acute Hematogenous Osteomyelitis (AHO) and Deep Venous Thrombosis (DVT) in children. It seeks to identify independent risk factors for DVT secondary to AHO, enhance understanding of the disease, and establish a predictive model to guide clinical practice.
Methods
A retrospective analysis of clinical data from AHO patients treated at our hospital (January 2017 to December 2023) was conducted. Patients were divided into thrombosis and non-thrombosis groups based on DVT occurrence.
Univariate analysis used independent samples t-tests, Mann–Whitney U tests, and chi-square tests to screen DVT-associated variables (P < 0.1). Significant variables were included in a binary logistic regression model to identify independent risk factors for DVT secondary to AHO. Model fit was assessed using the Hosmer–Lemeshow test, and Cox-Snell R2 was calculated through linear regression of P-values with the dependent variable. ROC curves were plotted, and AUC was recorded to evaluate model validity and reliability.
Results
Univariate analysis revealed significant correlations between DVT and several factors, including age, peak levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), and D-dimer. Additionally, the time for CRP to normalize, concurrent pneumonia, intensive care use, the severity of osteomyelitis (Lawson score), and femoral involvement in osteomyelitis were also significantly associated with DVT (P < 0.05).
Binary logistic regression identified age (OR = 1.248), D-dimer (OR = 1.600), and concurrent pneumonia (OR = 4.921) as significantly positively associated with secondary DVT (P < 0.05).
The model demonstrated good goodness-of-fit (P > 0.05), accurately explained the data (R2 = 0.449, Cox-Snell R2 = 0.413), and had strong predictive performance (AUC = 0.895).
Conclusion
Age, peak levels of CRP/ESR/PCT/D-dimer, osteomyelitis severity (Lawson score), femoral involvement in osteomyelitis, and concurrent pneumonia were significantly associated with secondary deep vein thrombosis (DVT) in pediatric acute hematogenous osteomyelitis (AHO). Among these, age, peak D-dimer levels, and concurrent pneumonia were identified as independent risk factors for DVT in children with AHO.
The current predictive model demonstrated good performance in this cohort, providing early warning for potential DVT risk in pediatric AHO patients. However, multicenter studies are needed to further validate its generalizability.
Pediatricians may use this model to assess the necessity of DVT screening, reduce missed diagnoses, and initiate timely antithrombotic therapy to prevent adverse outcomes.
Keywords: Acute hematogenous osteomyelitis, Deep vein thrombosis, Risk factors, Children
Introduction
Acute Hematogenous Osteomyelitis (AHO) is a single or multiple bone infection caused by bacteria spreading through the bloodstream to susceptible sites. In children, AHO commonly occurs in the metaphysis of long bones in the extremities, where the rich capillary network, slow blood flow, and lack of reticuloendothelial cells make it easier for bacteria to colonize [1]. In 2014, Lawson et al. established a scoring system to assess the severity of AHO in children based on objective clinical indicators and laboratory results (Table 1) [2]. Bacteremia can lead to endothelial cell damage and the release of inflammatory factors at nearby infection sites, activation of coagulation pathways and fibrinogen, or cause local soft tissue edema resulting in blood stasis, thereby providing a microenvironment for the formation of deep venous thrombosis (DVT). The incidence of deep vein thrombosis (DVT) in children is low, approximately less than 0.01% [3]. It typically occurs following the use of central venous catheters (CVCs), trauma, infection, or surgery. In children with AHO complicated by DVT, dislodged thrombi may carry pathogenic bacteria and lead to septic thromboembolism, promoting the spread of infection [4]. If a thrombus breaks off and migrates to the lungs, it can develop into pulmonary embolism (PE). DVT and PE are collectively referred to as venous thromboembolism (VTE). In children, DVT often manifests as localized pain, which significantly overlaps with the clinical symptoms of AHO. Additionally, MRI of the affected limb's soft tissues—typically performed for AHO evaluation—is not used to assess DVT, potentially leading to delayed diagnosis and treatment, thereby increasing therapeutic challenges [5–11]. This delay may result in adverse outcomes such as respiratory distress, multi-organ failure, or even death.
Table 1.
Lawson's Severity score for osteomyelitis
| CRP initial | CRP 48 h | CRP 96 h | Respiratory rate | Febrile dats on Antibiotic | ICU admission | Disseminated disease | |
|---|---|---|---|---|---|---|---|
| 0 | < 10 | < 5 | < 5 | < 125% | < 2 | No | No |
| 1 | 10–15 | 5–10 | 5–10 | > 125% | ≥ 2 | Yes | Yes |
| 2 | > 15 | > 10 | > 10 |
Materials and methods
Study subjects
Clinical data of AHO patients treated at our hospital from January 2017 to December 2023 were retrospectively collected.
Inclusion criteria
① Discharge date between January 2017 and December 2023 ② Age between 0 and 18 years ③ Primary diagnosis of AHO ④ No other systemic diseases.
Exclusion criteria
① Abnormal coagulation function ② Previous antithrombotic therapy ③ Use of central venous catheters (CVC) ④ Chronic osteomyelitis ⑤ No bacterial culture performed ⑥ Incomplete clinical data (Fig. 1).
Fig. 1.
Research flowchart
Admission management
Upon admission, patients immediately underwent comprehensive vital sign monitoring, including continuous observation of respiratory rate, body temperature, and blood pressure, to ensure physiological stability. Laboratory tests included complete blood count analysis to assess blood composition, C-reactive protein (CRP) and procalcitonin (PCT) levels to evaluate inflammatory response, D-dimer and fibrinogen tests to assess coagulation status, and erythrocyte sedimentation rate (ESR) to monitor inflammation or infection activity. Additionally, blood and pus cultures were performed to identify pathogens for precise antibiotic guidance. Preoperative routine chest X-rays were conducted to evaluate lung conditions and rule out complications. For patients with significant local swelling and pain, soft tissue and venous ultrasounds were performed to screen for abscesses or deep vein thrombosis (DVT). While awaiting culture results, empirical treatment with cephalosporins or vancomycin was initiated to control potential infections and prevent further deterioration. The entire treatment process strictly adhered to medical protocols to ensure optimal patient outcomes.
Evaluation metrics
After admission, a thorough review of the patient's previous medical records and history is conducted to comprehensively understand the progression of their condition and fluctuations in key laboratory indicators. This information is crucial for assessing disease progression trends and treatment responses. Understanding the patient's pre-admission antibiotic usage, including drug types, dosages, treatment duration, and efficacy, provides a reference for the current antibiotic treatment strategy and helps avoid potential drug interactions or resistance issues.
Additionally, existing magnetic resonance imaging (MRI) reports and venous ultrasound reports are collected to evaluate bone marrow edema and the progression of bone destruction at the AHO infection site, as well as to identify the location and severity of deep vein thrombosis (DVT).
All patients showed no evidence of DVT upon admission to our department. During hospitalization, routine blood tests and chest X-rays were performed for all patients upon admission. Ultimately, venous ultrasound identified AHO patients complicated with DVT.
Statistical methods
Statistical analysis was performed using SPSS 27.0. Clinical data (including age, gender, clinical features, thrombus location, laboratory results, bacteriological tests, and imaging findings) were organized, and patients were divided into the DVT group and the non-DVT group based on whether they developed secondary DVT.
For continuous variables with a normal distribution, independent samples t-tests were used, with results expressed as mean ± standard deviation (x̄ ± s). For continuous variables without a normal distribution, the Mann–Whitney U test was used, with results expressed as median and interquartile range (Q1-Q3). For categorical variables, the chi-square test was used, with results expressed as frequencies and percentages. In univariate analysis, variables with a P-value < 0.1 were included in subsequent multivariate analysis. A binary logistic regression model was used to further identify independent risk factors for acute hematogenous osteomyelitis (AHO) complicated by deep vein thrombosis (DVT), and the odds ratios (ORs) and their 95% confidence intervals were calculated. To evaluate the goodness-of-fit of the model, the Hosmer–Lemeshow test was performed, and the Cox-Snell R2 value was calculated through linear regression analysis to quantify the model's explanatory power. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated to assess the model's discriminative performance. Additionally, the optimal cutoff values for continuous variables were determined through ROC curve analysis.
Result
{Following continuous antibiotic therapy and low-molecular-weight heparin (LMWH) treatment, all patients improved without recurrence of DVT.}
According to the aforementioned inclusion and exclusion criteria, a total of 76 patients were included in this study, with 30 in the DVT group and 46 in the non-DVT group. All patients had positive blood culture results, with 72 cases (95%) caused by Staphylococcus aureus infection, including 24 cases (32%) of methicillin-resistant Staphylococcus aureus (MRSA). Among these, 12 cases (40%) were in the DVT group, a higher proportion compared to 12 cases (26.1%) in the non-DVT group (P = 0.202). The average age in the DVT group (9.18 ± 3.58) was significantly higher than that in the non-DVT group (6.07 ± 3.83) (P < 0.001). Males were predominant (63.3%) (P = 0.131). Osteomyelitis involved the femur in 41 cases (54%), the tibia in 14 cases (18%), the humerus in 10 cases (13%), and the pelvis in 7 cases (9%) (Fig. 2). DVT involved the common femoral vein in 14 cases (47%), the superficial femoral vein in 9 cases (30%), the deep femoral vein in 4 cases (13%), the external iliac vein in 4 cases (13%), the anterior tibial vein in 4 cases (13%), the small saphenous vein in 3 cases (10%), the popliteal vein in 7 cases (9%), the common iliac vein in 2 cases (6%), the great saphenous vein in 2 cases (6%), the dorsal foot vein in 2 cases (6%), the brachial vein in 2 cases (6%), the subclavian vein in 2 cases (6%), the axillary vein in 2 cases (6%), and the peroneal vein in 1 case (3%) (Fig. 3). All DVT cases originated near the AHO lesion, and one patient developed VTE (3.3%).
Fig. 2.
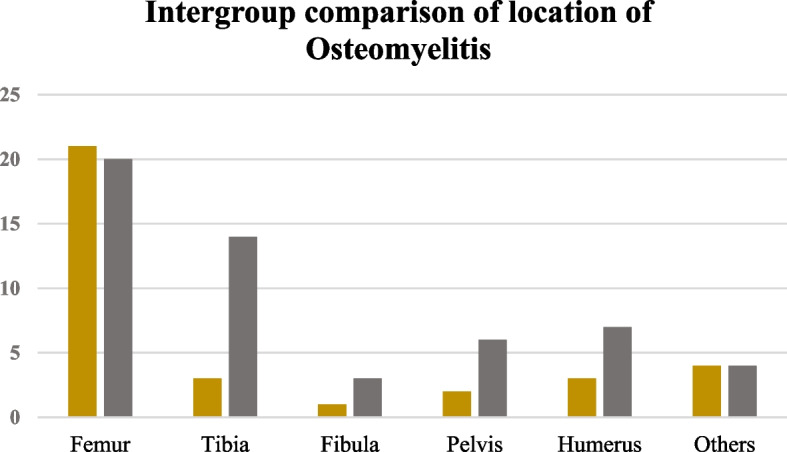
Comparison of groups based on the sites involved by osteomyelitis
Fig. 3.
Location of deep vein thrombosis
According to the pediatric osteomyelitis severity score provided by Lawson et al., in the DVT group, 25 cases (83%) were classified as"severe"and 5 cases (17%) as"moderate"(Fig. 4. Inner ring); in the non-DVT group, 31 cases (67%) were classified as"severe"and 4 cases (9%) as"mild"(Fig. 4. Outer ring) [2].
Fig. 4.
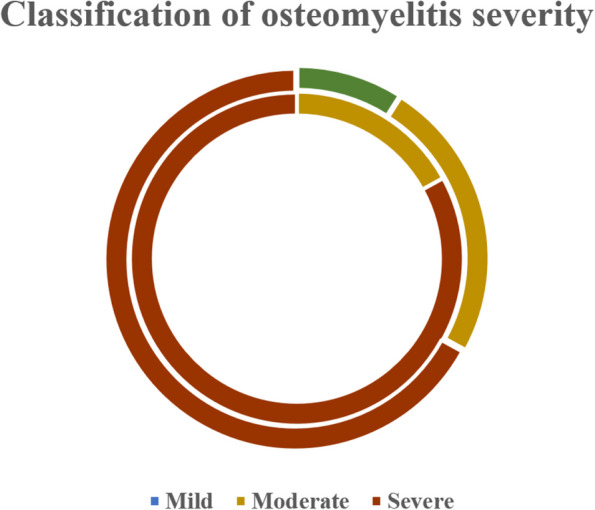
Classification of osteomyelitis severity
Univariate analysis using independent samples t-test revealed that age, CRP, and ESR were significantly associated with DVT secondary to pediatric AHO (P < 0.05).
Univariate analysis with the Mann–Whitney U test showed that PCT, peak D-dimer levels, and osteomyelitis severity (Lawson score) were significantly correlated with DVT secondary to pediatric AHO (P < 0.05).
Univariate analysis using the chi-square test indicated that concomitant pneumonia, femoral osteomyelitis, and ICU admission were significantly associated with DVT secondary to pediatric AHO (P < 0.05) (Table 2).
Table 2.
Univariate analysis results
| DVT(n = 30) | N-DVT(n = 46) | P | |
|---|---|---|---|
| Age | 9.18 ± 3.58 | 6.07 ± 3.83 | < 0.001* |
| Gender(n/%) male | 19(63.3%) | 21(45.7%) | 0.131 |
| Side(n/%) left | 15(50%) | 27(58.7%) | 0.456 |
| MRSA(n/%) | 12(40%) | 12(26.1%) | 0.202 |
| Hospital Stay | 23.5(16.7,27) | 19(13,25.5) | 0.114 |
| WBC | 18.67 ± 6.49 | 15.55(10.71,22.42) | 0.212 |
| CRP | 160.2 ± 65.62 | 117.55 ± 59.2 | 0.004* |
| CRP Normalization Time | 23.5(18,34.5) | 15.5(12,20.25) | < 0.001* |
| ESR | 95.3 ± 31.24 | 71.7 ± 28.21 | 0.001* |
| Fibrinogen | 5.95(4.56,6.5) | 4.88(4.55,6.08) | 0.172 |
| PCT | 3.54(1.57,11.52) | 1.1(0.2,8.72) | 0.006* |
| D-dimer | 3.42(2.13,4.97) | 1.16(0.62, 2.28) | < 0.001* |
| Complicated Pneumonia | 20(66.7%) | 9(19.6%) | < 0.001* |
| Multiple Infection | 6(20%) | 12(26.1%) | 0.542 |
| Femoral Osteomyelitis | 21(70%) | 20(43%) | 0.034* |
| Multiple Surgeries | 7 (23.3%) | 6 (13%) | 0.351 |
| ICU Admission | 7(23.3%) | 2(4.3%) | 0.012* |
| AHO Severity Score | 7(23.3%) | 2(4.3%) | 0.033* |
*represents a statistically significant variable
Using backward stepwise binary logistic regression (Table 3), the following independent variables were identified: age (OR = 1.248), PCT peak (OR = 1.035), D-dimer peak (OR = 1.600), and concurrent pneumonia (OR = 4.921). Among these, age, D-dimer peak, and concurrent pneumonia remained significantly positively correlated with DVT (P < 0.05), serving as independent risk factors for secondary DVT in children with AHO.
Table 3.
Binary logistic regression analysis
| (n = 1) | Wald | P | OR |
|---|---|---|---|
| Age | 5.534 | 0.019* | 1.248 (1.038, 1.501) |
| PCT | 3.221 | 0.073 | 1.035 (0.997, 1.074) |
| D-dimer | 3.898 | 0.048* | 1.600 (1.003, 2.552) |
| Complicated Pneumonia | 4.980 | 0.026* | 4.921 (1.214, 19.944) |
*represents a statistically significant variable
The goodness-of-fit test indicated that the model had a high fit (Table 4, P > 0.05). The model's R2 = 0.449, explaining 44% of the variability in the dependent variable (Cox-Snell R2 = 0.413) (Table 5), and demonstrated good predictive performance (AUC = 0.895) (Fig. 4, Table 6). The Youden index was calculated to determine the cutoff values for continuous variables within the model (age > 8.7 years, PCT peak > 1.44 ng/ml, D-dimer peak > 1.9 mg/L) (Fig. 5).
Table 4.
Hosmer–lemeshow goodness-of-fit test
| (n = 8) | Chi-square | P |
|---|---|---|
| Goodness-of-Fit Test | 4.890 | 0.769 |
Table 5.
Goodness-of-fit test using cox-snell R2
| R | R2 | Cox-Snell R2 | Sig. F |
|---|---|---|---|
| 0.67 | 0.449 | 0.413 | .000 |
Table 6.
Area under the combined ROC curve of the model
| Area | P |
|---|---|
| 0.895 | .000 |
Fig. 5.
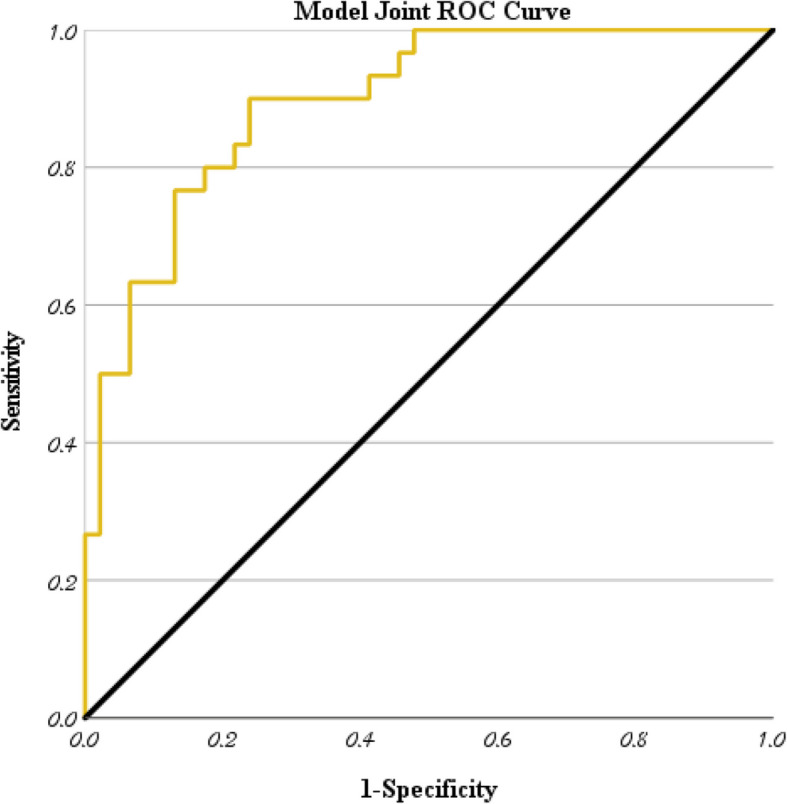
Joint ROC curve of four independent risk factors
Discussion
Given that AHO and DVT share overlapping clinical manifestations (such as local redness, swelling, and pain), a definitive diagnosis cannot be made based solely on physical examination. Analyzing the risk factors for DVT complicating AHO can provide clinicians with early warning signs, enabling timely anticoagulation therapy to prevent adverse outcomes.
Among the included AHO cases, 95% were caused by Staphylococcus aureus infections. Staphylococcus aureus can release various exotoxins, among which alpha-toxin acts on cell membranes and can induce vascular smooth muscle spasm, creating a hemodynamic environment conducive to thrombosis formation [12]. Moreover, PV toxin induces neutrophil lysis, releasing α-defensins and myeloperoxidase (MPO) products, which activate platelets and promote their binding to fibrinogen, thereby facilitating thrombus formation [13]. Many scholars believe that Staphylococcus aureus infection is a significant risk factor for the development of DVT in AHO, and multiple studies have confirmed that MRSA infection is more common in AHO patients complicated with DVT [8, 9, 14, 15]. In this study, the proportion of MRSA infections in DVT patients was higher than in non-DVT patients, but the correlation between MRSA infection and secondary DVT was not significant (P > 0.05). We believe this difference may be attributed to the single-center study design, limited sample size, patient environment, racial and cultural differences, as well as genetic variations in MRSA strains [16].
We identified two cases of AHO caused by α-hemolytic Streptococcus (1 case) and Klebsiella pneumoniae (1 case) that subsequently developed DVT. Relevant studies have demonstrated that infections with these microorganisms carry a risk of secondary DVT [17–19]. Three patients with AHO caused by pathogenic bacteria, including Streptococcus pyogenes (2 cases) and Streptococcus lactis (1 case), did not develop secondary DVT.
All patients with DVT had secondary occurrences near the site of AHO involvement, with AHO affecting the pelvis and lower limbs more likely to lead to secondary DVT. Some scholars have noted that DVT commonly occurs in the femoral vein, popliteal vein, and iliac vein [8, 14, 20]. We observed similar results, with most patients developing DVT in the femoral vein (common femoral vein, deep femoral vein, superficial femoral vein) and iliac vein. There was a significant correlation between AHO involving the femur and secondary DVT (P < 0.05). We analyzed that this may be due to the thicker thigh veins with slower blood flow, numerous branches prone to vortex formation, deeper location susceptible to compression, and greater gravitational pressure leading to poor venous return.
The results demonstrated that the median age of pediatric AHO patients who developed secondary DVT (9 years) was significantly higher than that of those without DVT (6 years). We identified age as an independent risk factor for secondary DVT in children with AHO, which may be associated with vascular development and hormonal effects. Several scholars have reached similar conclusions [8, 14]. However, two other studies [5, 20] did not show a significant correlation between age and secondary DVT in AHO patients. Although their experimental groups included only 7 and 9 cases, respectively, further increasing the sample size or conducting multicenter studies is still necessary to make the conclusions more scientifically robust.
Some studies suggest that the risk of secondary DVT in AHO is related to gender, occurring more frequently in male patients [15, 20–22]. In our results {thrombosis group (boys: girls) = (19:11), non-thrombosis group (boys: girls) = (21:25)}, analysis of the data showed that this difference was not statistically significant (P > 0.05).
CRP levels rise within 4 to 6 h after tissue injury and return to normal after inflammation subsides, serving as a non-specific but sensitive indicator for diagnosing AHO in children. Some studies Have found that the peak CRP level in AHO patients is closely related to the occurrence of DVT, concluding that for every 20 mg/L increase in peak CRP, the risk of secondary DVT increases by 28% [21]. Since all AHO patients included in this study underwent multiple CRP tests, we used the highest value as the"peak"CRP level for each patient to best reflect the CRP levels during their inflammatory process. The results showed that AHO children who developed secondary DVT had significantly higher CRP levels compared to those without DVT (P < 0.05), indicating more severe conditions in the DVT group. Additionally, the time for CRP to return to normal in pediatric AHO patients was significantly correlated with the development of secondary DVT (P < 0.05). Higher CRP levels and potential prolonged hospitalization could influence the time for CRP normalization, so we conducted further regression analysis to minimize bias from confounding factors. Ultimately, we found that peak CRP levels and the time for CRP to return to normal were not independent risk factors for secondary DVT in AHO patients.
Both PCT and CRP are important clinical indicators for evaluating infections. Due to their high sensitivity and specificity for bacterial infections, PCT can be used to assess the severity of infections and guide surgical treatment [23]. Our study results indicate a significant correlation between PCT peak levels and the occurrence of secondary DVT in children with AHO (P < 0.05). After further regression analysis to eliminate interference between factors, the result exceeded the pre-set significance level (P = 0.073 > 0.05). Although the statistical evidence level is slightly lower, we believe its statistical tendency cannot be ignored, and thus PCT peak levels were included in the model for analysis.
Lawson et al.'s [2] scoring system can effectively describe the severity of acute hematogenous osteomyelitis (AHO), and our statistical analysis also revealed a significant correlation between this score and secondary deep vein thrombosis (DVT). However, further binary logistic regression showed that this score could not be included in the final model. We speculate that this may be due to reasons similar to the exclusion of CRP and MRSA—many AHO patients had received multiple treatments at local hospitals before admission. Due to the limited medical resources in non-central cities, their conditions were often already severe by the time they were first diagnosed at our hospital, which reduced the baseline differences in infection severity between our control and experimental groups. Additionally, this may also be related to the fact that the covariates included in the Lawson score had already been incorporated into the univariate analysis. This represents a limitation of our study.
Some scholars believe that the prolonged hospitalization and bed rest during AHO treatment increase the risk of secondary DVT [20, 24]. We analyzed the length of hospitalization among the included cases and found that patients who developed secondary DVT had a longer hospital stay (23.5 days) compared to those without DVT (19 days). However, since many patients had previously been hospitalized at local medical institutions before admission to our hospital, the total duration of hospitalization and referral timelines were unclear. Additionally, the length of hospitalization could be influenced by multiple factors, including prolonged treatment due to DVT, increased patient healthcare-seeking behavior, and the timing of venous ultrasound detection [8] Therefore, we could not establish a definitive correlation between the duration of hospitalization in AHO patients and the occurrence of secondary DVT.
No studies have confirmed the correlation between ESR and the development of DVT in patients with AHO [5, 6, 8]. We identified a significant correlation in the univariate analysis (P < 0.05), but the peak ESR cannot be considered an independent risk factor for secondary DVT in AHO patients (P > 0.05).
Undergoing multiple surgeries indirectly reflects the severity of AHO. Studies have found that several admission indicators (vital signs, CRP, ESR, hemoglobin) in pediatric AHO patients are associated with secondary or multiple surgeries [25]. The correlation between secondary DVT and multiple surgeries in pediatric AHO patients has not been reported. We believe that AHO children who develop secondary DVT may require more surgeries subsequently, while factors such as prolonged treatment cycles or increased risk of postoperative complications due to multiple surgeries could also contribute to the occurrence of DVT. The two may have a bidirectional causal relationship, necessitating more comprehensive statistical analysis.
Coagulation-related markers are associated with hemodynamic changes. Fibrinogen can directly or indirectly promote coagulation, activate platelets, facilitate platelet adhesion, and promote thrombus formation [26]. D-dimer, a degradation product of fibrin, has blood levels directly correlated with thrombus formation [27, 28]. In our study results, D-dimer was identified as an independent risk factor for secondary DVT in children with AHO (P < 0.05). Our conclusions can enable orthopedic surgeons to have a more comprehensive understanding and appreciation of various examination indicators when dealing with AHO patients, clarify diagnostic thinking, and minimize the rate of missed diagnoses of DVT as much as possible.
AHO caused by Staphylococcus aureus often involves pulmonary complications [29]. Studies suggest that patients with AHO who develop DVT are more prone to pneumonia or VTE [9]. Through routine chest X-rays of newly admitted children, we found that the majority (66.7%) of AHO patients with secondary DVT had varying degrees of pneumonia (before DVT was suspected and diagnosed by venous ultrasound), while this proportion was much smaller (19.6%) in AHO patients without secondary DVT. We hypothesize that secondary DVT is associated with AHO complicated by pneumonia. In further logistic regression analysis, we found a significant correlation between the two (P < 0.05), indicating that pneumonia is an independent risk factor for secondary DVT in children with AHO. However, the exact chronological sequence between pulmonary lesions and secondary DVT remains undetermined. Due to the uneven distribution of healthcare resources in China, most patients admitted to our hospital were referrals from local institutions, making it difficult to obtain first-visit medical records. This may have obscured the temporal relationship between pulmonary abnormalities and DVT onset, representing a limitation of our study. Nevertheless, all patients with abnormal chest radiographs were diagnosed with pneumonia before developing overt DVT-related symptoms. We therefore speculate that radiographic abnormalities may still possess predictive value for DVT secondary to AHO and could potentially guide clinical management.
The limitations of this study lie in its retrospective design and relatively small sample size. Over the 7-year period, more than 200 children with AHO were hospitalized at our institution, but only 76 cases were ultimately included. While most exclusions were due to incomplete clinical data, the high exclusion rate may still potentially affect the generalizability of the results. Cases with incomplete clinical information were excluded based on indicators mentioned in relevant guidelines and literature, which enhanced the homogeneity of the study population and facilitated comparative analysis. This approach allowed us to identify statistically significant factors through regression analysis, thereby improving the standardization of the statistical results.
Conclusion
Age, peak levels of CRP/ESR/PCT/D-dimer, osteomyelitis severity (Lawson score), femoral involvement in osteomyelitis, and concurrent pneumonia were significantly associated with secondary deep vein thrombosis (DVT) in pediatric acute hematogenous osteomyelitis (AHO). Among these, age, peak D-dimer levels, and concurrent pneumonia were identified as independent risk factors for DVT in children with AHO.
The current predictive model demonstrated good performance in this cohort, providing early warning for potential DVT risk in pediatric AHO patients. However, multicenter studies are needed to further validate its generalizability.
Pediatricians may use this model to assess the necessity of DVT screening, reduce missed diagnoses, and initiate timely antithrombotic therapy to prevent adverse outcomes.
Acknowledgements
Firstly, I would like to express my gratitude to Professor Qiang Wang, my esteemed teacher, for his invaluable feedback on revisions and technical guidance. Jiale Guo, offering formal analysis and associated corrections. Hanwen Zhang, writing assistance, or proofreading the article. Baojian Song, providing spiritual motivation and empirical evidence support. Wei Feng/Danjiang Zhu/Yuwei Wen, assisting with ideas, language creating illustrations, and generating tables. The study was conducted without any financial support. Thank you immensely for your invaluable assistance.
Patient and public involvement statement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Authors’ contributions
Acknowledgments Firstly, I would like to express my gratitude to Professor Qiang Wang, my esteemed teacher, for his invaluable feedback on revisions and technical guidance. Jiale Guo, offering formal analysis and associated corrections. Hanwen Zhang, writing assistance, or proofreading the article. Baojian Song, providing spiritual motivation and empirical evidence support. Wei Feng/Danjiang Zhu/Yuwei Wen, assisting with ideas, language creating illustrations, and generating tables. All authors read and approved the final manuscript. The study was conducted without any financial support. Thank you immensely for your invaluable assistance.
Funding
Supported by Beijing Natural Science Foundation (L242167).
Data availability
Availability of Data and Materials The datasets and analysis materials used in this study are available from the corresponding author upon reasonable request. Due to patient privacy and ethical restrictions, some data may require anonymization or approval from the relevant ethics committee before being provided. If further adjustments are needed or if there are specific journal requirements, please let me know!
If further adjustments are needed or if there are specific journal requirements, please let me know!
Declarations
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of Beijing Children's Hospital, affiliated with Capital Medical University, in compliance with Chinese laws and regulations as well as the Declaration of Helsinki. Informed consent was obtained from all participants, and the study has been authorized to be conducted at our hospital (Ethics Approval Number: [2025] -E-082-R).
Consent for publication
All participants or their legal guardians provided written consent for the publication of the data and results obtained from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yeargan SA, Nakasone CK, Shaieb MD, et al. Treatment of chronic osteomyelitis in children resistant to previous therapy. J Pediatr Orthop. 2004;24:109–22. [DOI] [PubMed]
- 2.Copley LAB, Barton T, Garcia C, et al. A proposed scoring system for assessment of severity of illness in pediatric acute hematogenous osteomyelitis using objective clinical and laboratory findings. Pediatr Infect Dis J. 2014;33:35–41. [DOI] [PubMed]
- 3.Amaro E, Marvi TK, Posey SL, et al. C-Reactive Protein Predicts Risk of Venous Thromboembolism in Pediatric Musculoskeletal Infection. J Pediatr Orthop. 2019;39:e62–7. [DOI] [PubMed]
- 4.Gorenstein A, Gross E, Houri S, et al. The pivotal role of deep vein thrombophlebitis in the development of acute disseminated staphylococcal disease in children. Pediatrics. 2000;106:E87. [DOI] [PubMed]
- 5.Bouchoucha S, Benghachame F, Trifa M, et al. Deep venous thrombosis associated with acute hematogenous osteomyelitis in children. Orthop Traumatol Surg Res. 2010;96:890–3. [DOI] [PubMed]
- 6.Crary SE, Buchanan GR, Drake CE, et al. Venous thrombosis and thromboembolism in children with osteomyelitis. Pediatrics. 2006;149:537–41. [DOI] [PubMed]
- 7.Vander Have KL, Karmazyn B, Verma M, et al. Community-associated methicillin-resistant Staphylococcus aureus in acute musculoskeletal infection in children: a game changer. J Pediatr Orthop. 2009;29:927–31. [DOI] [PubMed]
- 8.Hollmig ST, Copley LA, Browne RH, et al. Deep venous thrombosis associated with osteomyelitis in children. J Bone Joint Surg Am. 2007;89:1517–23. [DOI] [PubMed]
- 9.Ligon JA, Journeycake JM, Josephs SC, et al. Differentiation of deep venous thrombosis among children with or without osteomyelitis. J Pediatr Orthop. 2018;38(10):e597–603. [DOI] [PubMed]
- 10.McDonald JE, Copley LA. volume. Upper-extremity deep venous thrombosis associated with proximal humeral osteomyelitis in a child: a case report. J Bone Joint Surg Am. 2010;92:2121–4. [DOI] [PubMed]
- 11.Yüksel H, Ozgüven AA, Akil I, et al. Septic pulmonary emboli presenting with deep venous thrombosis secondary to acute osteomyelitis. Pediatr Int. 2004;46:621–3. [DOI] [PubMed]
- 12.Todd JK. Staphylococcal infections. Pediatr Rev. 2005;26:444–50. [PubMed]
- 13.Niemann S, Bertling A, Brodde MF, et al. Panton-Valentine Leukocidin associated with S. aureus osteomyelitis activates platelets via neutrophil secretion products. Sci Rep. 2018;8:2185. [DOI] [PMC free article] [PubMed]
- 14.Gonzalez BE, Teruya J, Mahoney DH, et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117:1673–9. [DOI] [PubMed]
- 15.Citla Sridhar D, Maher OM, Rodriguez NI. Pediatric Deep Venous Thrombosis Associated With Staphylococcal Infections: Single Institutional Experience. J Pediatr Hematol Oncol. 2018;40:e73–76. [DOI] [PubMed]
- 16.Wade Shrader M, Nowlin M, Segal LS. Independent analysis of a clinical predictive algorithm to identify methicillin-resistant Staphylococcus aureus osteomyelitis in children. J Pediatr Orthop. 2013;33:759–62. [DOI] [PubMed]
- 17.G Mitsiakos, D Gialamprinou, C Tsakalidis, et al. Osteomyelitis and Thrombosis in a Newborn with Group A Streptococcus Infection. Prague Med Rep . 2023;124:293–300. [DOI] [PubMed]
- 18.Sheikh Najeeb M, Alshwaiki A, Martini N, et al. Acute osteomyelitis, thrombophlebitis, and pulmonary embolism: a case report. J Med Case Rep . 2023;17:471. [DOI] [PMC free article] [PubMed]
- 19.Shannon O, Hertzén E, Norrby-Teglund A, et al. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol Microbiol . 2007;65:1147–57. [DOI] [PubMed]
- 20.He Y, Liu S, Su Y. Risk factors of deep vein thrombosis in children with osteomyelitis. Ann Med. 2023;55:2249011. [DOI] [PMC free article] [PubMed]
- 21.Abdalla G, Matuk RF, Venugopal V, et al. The diagnostic accuracy of magnetic resonance venography in the detection of deep venous thrombosis: a systematic review and meta-analysis. Clin Radiol. 2015;70:858–71. [DOI] [PubMed]
- 22.Mantadakis E, Plessa E, Vouloumanou EK, et al. Deep venous thrombosis in children with musculoskeletal infections: the clinical evidence. Int J Infect Dis. 2012;16:e236–43. [DOI] [PubMed]
- 23.Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–8. [DOI] [PubMed]
- 24.Amaral A, Opal SM, Vincent JL. Coagulation in sepsis. Intensive Care Med. 2004;30:1032–40. [DOI] [PubMed]
- 25.Tuason DA, Gheen T, Sun D, et al. Clinical and laboratory parameters associated with multiple surgeries in children with acute hematogenous osteomyelitis. J Pediatr Orthop. 2014;34:565–70. [DOI] [PubMed]
- 26.Koster T, Rosendaal FR, Reitsma PH, et al. Factor VII and fibrinogen levels as risk factors for venous thrombosis. A case-control study of plasma levels and DNA polymorphisms--the Leiden Thrombophilia Study (LETS). Thromb Haemost. 1994;71:719–22. [PubMed]
- 27.Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ, et al. Elevated plasma factor VIII and D-dimer levels as predictors of poor outcomes of thrombosis in children. N Engl J Med Overseas Ed. 2004;351:1081–8. [DOI] [PubMed]
- 28.Kanis J, Hall CL, Pike J, et al. Diagnostic accuracy of the D-dimer in children. Arch Dis Child. 2018;103:832–4. [DOI] [PubMed]
- 29.Felman AH, Shulman ST. Staphylococcal osteomyelitis, sepsis, and pulmonary disease. Observations of 10 patients with combined osseous and pulmonary infections. Radiology. 1975;117:649–55. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of Data and Materials The datasets and analysis materials used in this study are available from the corresponding author upon reasonable request. Due to patient privacy and ethical restrictions, some data may require anonymization or approval from the relevant ethics committee before being provided. If further adjustments are needed or if there are specific journal requirements, please let me know!
If further adjustments are needed or if there are specific journal requirements, please let me know!




