Abstract
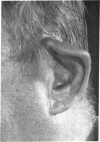
Serial studies have been performed on three patients with relapsing polychondritis in an attempt to define a potential immunopathologic role for degradation constituents of cartilage in the causation and/or perpetuation of the inflammation observed. Crude proteoglycan preparations derived by disruptive and differential centrifugation techniques from human costal cartilage, intact chondrocytes grown as monolayers, their homogenates and products of synthesis provided antigenic material for investigation. Circulating antibody to such antigens could not be detected by immunodiffusion, hemagglutination, immunofluorescence or complement mediated chondrocyte cytotoxicity as assessed by 51Cr release. Similarly, radiolabeled incorporation studies attempting to detect de novo synthesis of such antibody by circulating peripheral blood lymphocytes as assessed by radioimmunodiffusion, immune absorption to neuraminidase treated and untreated chondrocytes and immune coprecipitation were negative.
Delayed hypersensitivity to cartilage constituents was studied by peripheral lymphocyte transformation employing [3H]thymidine incorporation and the release of macrophage aggregation factor. Positive results were obtained which correlated with periods of overt disease activity. Similar results were observed in patients with classical rheumatoid arthritis manifesting destructive articular changes. This study suggests that cartilage antigenic components may facilitate perpetuation of cartilage inflammation by cellular immune mechanisms.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 1958 REVISION of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1959 Feb;2(1):16–20. doi: 10.1002/1529-0131(195902)2:1<16::aid-art1780020104>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Voynow N. K. One-way stimulation in mixed leukocyte cultures. Science. 1966 Jul 29;153(3735):545–547. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- CHRISMAN O. D., FESSEL J. M., SOUTHWICK W. O. EXPERIMENTAL PRODUCTION OF SYNOVITIS AND MARGINAL ARTICULAR EXOSTOSES IN THE KNEE JOINTS OF DOGS. Yale J Biol Med. 1965 Apr;37:409–412. [PMC free article] [PubMed] [Google Scholar]
- CRAIGMYLE M. B. A study of cartilage homografts in rabbits sensitised by a skin homograft from the cartilage donor. Transplant Bull. 1960 Jul;26:150–153. doi: 10.1097/00006534-196007000-00051. [DOI] [PubMed] [Google Scholar]
- DIFERRANTE N. PRECIPITINS IN THE RABBIT PRODUCED BY PROTEIN POLYSACCHARIDE FROM BOVINE NASAL CARTILAGE. Science. 1964 Jan 17;143(3603):250–252. doi: 10.1126/science.143.3603.250. [DOI] [PubMed] [Google Scholar]
- Dolan D. L., Lemmon G. B., Jr, Teitelbaum S. L. Relapsing polychondritis. Analytical literature review and studies on pathogenesis. Am J Med. 1966 Aug;41(2):285–299. doi: 10.1016/0002-9343(66)90023-4. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Coombs R. R., Dingle J. T. The breakdown of embryonic (chick) cartilage and bone cultivated in the presence of complement-sufficient antiserum. I. Morphological changes, their reversibility and inhibition. Int Arch Allergy Appl Immunol. 1966;30(2):146–176. doi: 10.1159/000229803. [DOI] [PubMed] [Google Scholar]
- GERBER B. R., FRANKLIN E. C., SCHUBERT M. Ultracentrifugal fractionation of bovine nasal chondromucoprotein. J Biol Chem. 1960 Oct;235:2870–2875. [PubMed] [Google Scholar]
- GLYNN L. E., HOLBOROW E. J. Conversion of tissue polysaccharides to auto-antigens by group-A beta-haemolytic streptococci. Lancet. 1952 Sep 6;2(6732):449–451. doi: 10.1016/s0140-6736(52)90242-0. [DOI] [PubMed] [Google Scholar]
- Goldman J. A., Litwin A., Adams L. E., Krueger R. C., Hess E. V. Cellular immunity to nuclear antigens in systemic lupus erythematosus. J Clin Invest. 1972 Oct;51(10):2669–2677. doi: 10.1172/JCI107085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman D. H., McFarland W. Inhibition of tuberculin-induced mitogenesis in cultures of lymphocytes from tuberculous donors. Int Arch Allergy Appl Immunol. 1966;30(1):58–66. doi: 10.1159/000229793. [DOI] [PubMed] [Google Scholar]
- Herman J. H., Bradley J., Ziff M., Smiley J. D. Response of the rheumatoid synovial membrane to exogenous immunization. J Clin Invest. 1971 Feb;50(2):266–273. doi: 10.1172/JCI106491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyner S. The significance of the intercellular matrix in the survival of cartilage allografts. Transplantation. 1969 Nov;8(5):666–677. doi: 10.1097/00007890-196911000-00011. [DOI] [PubMed] [Google Scholar]
- Jasin H. E., Ziff M. Immunoglobulin and specific antibody synthesis in a chronic inflammatory focus: antigen-induced synovitis. J Immunol. 1969 Feb;102(2):355–369. [PubMed] [Google Scholar]
- Knowles M., Hughes D., Caspary E. A., Field E. J. Lymphocyte transformation in multiple sclerosis. Inhibition of unstimulated thymidine uptake by a serum factor. Lancet. 1968 Dec 7;2(7580):1207–1209. doi: 10.1016/s0140-6736(68)91691-7. [DOI] [PubMed] [Google Scholar]
- LACK C. H. Chondrolysis in arthritis. J Bone Joint Surg Br. 1959 May;41-B(2):384–387. doi: 10.1302/0301-620X.41B2.384. [DOI] [PubMed] [Google Scholar]
- LLOYD-ROBERTS G. C. The role of capsular changes in osteoarthritis of the hip joint. J Bone Joint Surg Br. 1953 Nov;35-B(4):627–642. doi: 10.1302/0301-620X.35B4.627. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Coombs R. R., Fell H. B., Dingle J. T. The breakdown of embryonic (chick) cartilage and bone cultivated in the presence of complement-sufficient antiserum. 3. Immunological analysis. Int Arch Allergy Appl Immunol. 1969;36(5):469–485. doi: 10.1159/000230766. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Loewi G., Muir H. The antigenicity of chondromucoprotein. Immunology. 1965 Aug;9(2):119–127. [PMC free article] [PubMed] [Google Scholar]
- Lolekha S., Dray S., Gotoff S. P. Macrophage aggregation in vitro: a correlate of delayed hypersensitivity. J Immunol. 1970 Feb;104(2):296–304. [PubMed] [Google Scholar]
- MALAWISTA I., SCHUBERT M. Chondromucoprotein: new extraction method and alkaline degradation. J Biol Chem. 1958 Jan;230(1):535–544. [PubMed] [Google Scholar]
- Manning W. K., Bonner W. M., Jr Isolation and culture of chondrocytes from human adult articular cartilage. Arthritis Rheum. 1967 Jun;10(3):235–239. doi: 10.1002/art.1780100309. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Schwartz H. J., Michel B., Ratnoff O. D., Astrup T. Generation of kinin-like agents by chondroitin sulfate, heparin, chitin sulfate, and human articular cartilage: possible pathophysiologic implications. J Lab Clin Med. 1970 Nov;76(5):790–798. [PubMed] [Google Scholar]
- Paronetto F., Popper H. Lymphocyte stimulation induced by halothane in patients with hepatitis following exposure to halothane. N Engl J Med. 1970 Aug 6;283(6):277–280. doi: 10.1056/NEJM197008062830602. [DOI] [PubMed] [Google Scholar]
- Phair J. P., Kantor F. S. Delayed hypersensitivity. II. Persistence and conjugate specificity of the transfer reaction. J Immunol. 1968 Feb;100(2):302–306. [PubMed] [Google Scholar]
- Sandson J., Hamerman D., Janis R., Rojkind M. Immunologic and chemical similarities between the streptococcus and human connective tissue. Trans Assoc Am Physicians. 1968;81:249–257. [PubMed] [Google Scholar]
- Sandson J., Rosenberg L., White D. The antigenic determinants of the protein-polysaccharides of cartilage. J Exp Med. 1966 May 1;123(5):817–828. doi: 10.1084/jem.123.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk M. Effect of plasma from patients with carcinoma on in vitro lymphocyte transformation. Cancer. 1967 Dec;20(12):2088–2089. doi: 10.1002/1097-0142(196712)20:12<2088::aid-cncr2820201205>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- THOMAS L., McCLUSKEY R. T., POTTER J. L., WEISSMANN G. Comparison of the effects of papain n vitamin A on cartilage. I. The effects in rabbits. J Exp Med. 1960 May 1;111:705–718. doi: 10.1084/jem.111.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956 Aug 1;104(2):245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I. Breakdown of cartilage proteinpolysaccharide by lysosomes. Arthritis Rheum. 1968 Apr;11(2):162–169. doi: 10.1002/art.1780110206. [DOI] [PubMed] [Google Scholar]
- Winter G. C., McCarthy C. F., Read A. E., Yoffey J. M. Development of macrophages in phytohaemagglutinin cultures of blood from patients with idiopathic steatorrhoea and with cirrhosis. Br J Exp Pathol. 1967 Feb;48(1):66–80. [PMC free article] [PubMed] [Google Scholar]
- ZIFF M., GRIBETZ H. J., LOSPALLUTO J. Effect of leukocyte and synovial membrane extracts on cartilage mucoprotein. J Clin Invest. 1960 Feb;39:405–412. doi: 10.1172/JCI104051. [DOI] [PMC free article] [PubMed] [Google Scholar]