Abstract
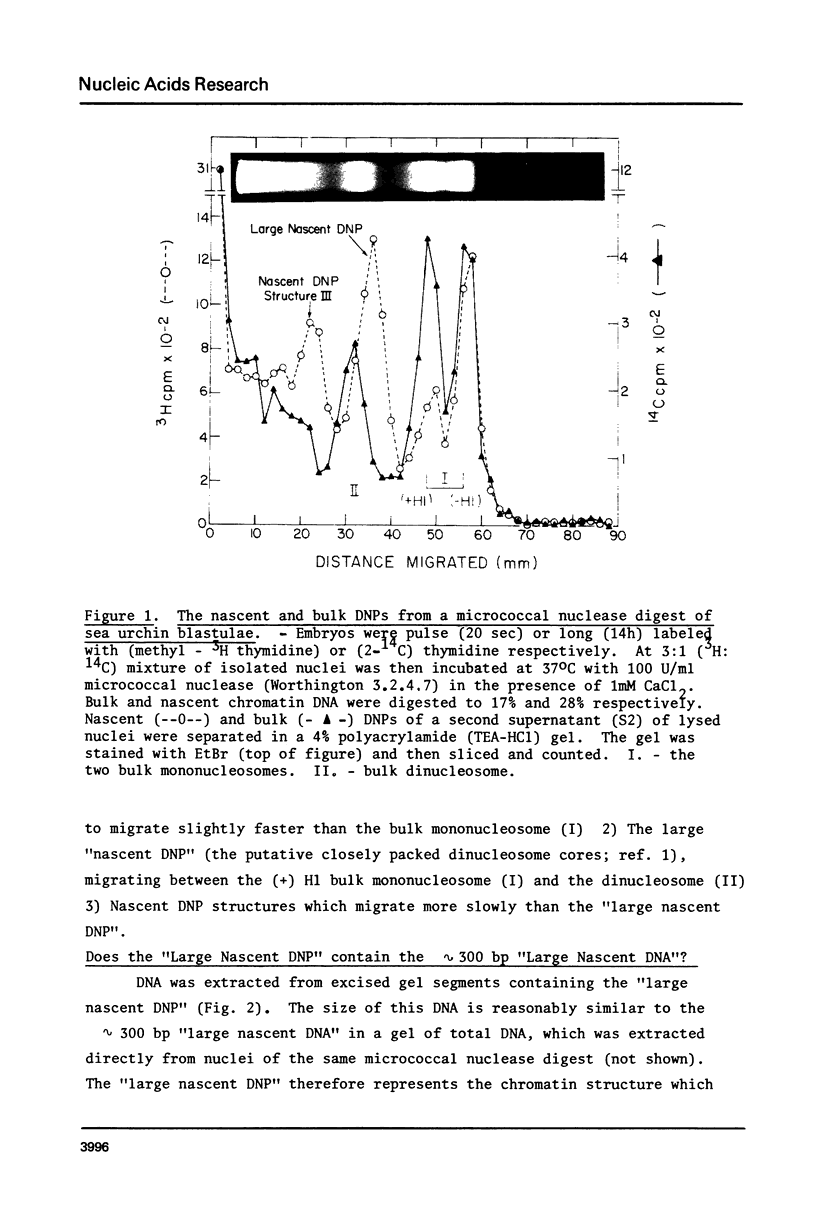
Discrete deoxyribonucleoproteins (DNPs) containing nascent and/or bulk DNA, were obtained by fractionating micrococcal nuclease digests of nuclei form 3H-thymidine pulse (15-20 sec) and 14C-thymidine long (16 h) labeled sea urchin embryos in polyacrylamide gels. One of these DNPs was shown to contain the micrococcal nuclease resistant 300 bp "large nascent DNA" described in Cell 14, 259-267, 1978. The bulk and nascent mononucleosome fractions provided evidence for the preferential digestion by micrococcal nuclease of nascent over bulk linker regions to yield mononucleosome cores with nascent DNA. DNAase I was used to probe whether any nascent DNA is in nucleosomes. Nascent as well as bulk single-stranded DNA fragments occurred in multiples of 10.4 bases with higher than random frequencies of certain fragment sizes (for instance 83 bases) as expected from a nucleosome structure. However, a striking background of nascent DNA between nascent DNA peaks was observed. This was eliminated by a pulse-chase treatment or by digestion of pulse-labeled nuclei with micrococcal nuclease together with DNAase I. One of several possible interpretations of these results suggests that a transient change in nucleosome structure may have created additional sites for the nicking of nascent DNA by DNAase I; the micrococcal nuclease sensitivity of the interpeak radioactivity suggest its origin from the linker region. Endogenous nuclease of sea urchin embryos cleaves chromatin DNA in a manner similar to that of DNAase I.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: location of the first nucleosomes on newly synthesized simian virus 40 deoxyribonucleic acid. Biochemistry. 1981 Feb 3;20(3):621–630. doi: 10.1021/bi00506a027. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Levy A., Jakob K. M., Moav B. Metrizamide density gradients of sea urchin chromatin: fractions rich and poor in nascent DNA. Nucleic Acids Res. 1975 Dec;2(12):2299–2303. doi: 10.1093/nar/2.12.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Jakob K. M. Nascent DNA in nucleosome like structures from chromatin. Cell. 1978 Jun;14(2):259–267. doi: 10.1016/0092-8674(78)90112-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Murphy R. F., Wallace R. B., Bonner J. Isolation of newly replicated chromatin by using shallow metrizamide gradients. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3336–3340. doi: 10.1073/pnas.77.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. DNA folding in the nucleosome. J Mol Biol. 1977 Oct 15;116(1):49–71. doi: 10.1016/0022-2836(77)90118-8. [DOI] [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Parisi E., De Petrocellis B. Properties of a deoxyribonuclease from a nuclear extract of Paracentrotus lividus embryos. Biochem Biophys Res Commun. 1972 Nov 1;49(3):706–712. doi: 10.1016/0006-291x(72)90468-8. [DOI] [PubMed] [Google Scholar]
- Prunell A., Kornberg R. D., Lutter L., Klug A., Levitt M., Crick F. H. Periodicity of deoxyribonuclease I digestion of chromatin. Science. 1979 May 25;204(4395):855–858. doi: 10.1126/science.441739. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Assembly of DNA and protein during replication in HeLa cells. Nature. 1975 May 15;255(5505):247–249. doi: 10.1038/255247a0. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Nucleosomes associated with newly replicated DNA have an altered conformation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2717–2721. doi: 10.1073/pnas.75.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]
- Yakura K., Tanifuji S. Chromatin replication in a higher plant, Vicia faba. Biochim Biophys Acta. 1980 Oct 17;609(3):448–455. doi: 10.1016/0005-2787(80)90118-5. [DOI] [PubMed] [Google Scholar]