Abstract
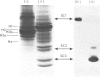
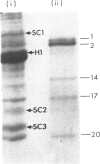
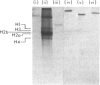
Acceptor proteins for poly(ADP-ribose) have been purified from mouse testis nuclei. Nuclear proteins were labelled in vitro with [14C]ribose and [3H]adenine, extracted with 5% (v/v) HClO4 and 0.25 M-HCl and separated by ion-exchange chromatography. Non-histone proteins were found to be the major acceptors in both the 5% (w/v)-HClO4-soluble and 5%-HClO4-insoluble HCl-extractable fractions. Of the two groups of non-histone proteins associated with chromatin, the LMG (low-mobility-group) proteins were preferentially ADP-ribosylated. HMG (high-mobility group) proteins were labelled to lower specific radioactivity. Six LMG proteins were purified to approx. 90% homogeneity and were identified from their mobility on polyacrylamide gels at pH 2.9 and from their amino acid composition. The average length of the poly(ADP-ribose) chain was estimated to be four to six repeating ADP-ribose units. It is suggested that ADP-ribosylation of LMG proteins, a long-neglected group of chromatin-associated proteins, is important during spermatogenesis for the production of spermatozoa with intact and competent DNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Brown E., Goodwin G. H. Comparison of the high-mobility-group chromosomal proteins in rainbow-trout (Salmo gairdnerii) liver and testis. Biochem J. 1983 Dec 1;215(3):531–538. doi: 10.1042/bj2150531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Goodwin G. H., Mayes E. L., Hastings J. R., Johns E. W. Heterogeneity of proteins resembling high-mobility-group protein HMG-T in trout testes nuclei. Biochem J. 1980 Nov 1;191(2):661–664. doi: 10.1042/bj1910661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. Activation of the template activity of isolated rat liver nuclei for DNA synthesis and its inhibition by NAD. Biochem Biophys Res Commun. 1973 Jul 17;53(2):572–579. doi: 10.1016/0006-291x(73)90700-6. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Brothers J. F., Giri C. P., Smulson M. E. A nuclear protein-modifying enzyme is responsive to ordered chromatin structure. Nucleic Acids Res. 1978 Aug;5(8):2775–2788. doi: 10.1093/nar/5.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary P. D., Turner C. H., Mayes E., Crane-Robinson C. Conformation and domain structure of the non-histone chromosomal proteins, HMG 1 and 2. Isolation of two folded fragments from HMG 1 and 2. Eur J Biochem. 1983 Mar 15;131(2):367–374. doi: 10.1111/j.1432-1033.1983.tb07272.x. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Comparison of the high mobility group proteins and their chromatin distribution in trout testis and liver. J Biol Chem. 1981 Jul 25;256(14):7549–7556. [PubMed] [Google Scholar]
- Cooper E., Spaulding S. W. HMG (high-mobility-group)-14/17-like proteins in calf thyroid. Thyrotropin-dependent phosphorylation and comparison with calf thymus proteins. Biochem J. 1983 Dec 1;215(3):643–649. doi: 10.1042/bj2150643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Sanders C., Johns E. W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973 Sep 21;38(1):14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Gordon J. S., Rosenfeld B. I., Kaufman R., Williams D. L. Evidence for a quantitative tissue-specific distribution of high mobility group chromosomal proteins. Biochemistry. 1980 Sep 16;19(19):4395–4402. doi: 10.1021/bi00560a003. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Miura K., Murachi T. Identification of a protease inhibitor from rat peritoneal macrophages as poly(ADP-ribose). J Biol Chem. 1980 Aug 25;255(16):7746–7750. [PubMed] [Google Scholar]
- James G. T., Yeoman L. C., Matsui S. i., Goldberg A. H., Busch H. Isolation and characterization of nonhistone chromosomal protein C-14 which stimulates RNA synthesis. Biochemistry. 1977 May 31;16(11):2384–2389. doi: 10.1021/bi00630a012. [DOI] [PubMed] [Google Scholar]
- Jones R., Brown C. R., Von Glós K. I., Parker M. G. Hormonal regulation of protein synthesis in the rat epididymis. Characterization of androgen-dependent and testicular fluid-dependent proteins. Biochem J. 1980 Jun 15;188(3):667–676. doi: 10.1042/bj1880667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. P., Davies P. L. Acid-soluble nuclear proteins of the testis during spermatogenesis in the winter flounder. Loss of the high mobility group proteins. J Biol Chem. 1980 Mar 25;255(6):2533–2539. [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B. ADP-ribosylation of trout testis chromosomal proteins: distribution of ADP-ribosylated proteins among DNase I-sensitive and -resistant chromatin domains. Arch Biochem Biophys. 1981 May;208(2):528–534. doi: 10.1016/0003-9861(81)90541-5. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Watson D. C., Dixon G. H. Multiacetylated forms of H4 are found in a putative transcriptionally competent chromatin fraction from trout testis. Nucleic Acids Res. 1979 Jan;6(1):259–274. doi: 10.1093/nar/6.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loir M., Lanneau M. Transformation of ram spermatid chromatin. Exp Cell Res. 1978 Sep;115(2):231–243. doi: 10.1016/0014-4827(78)90277-x. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Igo-Kemenes T., Johns E. W. The protein composition of rat satellite chromatin. FEBS Lett. 1981 Mar 9;125(1):25–29. doi: 10.1016/0014-5793(81)80988-x. [DOI] [PubMed] [Google Scholar]
- Mennella M. R., Quesada P., Farina B., Leone E., Jones R. ADP-ribosylation of nuclear proteins in mouse testis. Biochem J. 1982 Jul 1;205(1):245–248. doi: 10.1042/bj2050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaga T., Romaschin A. D., Kirsten E., Kun E. The in vivo distribution of immunoreactive larger than tetrameric polyadenosine diphosphoribose in histone and non-histone protein fractions of rat liver. J Biol Chem. 1979 Oct 10;254(19):9663–9668. [PubMed] [Google Scholar]
- Mullins D. W., Jr, Giri C. P., Smulson M. Poly(adenosine diphosphate-ribose) polymerase: the distribution of a chromosome-associated enzyme within the chromatin substructure. Biochemistry. 1977 Feb 8;16(3):506–513. doi: 10.1021/bi00622a026. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K. Poly ADP-ribosylation of DNA-dependent RNA polymerase I from quail oviduct. Dependence on progesterone stimulation. Mol Cell Biochem. 1976 Sep 30;12(3):147–159. doi: 10.1007/BF01741713. [DOI] [PubMed] [Google Scholar]
- Nduka N., Shall S. 5-Methylnicotinamide-resistant variant of mouse lymphoma L1210 cells. Biochem Biophys Res Commun. 1980 Oct 16;96(3):997–1002. doi: 10.1016/0006-291x(80)90051-0. [DOI] [PubMed] [Google Scholar]
- Ohgushi H., Yoshihara K., Kamiya T. Bovine thymus poly(adenosine diphosphate ribose) polymerase. Physical properties and binding to DNA. J Biol Chem. 1980 Jul 10;255(13):6205–6211. [PubMed] [Google Scholar]
- Okayama H., Hayaishi O. ADP-ribosylation of nuclear protein A24. Biochem Biophys Res Commun. 1978 Oct 16;84(3):755–762. doi: 10.1016/0006-291x(78)90769-6. [DOI] [PubMed] [Google Scholar]
- Okayama H., Ueda K., Hayaishi O. Purification of ADP-ribosylated nuclear proteins by covalent chromatography on dihydroxyboryl polyacrylamide beads and their characterization. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1111–1115. doi: 10.1073/pnas.75.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrick L. R., Olson M. O., Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973 May;70(5):1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Walker J. M., Brown E., Johns E. W. Trout liver high mobility group non-histone chromosomal proteins. FEBS Lett. 1980 Jan 14;109(2):294–298. doi: 10.1016/0014-5793(80)81108-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Kistler W. S. Levels of chromosomal protein high mobility group 2 parallel the proliferative activity of testis, skeletal muscle, and other organs. J Biol Chem. 1979 Nov 25;254(22):11264–11271. [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. Chemical and metabolic properties of adenosine diphosphate ribose derivatives of nuclear proteins. Biochem J. 1975 Jun;147(3):523–529. doi: 10.1042/bj1470523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanuma S., Johnson G. S. ADP-ribosylation of nonhistone high mobility group proteins in intact cells. J Biol Chem. 1983 Apr 10;258(7):4067–4070. [PubMed] [Google Scholar]
- Ueda K., Omachi A., Kawaichi M., Hayaishi O. Natural occurrence of poly(ADP-ribosyl) histones in rat liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):205–209. doi: 10.1073/pnas.72.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N. C., Poirier G. G., Dixon G. H. Adenosine diphosphoribosylation of certain basic chromosomal proteins in isolated trout testis nuclei. Eur J Biochem. 1977 Jul 1;77(1):11–21. doi: 10.1111/j.1432-1033.1977.tb11635.x. [DOI] [PubMed] [Google Scholar]