Abstract
1. Superior cervical ganglia of adult rats were excised and maintained in vitro in stable conditions. Potentials were recorded with external electrodes. After transmission was blocked by mecamylamine, a small potential change was recorded from the rostral area of the ganglion in response to preganglionic stimulation.
2. This electrical response was identified as the presynaptic action potential recorded from the nerve terminals by a number of criteria based on histological and physiological considerations including the disappearance of the spike in a glucose free solution. As shown by Nicolescu, Dolivo, Rouiller & Foroglou-Kerameus (1966) on the same preparation this condition causes an irreversible and selective lesion of the presynaptic nerve endings.
3. A suitable concentration of mecamylamine permitted the presynaptic response and the excitatory post-synaptic potential (EPSP) to be recorded simultaneously. As the stimulus was increased, the EPSP increased linearly with the amplitude of the presynaptic response.
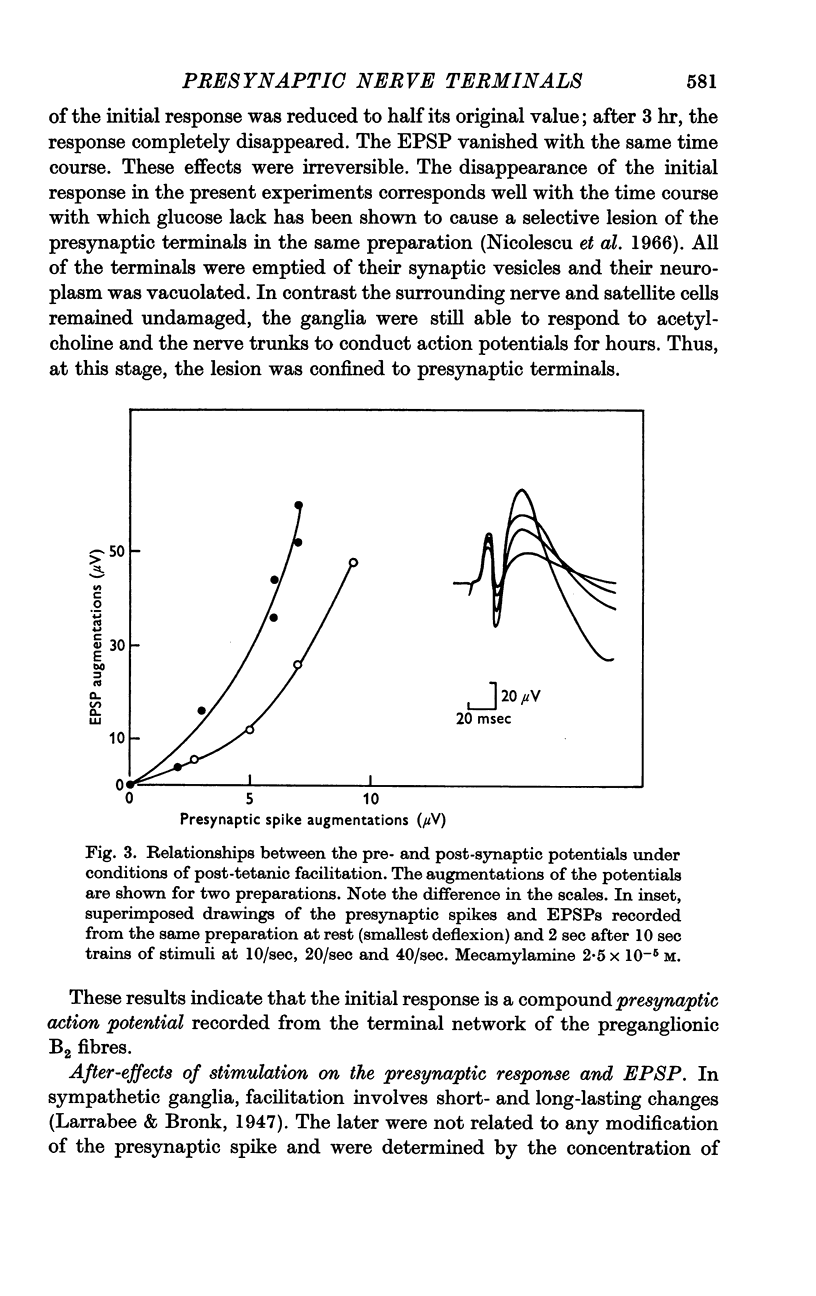
4. After replacement of potassium ions in the bathing solution by caesium and during the early phase of post-tetanic facilitation there was an increase in the presynaptic response accompanied by a disproportionate increase in the EPSP.
5. No changes in the presynaptic response were found in the presence of the following drugs, all of which depressed the EPSP: acetylcholine, hemicholinium, curare, further doses of ganglion-blocking agents, and high Mg2+ and low Ca2+ concentrations.
6. Ouabain (4·5 × 10-4 M) reversibly decreased the amplitude of the presynaptic response and increased the spontaneous release of transmitter. The EPSP was at first enhanced and then depressed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J Physiol. 1962 Nov;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- De Ribaupierre F., Dunant Y., Dolivo M., Foroglou-Kerameus C. L'action présynaptique de l'ouabaïne sur le ganglion sympathique. J Physiol (Paris) 1968;60 (Suppl 2):531–532. [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Presynaptic recording in excised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):268–270. doi: 10.1016/0006-8993(68)90133-9. [DOI] [PubMed] [Google Scholar]
- Dunant Y. Organisation topographique et fonctionnelle du ganglion cervical supérieur chez le Rat. J Physiol (Paris) 1967 Jan-Feb;59(1):17–38. [PubMed] [Google Scholar]
- Dunant Y. Presynaptic spike and excitatory postsynaptic potential in sympathetic ganglion: their modifications by pharmacological agents. Prog Brain Res. 1969;31:131–139. doi: 10.1016/S0079-6123(08)63234-3. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSSMANN W. G. STUDIEN UEBER DEN FEINBAU DES GANGLION CERVICALE SUPERIUS DER RATTE. I. NORMALE STRUKTUR. Acta Anat (Basel) 1964;59:106–140. [PubMed] [Google Scholar]
- Ginsborg B. L., Hamilton J. T. The effect of caesium ions of neuromuscular transmission in the frog. Q J Exp Physiol Cogn Med Sci. 1968 Apr;53(2):162–169. doi: 10.1113/expphysiol.1968.sp001955. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. C., Volle R. L. Enhancement by cesium ions of ganglionic hyperpolarization induced by dimethylphenylpiperazinium (DMPP) and repetitive preganglionic stimulation. J Pharmacol Exp Ther. 1969 Oct;169(2):201–210. [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Cholinergic receptors at sympathetic preganglionic nerve terminals. J Physiol. 1968 May;196(2):293–310. doi: 10.1113/jphysiol.1968.sp008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu P., Dolivo M., Rouiller C., Foroglou-Kerameus C. The effect of deprivation of glucose on the ultrastructure and function of the superior cervical ganglion of the rat in vitro. J Cell Biol. 1966 May;29(2):267–285. doi: 10.1083/jcb.29.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The after-effects of repetitive stimulation on mammalian non-medullated fibres. J Physiol. 1956 Dec 28;134(3):698–711. doi: 10.1113/jphysiol.1956.sp005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A. Long duration responses in squid giant axons injected with 134cesium sulfate solutions. J Gen Physiol. 1966 Nov;50(2):269–278. doi: 10.1085/jgp.50.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]


