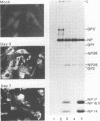
Abstract
A stock of plaque-purified Pichinde virus, prepared under conditions designed to limit the amounts of defective interfering virus, was used to infect BHK cells. At daily intervals after infection, cells were examined for infectious and radiolabeled virus particle production and for the synthesis of virus-specific polypeptides. Quantitative comparisons were also made of the concentrations of genomic Pichinde virus L and S RNAs in the cytoplasm of infected cells on different days after infection. Our results showed that virus particle production, rates of protein synthesis, and the intracellular levels of viral genomic RNAs all increased and decreased with similar kinetics, and that this regulation was independent of the cell growth cycle. We were unable to relate these changes in viral macromolecule and virus production to the appearance of readily identifiable defective interfering particles. Our findings suggest that regulation of virus replication early during the replicative cycle of Pichinde virus may not be dependent upon the generation of defective interfering virus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D., Dimock K., Cash P., Rawls W. E., Leung W. C., Bishop D. H. Analyses of the genomes of prototype pichinde arenavirus and a virulent derivative of Pichinde Munchique: evidence for sequence conservation at the 3' termini of their viral RNA species. Virology. 1982 Jan 15;116(1):363–367. doi: 10.1016/0042-6822(82)90429-9. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Gee S. R., Rawls W. E. Antigens of Pichinde virus I. Relationship of soluble antigens derived from infected BHK-21 cells to the structural components of the virion. J Virol. 1977 Apr;22(1):175–186. doi: 10.1128/jvi.22.1.175-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Rawls W. E. Variation between strains of hamsters in the lethality of Pichinde virus infections. Infect Immun. 1977 May;16(2):413–421. doi: 10.1128/iai.16.2.413-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Wright E. A., Pfau C. J. The RNAs of the defective interfering Pichinide virus. J Gen Virol. 1976 Jun;31(3):417–427. doi: 10.1099/0022-1317-31-3-417. [DOI] [PubMed] [Google Scholar]
- Gimenez H. B., Compans R. W. Defective interfering Tacaribe virus and persistently infected cells. Virology. 1980 Nov;107(1):229–239. doi: 10.1016/0042-6822(80)90288-3. [DOI] [PubMed] [Google Scholar]
- Harnish D. G., Leung W. C., Rawls W. E. Characterization of polypeptides immunoprecipitable from Pichinde virus-infected BHK-21 cells. J Virol. 1981 Jun;38(3):840–848. doi: 10.1128/jvi.38.3.840-848.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin J. Transient virus infection: spontaneous recovery mechanism of lymphocytic choriomeningitis virrus-infected cells. Nat New Biol. 1973 Feb 28;241(113):270–272. doi: 10.1038/newbio241270a0. [DOI] [PubMed] [Google Scholar]
- Howard C. R., Simpson D. I. Review article the biology of the arenaviruses. J Gen Virol. 1980 Nov;51(Pt 1):1–14. doi: 10.1099/0022-1317-51-1-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Ramsingh A., Dimock K., Rawls W. E., Petrovich J., Leung M. Pichinde virus L and S RNAs contain unique sequences. J Virol. 1981 Jan;37(1):48–54. doi: 10.1128/jvi.37.1.48-54.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Structural components and replication of arenaviruses. Adv Virus Res. 1979;24:277–330. doi: 10.1016/s0065-3527(08)60396-6. [DOI] [PubMed] [Google Scholar]
- Ramsingh A. I., Dimock K., Rawls W. E., Leung W. C. Size estimation of Pichinde virus RNA by gel electrophoresis under denaturing conditions. Intervirology. 1980;14(1):31–36. doi: 10.1159/000149159. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Chan M. A., Gee S. R. Mechanisms of persistence in arenavirus infections: a brief review. Can J Microbiol. 1981 Jun;27(6):568–574. doi: 10.1139/m81-086. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Rawls W. E. Pseudotypes of vesicular stomatitis virus and Pichinde virus. J Gen Virol. 1979 Jan;42(1):141–148. doi: 10.1099/0022-1317-42-1-141. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza A. C., Cash P., Jahrling P., Eddy G., Bishop D. H. Arenavirus recombination: the formation of recombinants between prototype pichinde and pichinde munchique viruses and evidence that arenavirus S RNA codes for N polypeptide. Virology. 1980 Oct 30;106(2):250–260. doi: 10.1016/0042-6822(80)90248-2. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Pfau C. J. Determinants of lymphocytic choriomeningitis interference. J Gen Virol. 1972 Feb;14(2):177–187. doi: 10.1099/0022-1317-14-2-177. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]