Introduction
Up to 78% of cases of autosomal dominant polycystic kidney disease are caused by heterozygous variants of PKD1.1 PKD1 is located on chromosome 16 (16p13.3), and encodes polycystin-1, a large multidomain glycoprotein that undergoes cleavage at the G-protein-coupled receptor proteolytic site.2 Disruption of polycystin-1 reduces intracellular Ca2+ levels, leading to the upregulation of cAMP signaling and promotion of cell proliferation.S1 Various variants have been detected at the DNA level, including in introns; however, the functional importance of these variants remain unclear.
Splice variants typically lead to improper exon and intron recognition in the mRNA, leading to the generation of an aberrant transcript of the mutated gene. Changes in introns and exons can disrupt the existing splicing sites, create new sites, and activate cryptic sites.S2 Such variants often lead to exon skipping or exon fragment skipping, intron fragment inclusion, or exon fragment removal when a cryptic splice site is within an intron or exon during pre-mRNA splicing.3 The impact of splicing variants can be evaluated at various levels, including DNA, RNA, RNA-protein interaction, and protein levels. The simplest and most effective approach for assessing whether a selected variant affects splicing is to analyze RNA extracted from relevant patient tissues or patient-derived cell lines.4 Ideally, reverse transcription–polymerase chain reaction followed by sequencing of RNA or cDNA can confirm whether an identified variant affects the mRNA sequence.3,5 However, obtaining optimal tissues from patients for measuring RNA expression is often challenging or unfeasible. In addition, this approach is limited by the propensity of RNA to undergo nonsense-mediated decay.S3 In such situations, the effects of potential splicing variants can easily be overlooked. To overcome these limitations, patient cells can be treated with nonsense-mediated decay inhibitors such as puromycin, which blocks RNA degradation.S4
In silico prediction tools are ancillary tools for assessing the effects of identified changes. However, the results of these tests are only useful for prediction, and the precise effects of the variants must be verified in functional studies.3 When suitable materials for functional RNA sequencing are unavailable, minigene assays can serve as an alternative approach6 (Supplementary Figure S1).
This study was conducted to evaluate whether a minigene assay can be used to analyze whether intronic variants in PKD1 affect splicing (Supplementary Methods). In addition, we evaluated how well the in silico prediction tools predicted the splicing effects of the variants. This study was approved by the Institutional Review Board of Seoul National University Hospital (H-1907-067-1047).
Results
Reverse Transcription–Polymerase Chain Reaction and Sanger Sequencing
Of the 5 intronic PKD1 variants analyzed, 4 disrupted normal splicing, whereas the splicing outcome of the remaining variant (c.288-12C>A) could not be clearly interpreted using the minigene assay (Supplementary Tables S1 and S2).
The variant (c.2097+5G>A) showed an electrophoretic banding pattern similar to that of the control. However, unlike the splicing product of the normal control (1267 bp), a 2 bp GT was confirmed to remain in the 5' splice site region of intron 10 in the proband. Splicing at c.2097+3G is facilitated by a novel splice donor site and splice acceptor site in intron 10. In addition, the 1019 bp sequence observed in both the normal control and proband can be attributed to alternative splicing, likely resulting from the insertion of random exon and intron sequences into the vector during in vitro experiments (Figure 1).
Figure 1.
Minigene assay results of NM_001009944.3(PKD1):c.2097+5G>A. (a) Schematic illustration of pSPL3-PKD1 c.2097+5G>A and c.2853+5G>C minigenes. Exons 10 and 11 of PKD1 and flanking introns were cloned into the pSPL3 vector with a wild-type or mutant c.2097+5G>A and c.2853+5G>C between 2 pSPL3 vectors. After reverse transcription, the splicing products were amplified by polymerase chain reaction using vector exon–specific primers, and (b) the c.2097+5G>A variant was visualized by agarose gel electrophoresis. (c) Sanger sequencing confirmed the reverse transcription–polymerase chain reaction products, and the results in intron 10 showed a 2 bp retention. The clinical profile of the patient harboring the PKD1 variant illustrated in this figure includes the following: onset age of 34 years, an eGFR of 88.09 ml/min per 1.73 m2, classification as Mayo class 1D, and no family history of ADPKD. ADPKD, autosomal dominant polycystic kidney disease; eGFR, estimated glomerular filtration rate; EV, empty vector; NC, normal control; P, proband; SA, splice acceptor exon; SD, splice donor exon; TE, TE buffer.
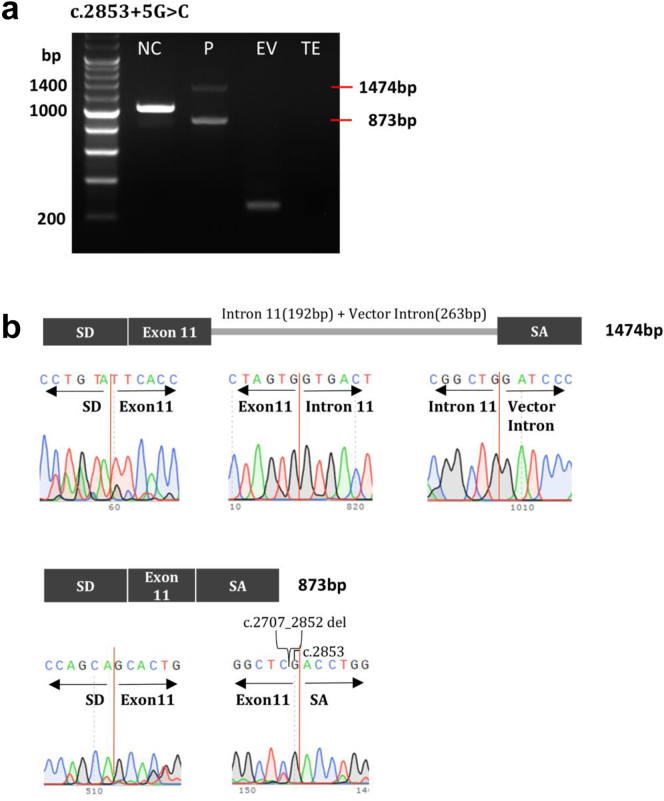
Another variant (c.2853+5G>A) showed 2 amplicons with longer and shorter DNA fragments than those of the normal control (1019 bp). Sequencing revealed a longer amplicon of 1474 bp, with retention of intron 11 (192 bp) of the insert DNA and vector intron (263 bp), and a shorter amplicon of 873 bp, with deletion of c.2707_2852 (192 bp) in exon 11 of the insert DNA (Figure 2).
Figure 2.
Minigene assay results of NM_001009944.3(PKD1):c.2853+5G>C. (a) HEK293T cells were transfected with wild-type or mutant PKD1 minigene or an empty pSPL3 vector. After reverse transcription, splicing products were amplified by polymerase chain reaction using vector exon–specific primers and (b) visualized by agarose gel electrophoresis. (c) Sanger sequencing confirmed the reverse transcription–polymerase chain reaction products, and the results showed that part of the intron 11 and the vector intron (263 bp) were retained, exon 10 was skipped, and part of exon 11 was deleted because of the presence of a cryptic splicing site. The clinical profile of the patient harboring the PKD1 variant depicted in this figure includes the following: onset age of 44 years, an eGFR of 84 ml/min per 1.73 m2, classification as Mayo class 1C, and a positive family history of ADPKD. ADPKD, autosomal dominant polycystic kidney disease; eGFR, estimated glomerular filtration rate; EV, empty vector; NC, normal control; P, proband; SA, splice acceptor exon; SD, splice donor exon; TE, Tris-EDTA buffer.
Both variants (c.7490-3C>G and c.7490-11C>G) were 850 bp longer than the normal control (757 bp) because of intron 18 retention (93 bp). The c.7490-3C>G variant was generated by site-directed mutagenesis, as no patient-derived sample was available (Supplementary Figure S2). In addition, both variants were 850 bp in the normal control group, possibly because of the presence of an artificial vector sequence. Because the proband did not show the splicing product of the normal control, both variants appeared to cause aberrant splicing (Supplementary Figures S3 and S4).
Splicing Prediction Tools
The results of the minigene assays for the 5 variants were compared with those of the in silico prediction tool (Supplementary Table S3). For the 4 intronic variants with abnormal splicing patterns in the minigene assays, most of the predicted splicing effects aligned with the minigene assay results. However, for c.288-12C>A, with the exception of the results obtained using NNSPLICE, all in silico prediction tools predicted aberrant splicing, whereas the minigene assay predicted a normal splicing pattern. This result suggests that prediction tools can only be used as auxiliary approaches and that functional studies are necessary to obtain accurate results.
Discussion
Analysis of hybrid minigenes is a quick and effective approach for examining the impact of genomic variations on splicing and has been widely used to functionally characterize variants across numerous genes, including CNGA3 and EYS.S11,S12 The minigene assay can be employed even in the absence of patient RNA, and thus is effective when expression profiles are limited, specific tissues are difficult to obtain,7 or transcript analysis is complicated by nonsense-mediated mRNA decay.8 In this study, we tested 5 potential splicing variants of PKD1 using minigene assays to investigate their effects on splicing. These results indicate that the 4 variants led to aberrant splicing, confirming the predictions of the splicing prediction tools. Specifically, intron retention (c.7490-3C>G and c.7490-11C>G) and cryptic splice site activation (c.2097+5G>A and c.2853+5G>C) were observed. These findings highlight the value of minigene assays in confirming in silico predictions and revealing the nuanced nature of splicing regulation. Importantly, by providing functional evidence, this approach may help clarify the clinical significance of PKD1 variants, particularly those classified as variants of unknown significance, thereby contributing to more confident genetic diagnoses and better-informed care for patients with autosomal dominant polycystic kidney disease.
However, in silico tools have some limitations. Currently available prediction tools can cover only cis-acting elements containing the 5′ splice site, 3′ splice site, branch point, exonic splicing enhancers, intronic splicing enhancers, exonic splicing silencers, and intronic splicing silencers. Another drawback of these tools is that they can only analyze sequences of limited lengths. Therefore, improvements are required to predict the vicinity (consensus sequence) of the splice site and more distant sites.4 Therefore, functional analysis using minigene assays is crucial for overcoming these limitations.
The limitations of this study include the use of a single cell line and the absence of patient RNA samples. Different splicing patterns may be observed depending on the cell line, and it is unclear how closely these patterns correspond to those observed in actual patients.9 Future studies that extend our minigene-based findings using genome editing or patient-derived cells could help validate the results in more physiologically relevant contexts and improve our understanding of these variants.
Disclosure
All the authors declared no competing interests.
Funding
This work was supported by the Research Program funded by the Korea National Institute of Health (KNIH) (2019-ER-7304-00, 2019-ER-7304-01, 2019-ER-7304-02, 2022-ER-0703-00, 2022-ER-0703-01, 2022-ER-0703-02). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding institution.
Author Contributions
The study was conceived and designed by M-WS. Patients were recruited by HCP, YCK, EHB, and YKO and interpretation of variants was performed by BK, JWJ, and SWC. The minigene splicing assay and in silico analyses were performed by SHY. The manuscript was drafted by SHY, J-SL, and M-WS. All the authors approved the final version of the manuscript.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Minigene assay workflow.
Figure S2. PKD1 c.7490-3C>G by site-directed mutagenesis.
Figure S3. Minigene assay results of NM_001009944.3(PKD1):c.7490-3C>G.
Figure S4. Minigene assay results of NM_001009944.3(PKD1):c.7490-11C>G.
Table S1. Phenotype summary of patients with PKD1 variant of uncertain signification.
Table S2. Minigene assay results and revised pathogenicity classification of 5 variants in this study.
Table S3. In silico prediction of 5 variants on RNA splicing.
Contributor Information
Jee-Soo Lee, Email: leciel85@snu.ac.kr.
Moon-Woo Seong, Email: mwseong@snu.ac.kr.
Supplementary Material
Supplementary Methods. Supplementary References. Figure S1. Minigene assay workflow. Figure S2. PKD1 c.7490-3C>G by site-directed mutagenesis. Figure S3. Minigene assay results of NM_001009944.3(PKD1):c.7490-3C>G. Figure S4. Minigene assay results of NM_001009944.3(PKD1):c.7490-11C>G. Table S1. Phenotype summary of patients with PKD1 variant of uncertain signification. Table S2. Minigene assay results and revised pathogenicity classification of 5 variants in this study. Table S3.In silico prediction of 5 variants on RNA splicing.
References
- 1.Lakhia R., Ramalingam H., Chang C.M., et al. PKD1 and PKD2 mRNA cis-inhibition drives polycystic kidney disease progression. Nat Commun. 2022;13:4765. doi: 10.1038/s41467-022-32543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E., Alam A., Perrone R.D. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919–935. doi: 10.1016/S0140-6736(18)32782-X. [DOI] [PubMed] [Google Scholar]
- 3.Anna A., Monika G. Splicing mutations in human genetic disorders: examples, detection, and confirmation. J Appl Genet. 2018;59:253–268. doi: 10.1007/s13353-018-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jian X., Boerwinkle E., Liu X. In silico tools for splicing defect prediction: a survey from the viewpoint of end users. Genet Med. 2014;16:497–503. doi: 10.1038/gim.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BinEssa H.A., Zou M., Al-Enezi A.F., et al. Functional analysis of 22 splice-site mutations in the PHEX, the causative gene in X-linked dominant hypophosphatemic rickets. Bone. 2019;125:186–193. doi: 10.1016/j.bone.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Singh G., Cooper T.A. Minigene reporter for identification and analysis of cis elements and trans factors affecting pre-mRNA splicing. Biotechniques. 2006;41:177–181. doi: 10.2144/000112208. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Munoz A., Liquori A., Garcia-Bohorquez B., et al. Functional assays of non-canonical splice-site variants in inherited retinal dystrophies genes. Sci Rep. 2022;12:68. doi: 10.1038/s41598-021-03925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Shi X., Xin Q., et al. Identified eleven exon variants in PKD1 and PKD2 genes that altered RNA splicing by minigene assay. BMC Genomics. 2023;24:407. doi: 10.1186/s12864-023-09444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin Q., Liu Q., Liu Z., et al. Twelve exonic variants in the SLC12A1 and CLCNKB genes alter RNA splicing in a minigene assay. Front Genet. 2022;13 doi: 10.3389/fgene.2022.961384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary References. Figure S1. Minigene assay workflow. Figure S2. PKD1 c.7490-3C>G by site-directed mutagenesis. Figure S3. Minigene assay results of NM_001009944.3(PKD1):c.7490-3C>G. Figure S4. Minigene assay results of NM_001009944.3(PKD1):c.7490-11C>G. Table S1. Phenotype summary of patients with PKD1 variant of uncertain signification. Table S2. Minigene assay results and revised pathogenicity classification of 5 variants in this study. Table S3.In silico prediction of 5 variants on RNA splicing.