Abstract
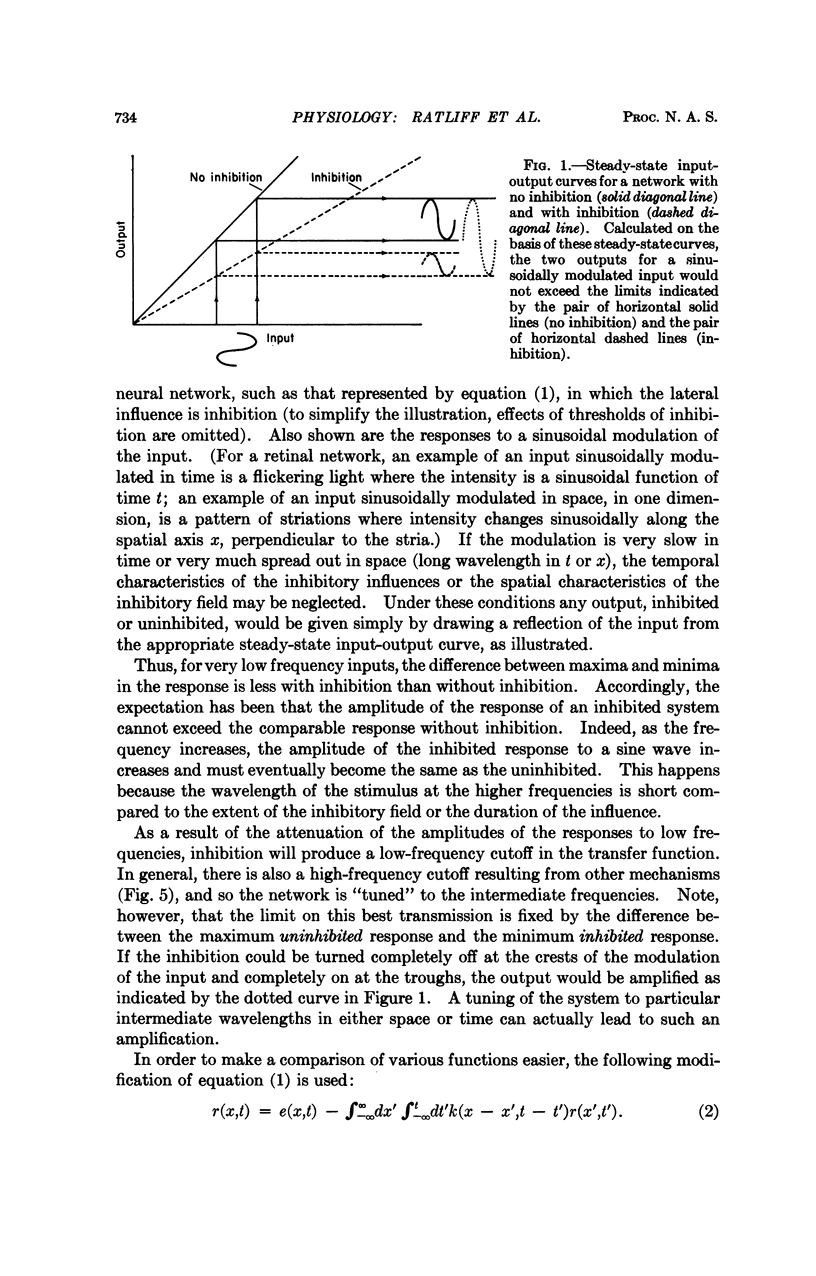
Lateral inhibition in a neural network generally attenuates the amplitudes of the responses to sinusoidal stimuli—both spatial and temporal. For an inhibitory influence with an abrupt onset and an exponential decay in time, and with a Gaussian distribution in space (the forms often assumed in theoretical calculations), the attenuation is greatest at low temporal and spatial frequencies. The attenuation diminishes with increasing frequencies until ultimately the amplitudes of inhibited responses become equal to, but never exceed, the amplitudes of the uninhibited.
For an inhibitory influence with a delay to the maximum in time or with eccentric maxima in space, however, the amplitudes of inhibited responses to certain intermediate frequencies may be greater than those of the uninhibited respones. This “amplification” results because the delay and the spatial separation “tune” the network to particular temporal and spatial frequencies; the inhibition is turned on at the trough of the response and off at the crest, thus tending to produce the greatest possible amplitude. The amplification has been observed in one neural network, the retina of the lateral eye of Limulus. The basic principles are general, and the effects may be expected in any system with negative feedback.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsellino A., Poppele R. E., Terzuolo C. A. Transfer functions of the slowly adapting stretch receptor organ of Crustacea. Cold Spring Harb Symp Quant Biol. 1965;30:581–586. doi: 10.1101/sqb.1965.030.01.056. [DOI] [PubMed] [Google Scholar]
- HARTLINE H. K., RATLIFF F. Inhibitory interaction of receptor units in the eye of Limulus. J Gen Physiol. 1957 Jan 20;40(3):357–376. doi: 10.1085/jgp.40.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLINE H. K., RATLIFF F. Spatial summation of inhibitory influences in the eye of Limulus, and the mutual interaction of receptor units. J Gen Physiol. 1958 May 20;41(5):1049–1066. doi: 10.1085/jgp.41.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY D. H. Effects of sharp edges in a flickering field. J Opt Soc Am. 1959 Jul;49(7):730–732. doi: 10.1364/josa.49.000730. [DOI] [PubMed] [Google Scholar]
- RATLIFF F., HARTLINE H. K., MILLER W. H. Spatial and temporal aspects of retinal inhibitory interaction. J Opt Soc Am. 1963 Jan;53:110–120. doi: 10.1364/josa.53.000110. [DOI] [PubMed] [Google Scholar]
- RATLIFF F., HARTLINE H. K. The responses of Limulus optic nerve fibers to patterns of illumination on the receptor mosaic. J Gen Physiol. 1959 Jul 20;42(6):1241–1255. doi: 10.1085/jgp.42.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff F., Knight B. W., Toyoda J., Hartline H. K. Enhancement of flicker by lateral inhibition. Science. 1967 Oct 20;158(3799):392–393. doi: 10.1126/science.158.3799.392. [DOI] [PubMed] [Google Scholar]


