Abstract
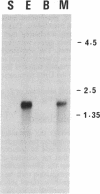
DNA sequences coding for the muscle-specific subunit of creatine kinase have been isolated from cDNA libraries constructed from Torpedo marmorata electric organ. Clones were screened by differential in situ hybridization and hybrid-selected translation. The in vitro translation product of the selected mRNA was immunoprecipitated by anti-chicken creatine kinase antibodies and comigrated with Torpedo muscle creatine kinase on two-dimensional gels at the same position as the cytosolic 43,000-dalton protein referred to as nu 2. The cDNA inserts hybridized to a mRNA species present in adult Torpedo muscle but not in brain. The complete sequence of the mRNA was determined on one of the clones except for the 78 nucleotides of the mRNA 5' terminal sequence, which were identified by the primer extension method. The amino acid sequence of muscle-specific creatine kinase from T. marmorata was deduced and analyzed. It includes the known sequence of a peptide from the active site of rabbit muscle-specific creatine kinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton R. S., Laws J. F., Miles B. J., Thomson A. R. Brain adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1970 Dec;120(3):589–600. doi: 10.1042/bj1200589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J., Mieskes G., Wallimann T. Creatine kinase activity in the Torpedo electrocyte and in the nonreceptor, peripheral v proteins from acetylcholine receptor-rich membranes. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5440–5444. doi: 10.1073/pnas.80.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Auffray C., Rougeon F., Changeux J. P. Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1982;1(6):713–717. doi: 10.1002/j.1460-2075.1982.tb01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R., Wirth M., Flanagan S. D. Structural heterogeneity and subcellular distribution of nicotinic synapse-associated proteins. J Biol Chem. 1981 Nov 25;256(22):11373–11376. [PubMed] [Google Scholar]
- Gysin R., Yost B., Flanagan S. D. Immunochemical and molecular differentiation of 43 000 molecular weight proteins associated with Torpedo neuroelectrocyte synapses. Biochemistry. 1983 Dec 6;22(25):5781–5789. doi: 10.1021/bi00294a016. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nghiêm H. O., Cartaud J., Dubreuil C., Kordeli C., Buttin G., Changeux J. P. Production and characterization of a monoclonal antibody directed against the 43,000-dalton v1 polypeptide from Torpedo marmorata electric organ. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6403–6407. doi: 10.1073/pnas.80.20.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OLSON O. E., KUBY S. A. STUDIES ON ADENOSINE TRIPHOSPHATE-ADENOSINE TRIPHOSPHATE TRANSPHOSPHORYLASES. V. CARBOXYL-TERMINAL SEQUENCES OF ADENOSINE TRIPHOSPHATE-CREATINE TRANSPHOSPHORYLASE AND OF ADENOSINE 5'-PHOSPHATE TRANSPHOSPHORYLASE (MYOKINASE). J Biol Chem. 1964 Feb;239:460–467. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Perriard E. R., Eppenberger H. M. Detection and relative quantitation of mRNA for creatine kinase isoenzymes in mRNA from myogenic cell cultures and embryonic chicken tissues. J Biol Chem. 1978 Sep 25;253(18):6529–6535. [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Eppenberger H. M., Perriard J. C. Occurrence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur J Biochem. 1981 May;116(1):87–92. doi: 10.1111/j.1432-1033.1981.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Changeux J. P. Phosphorylation in vitro of membrane fragments from Torpedo marmorata electric organ. Effect on membrane solubilization by detergents. Eur J Biochem. 1980 Mar;105(1):51–62. doi: 10.1111/j.1432-1033.1980.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Oswald R., Wennogle L. P., Changeux J. P. Conditions for the selective labelling of the 66 000 dalton chain of the acetylcholine receptor by the covalent non-competitive blocker 5-azido-[3H]trimethisoquin. FEBS Lett. 1980 Jul 11;116(1):30–36. doi: 10.1016/0014-5793(80)80522-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]