Abstract
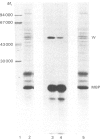
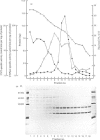
Cyclic AMP-stimulated phosphorylation of membrane proteins in central-nervous-system myelin was investigated, with rabbit brain myelin. Subfractionation of a myelin membrane preparation by sucrose-density-gradient centrifugation produced a rapidly sedimenting population of membrane vesicles containing 5'-nucleotidase and acetylcholinesterase, a light membrane fraction containing myelin basic protein and 2',3'-cyclic nucleotide 3'-phosphodiesterase, and an intermediate membrane fraction containing the highest specific activity of 2',3'-cyclic nucleotide 3'-phosphodiesterase and a small proportion of myelin basic protein. Cyclic AMP stimulation of protein phosphorylation was confined to a protein of Mr 49 700, which co-electrophoresed with the upper component of the Wolfgram protein doublet. Cyclic AMP did not affect the phosphorylation of myelin basic protein. Cyclic AMP-stimulated phosphorylation of this protein followed 2',3'-cyclic nucleotide 3'-phosphodiesterase activity on subcellular fractionation and was correspondingly high in the intermediate or 'myelin-like' fraction on sucrose-density-gradient centrifugation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., O'Connell K., Randle C. L., Agrawal D. Phosphorylation in vivo of four basic proteins of rat brain myelin. Biochem J. 1982 Jan 1;201(1):39–47. doi: 10.1042/bj2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik N. L., Davison A. N. Enzyme activity and composition of myelin and subcellular fractions in the developing rat brain. Biochem J. 1969 Dec;115(5):1051–1062. doi: 10.1042/bj1151051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer W., Sirota S. R., Zimmerman T. R., Jr, Norton W. T. 5'-nucleotidase in rat brain myelin. J Neurochem. 1980 Aug;35(2):367–373. doi: 10.1111/j.1471-4159.1980.tb06273.x. [DOI] [PubMed] [Google Scholar]
- Carnegie P. R. Amino acid sequence of the encephalitogenic basic protein from human myelin. Biochem J. 1971 Jun;123(1):57–67. doi: 10.1042/bj1230057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie P. R., Kemp B. E., Dunkley P. R., Murray A. W. Phosphorylation of myelin basic protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem J. 1973 Nov;135(3):569–572. doi: 10.1042/bj1350569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond R. J., Dean G. Comparison of 2',3'-cyclic nucleotide 3'-phosphodiesterase and the major component of Wolfgram protein W1. J Neurochem. 1980 Nov;35(5):1155–1165. doi: 10.1111/j.1471-4159.1980.tb07871.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Endo T., Hidaka H. Ca2+-calmodulin dependent phosphorylation of myelin isolated from rabbit brain. Biochem Biophys Res Commun. 1980 Nov 28;97(2):553–558. doi: 10.1016/0006-291x(80)90299-5. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. Amino acid sequence of the basic protein of the myelin membrane. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1425–1431. doi: 10.1073/pnas.67.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Jackson P., Hales C. N. Isolation and characterization of calmodulin from an insulin-secreting tumour. Biochem J. 1981 Mar 1;193(3):875–885. doi: 10.1042/bj1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. K. The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J. 1960 Dec;77:610–618. doi: 10.1042/bj0770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Maeno H., Greengard P. Phosphorylation of endogenous protein of rat brain by cyclic adenosine 3',5'-monophosphate-dependent protein kinase. J Biol Chem. 1971 Dec 25;246(24):7731–7739. [PubMed] [Google Scholar]
- Kurihara T., Tsukada Y. The regional and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphohydrolase in the central nervous system. J Neurochem. 1967 Dec;14(12):1167–1174. doi: 10.1111/j.1471-4159.1967.tb06164.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto E., Kakiuchi S. In vitro and in vivo phosphorylation of myelin basic protein by exogenous and endogenous adenosine 3':5'-monophosphate-dependent protein kinases in brain. J Biol Chem. 1974 May 10;249(9):2769–2777. [PubMed] [Google Scholar]
- Miyamoto E., Kakiuchi S., Kakimoto Y. In vitro and in vivo phosphorylation of myelin basic protein by cerebral protein kinase. Nature. 1974 May 10;249(453):150–151. doi: 10.1038/249150a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Protein kinases in myelin of rat brain: solubilization and characterization. J Neurochem. 1975 Mar;24(3):503–512. doi: 10.1111/j.1471-4159.1975.tb07668.x. [DOI] [PubMed] [Google Scholar]
- Sogin D. C. 2',3'-Cyclic NADP as a substrate for 2',3'-cyclic nucleotide 3'-phosphohydrolase. J Neurochem. 1976 Dec;27(6):1333–1337. doi: 10.1111/j.1471-4159.1976.tb02612.x. [DOI] [PubMed] [Google Scholar]
- Sprinkle T. J., Wells M. R., Garver F. A., Smith D. B. Studies on the Wolfgram high molecular weight CNS myelin proteins: relationship to 2',3'-cyclic nucleotide 3'-phosphodiesterase. J Neurochem. 1980 Nov;35(5):1200–1208. doi: 10.1111/j.1471-4159.1980.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck A. J., Appel S. H. Phosphorylation of myelin basic protein. J Biol Chem. 1974 Sep 10;249(17):5416–5420. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Petrali E. H., Davis E. R., Thiessen B. J. Calcium ion stimulated endogenous protein kinase catalyzed phosphorylation of basic proteins in myelin subfractions and myelin-like membrane fraction from rat brain. Biochemistry. 1980 Nov 11;19(23):5363–5371. doi: 10.1021/bi00564a034. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Petrali E. H., Thiessen B. J., Davis E. R. Calcium ion-stimulated phosphorylation of myelin proteins. Biochem J. 1980 Feb 15;186(2):469–473. doi: 10.1042/bj1860469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. S., Chou C. H., Kibler R. F., Kuo J. F. Basic protein in brain myelin is phosphorylated by endogenous phospholipid-sensitive Ca2+-dependent protein kinase. J Neurochem. 1982 Nov;39(5):1397–1404. doi: 10.1111/j.1471-4159.1982.tb12583.x. [DOI] [PubMed] [Google Scholar]
- Waehneldt T. V., Matthieu J. M., Neuhoff V. Characterization of a myelin-related fraction (SN 4) isolated from rat forebrain at two developmental stages. Brain Res. 1977 Dec 9;138(1):29–43. doi: 10.1016/0006-8993(77)90782-x. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]
- Whittaker V. P. The application of subcellular fractionation techniques to the study of brain function. Prog Biophys Mol Biol. 1965;15:39–96. doi: 10.1016/0079-6107(65)90004-0. [DOI] [PubMed] [Google Scholar]
- Wolfgram F., Kotorii K. The composition of the myelin proteins of the central nervous system. J Neurochem. 1968 Nov;15(11):1281–1290. doi: 10.1111/j.1471-4159.1968.tb05905.x. [DOI] [PubMed] [Google Scholar]