Abstract
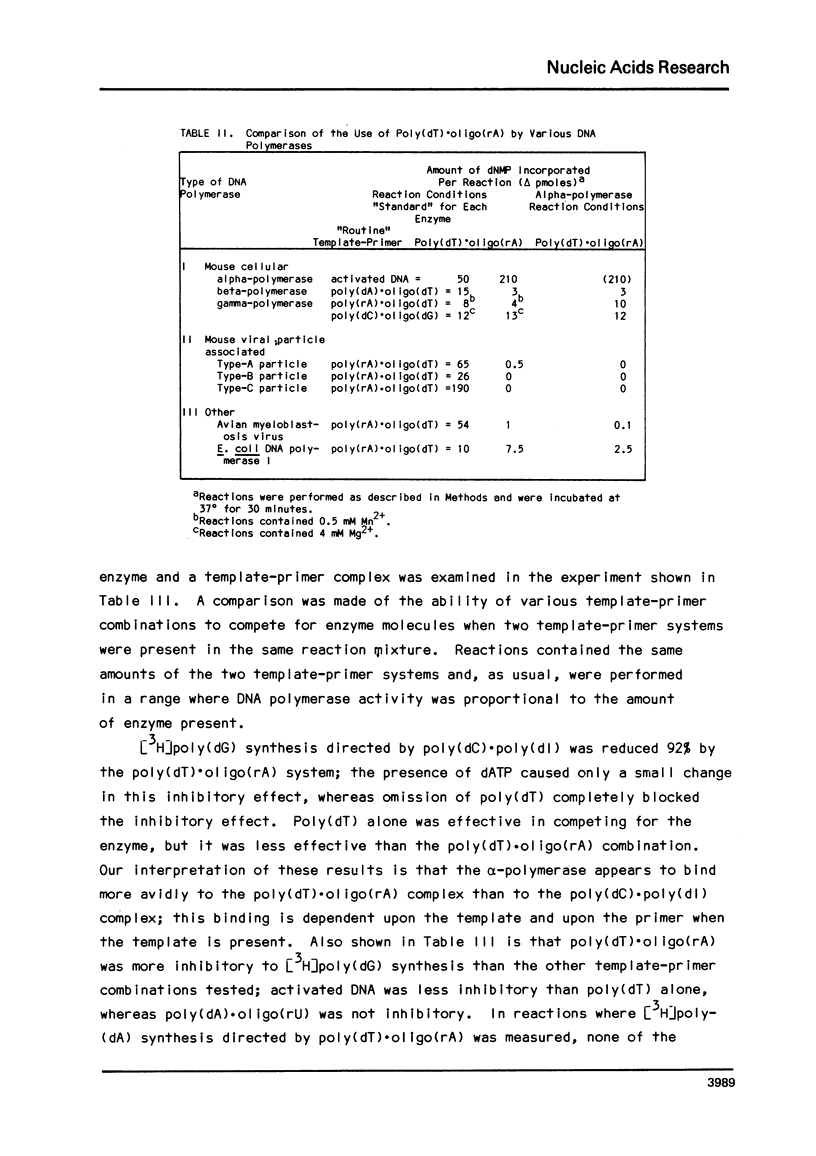
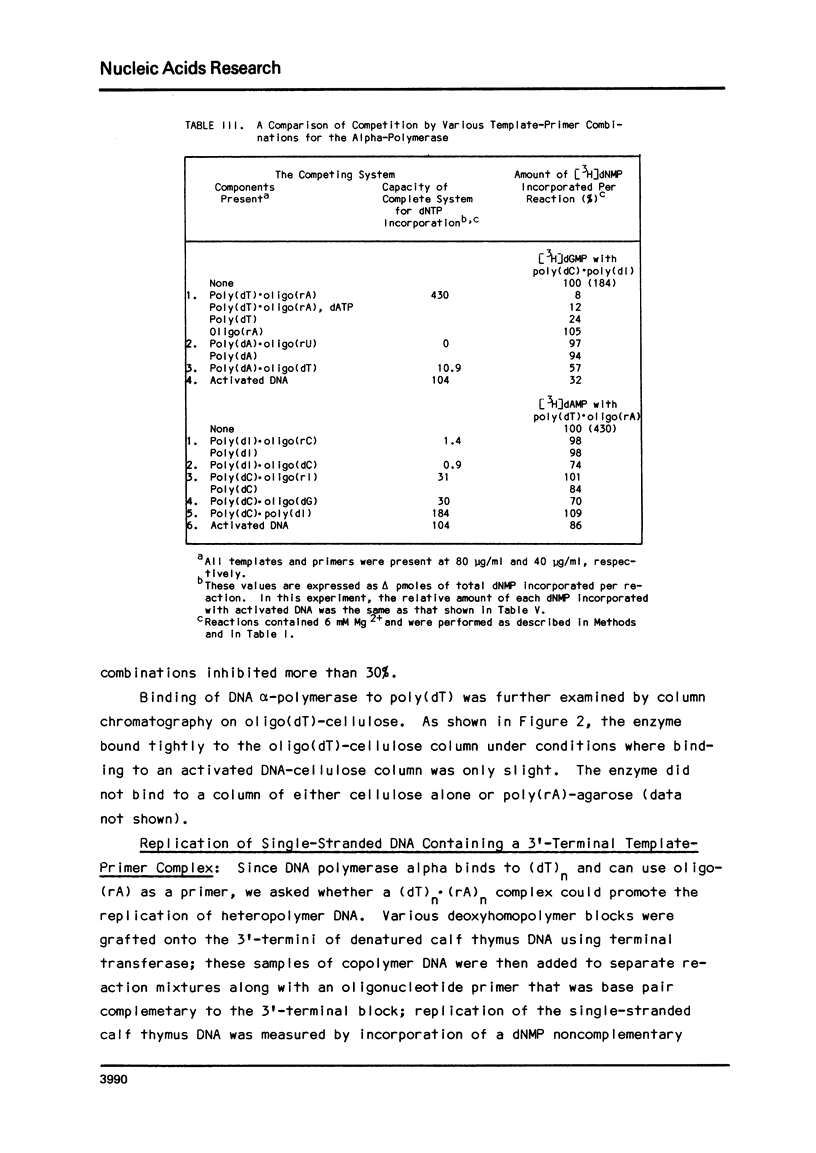
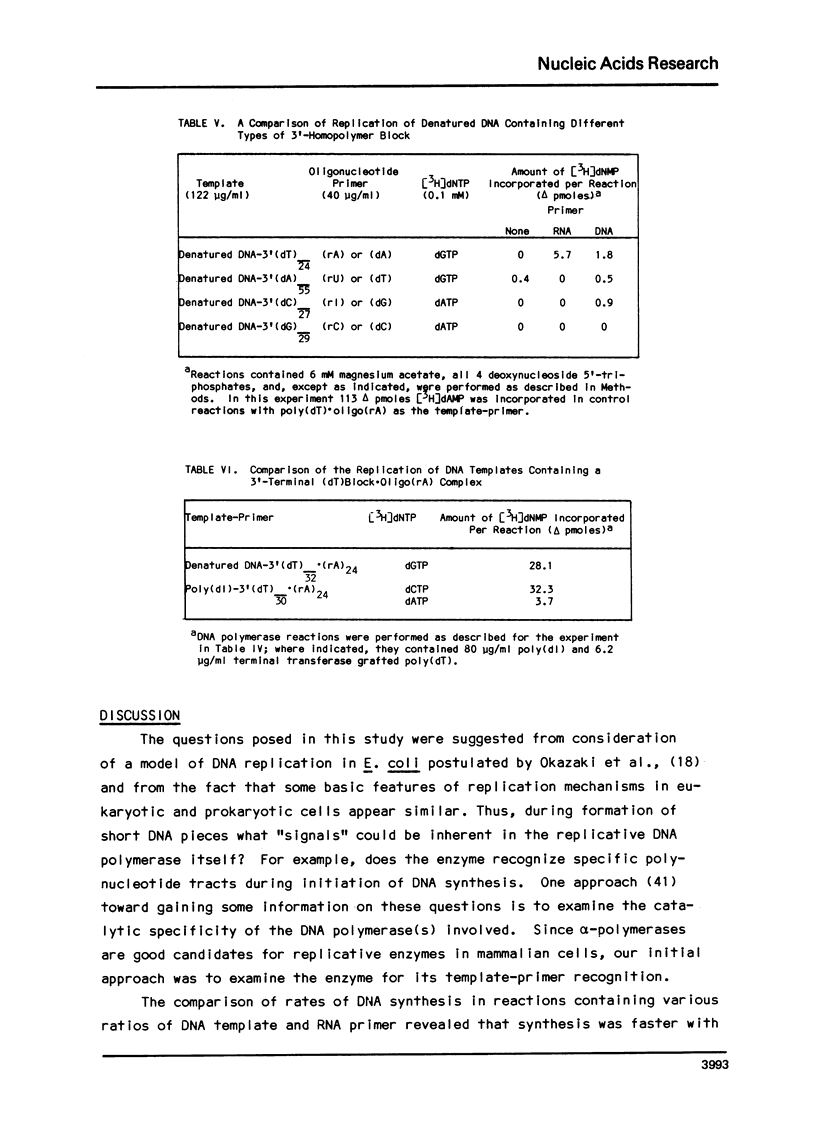
In a survey of template-primer preference of a mouse myeloma DNA alpha-polymerase, the fastest rate of DNA synthesis was with poly(dT) as template and (rA)24 as primer. Such a preference for poly(dT).oligo(rA) was not observed with other DNA polymerases of mouse origin. DNA synthesis in this system resulted in formation of oligo(dA) chains, not template-length poly(dA); thus, the average enzyme molecule bound to a poly(dT).(rA)24 complex and initiated a new oligo(dA) chain many times during the incubation. Binding experiments revealed that the alpha-polymerase had high affinity for poly(dT). Although the alpha-polymerase did not bind to poly(dl) and failed to replicate it inreactions with a base pair complementary primer, poly(dl) was replicated after a (dT) block had been grafted to its 3'-end and the oligo(rA) primer had been added. In similar experiments, the (dT) block was found to be much more effective than other 3'-terminal blocks in promoting replication of denatured calf thymus DNA. The results indicate that specific base sequences may regulate initiation of DNA syntehsis by this alpha-polymerase.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Oligodeoxyribonucleotide-primed reactions catalyzed by calf thymus polymerase. J Biol Chem. 1962 Jun;237:1945–1949. [PubMed] [Google Scholar]
- Bandyopadhyay A. K. Purification and properties of nuclear and cytoplasmic DNA polymerases from JLS-V9 cells. Arch Biochem Biophys. 1975 Jan;166(1):72–82. doi: 10.1016/0003-9861(75)90367-7. [DOI] [PubMed] [Google Scholar]
- Berger H., Jr Studies on nascent DNA in mouse myeloma. Cell. 1974 May;2(1):23–30. doi: 10.1016/0092-8674(74)90004-x. [DOI] [PubMed] [Google Scholar]
- Bollum F. J. Mammalian DNA polymerases. Prog Nucleic Acid Res Mol Biol. 1975;15(0):109–144. doi: 10.1016/s0079-6603(08)60118-x. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Dulbecco R. The replication of the ring-shaped DNA of polyoma virus. I. Identification of the replicative intermediate. Proc Natl Acad Sci U S A. 1969 Oct;64(2):701–708. doi: 10.1073/pnas.64.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd J. F., Wells R. D. Synthesis and characterization of the duplex block polymer d(C15A15)-d(T15G15). J Biol Chem. 1974 Nov 25;249(22):7094–7801. [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A chemical model for transcriptional initiation of DNA replication. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1354–1360. doi: 10.1016/s0006-291x(72)80124-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A comparison of associated enzyme activities in various deoxyribonucleic acid polymerases. J Biol Chem. 1973 May 25;248(10):3398–3404. [PubMed] [Google Scholar]
- Deepak J., Comer J., Bowling M., Dobbs J., Aldenderfer P. H., Fish D. C., Bandyopadhyay K. Simultaneous purification of RNA-dependent DNA polymerase and gs-antigen from Rauscher leukemia virus. Biochem Biophys Res Commun. 1975 Mar 17;63(2):400–408. doi: 10.1016/0006-291x(75)90702-0. [DOI] [PubMed] [Google Scholar]
- Dingman C. W. A convenient program for the rapid calculation of sedimentation coefficients in linear salt or sucrose gradients. Anal Biochem. 1972 Sep;49(1):124–133. doi: 10.1016/0003-2697(72)90249-7. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Fox R. M., Mendelsohn J., Barbosa E., Goulian M. RNA in nascent DNA from cultured human lymphocytes. Nat New Biol. 1973 Oct 24;245(147):234–237. doi: 10.1038/newbio245234a0. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Clarkson J. M. Discontinuous DNA replication in mouse P-815 cells. Eur J Biochem. 1975 Jan 2;50(2):403–412. doi: 10.1111/j.1432-1033.1975.tb09816.x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Holmer A. M., Hesslewood I. P., Johnston I. R. The occurrence of multiple activities in the high-molecular-weight DNA polymerase fraction of mammalian tissues. A preliminary study of some of their properties. Eur J Biochem. 1974 Apr 16;43(3):487–499. doi: 10.1111/j.1432-1033.1974.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Tsai A. Direction of DNA replication in mammalian cells. J Mol Biol. 1973 Mar 25;75(1):5–12. doi: 10.1016/0022-2836(73)90525-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Francke B. Letter: In vitro polyoma DNA synthesis: involvement of RNA in discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):123–130. doi: 10.1016/0022-2836(74)90427-6. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Kirschner R. H., Wolstenholme D. R., Gross N. J. Replicating molecules of circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1466–1472. doi: 10.1073/pnas.60.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukage A., Bohn E. W., Wilson S. H. On the DNA polymerase III of mouse myeloma: partial purification and characterization. Biochemistry. 1975 Mar 11;14(5):1006–1020. doi: 10.1021/bi00676a020. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Sivarajan M., Wilson S. H. Studies on DNA alpha-polymerase of mouse myeloma: partial purification and comparison of three molecular forms of the enzyme. Biochemistry. 1976 Nov 30;15(24):5305–5314. doi: 10.1021/bi00669a017. [DOI] [PubMed] [Google Scholar]
- Pero R. W., Bryngelsson T., Bryngelsson C., Deutsch A., Nordén A., Norgren A. Hybridization of polyuridylic acid to human DNA immobilized onto nitrocellulose filters. Nucleic Acids Res. 1975 Jul;2(7):1163–1176. doi: 10.1093/nar/2.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Wilson S. H. On the measurement of tritium in DNA and its applications to the assay of DNA polymerase activity. Methods Cell Biol. 1976;13:105–120. doi: 10.1016/s0091-679x(08)61799-9. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Shu-Fong Wang T., Korn D. "Cytoplasmic" deoxyribonucleic acid polymerase. Structure and properties of the highly purified enzyme from human KB cells. J Biol Chem. 1975 Sep 10;250(17):7045–7056. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. RNA-primed DNA synthesis: specific catalysis by HeLa cell DNA polymerase alpha. Proc Natl Acad Sci U S A. 1975 Feb;72(2):503–507. doi: 10.1073/pnas.72.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamblyn T. M., Wells R. D. Comparative ability of RNA and DNA to prime DNA synthesis in vitro: role of sequence, sugar, and structure of template-primer. Biochemistry. 1975 Apr 8;14(7):1412–1425. doi: 10.1021/bi00678a011. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Rates of chain growth and units of replication in DNA of mammalian chromosomes. J Mol Biol. 1968 Feb 14;31(3):579–594. doi: 10.1016/0022-2836(68)90429-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Tsukada K., Moriyama T., Lynch W. E., Lieberman I. Polydeoxynucleotide intermediates in DNA replication in regenerating liver. Nature. 1968 Oct 12;220(5163):162–164. doi: 10.1038/220162a0. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Huberman J. A. Evidence for the attachment of RNA to pulse-labeled DNA in the slime mold, Physarum polycephalum. Biochem Biophys Res Commun. 1973 Mar 5;51(1):174–180. doi: 10.1016/0006-291x(73)90524-x. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Bohn E. W., Matsukage A., Lueders K. K., Kuff E. L. Studies on the relationship between deoxyribonucleic acid polymerase activity and intracisternal A-type particles in mouse myeloma. Biochemistry. 1974 Mar 12;13(6):1087–1094. doi: 10.1021/bi00703a005. [DOI] [PubMed] [Google Scholar]
- de Recondo A. M., Lepesant J. A., Fichot O., Grasset L., Rossignol J. M., Cazillis M. Synthetic template specificity of a deoxyribonucleic acid polymerase from regenerating rat liver. J Biol Chem. 1973 Jan 10;248(1):131–137. [PubMed] [Google Scholar]


