Abstract
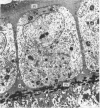
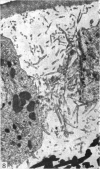
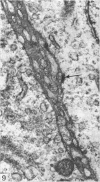
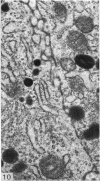
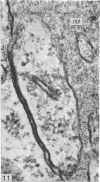
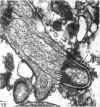
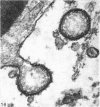
A survey has been made of the ultrastructural features of the oocyte surface and its enveloping layers, comprising the theca, the granulosa and the perivitelline layer, during the final growth phase of 'yellow yolk' deposition. The following observations suggest that many macromolecular components of the blood plasma have free access from the vasculature to the oolemma: the fenestrated structure of the endothelium of the thecal capillaries, the intercellular spaces containing several erythrocytes in the theca interna, the 20-30 nm particles in the granulosa basal lamina, the wide spaces between the granulosa cells, and the open meshwork of fibres in the perivitelline layer. Numerous coated pits and vesicles, of 0.25-0.35 micron diameter, in the highly convoluted surface layer of the oocyte provide a mechanism for the incorporation of yolk precursors by pinocytosis. Such large coated vesicles and wide spaces between the granulosa cells are lacking in follicles in the preceding growth phase, which is concerned with the deposition of 'white yolk'. Considerable metabolic activity of the granulosa cells is indicated by their prominent Golgi elements, diverse granules, vesicles and villus processes. Cell junctions at the tips of the macrovilli anchor the granulosa to the oocyte. The theca externa, which provides mechanical support for the oocyte and its vascular and nervous elements, consists of sheets of collagen fibrils and fibroblast-like cells. Microfilaments in these cells may contribute to the contractility of the theca. Groups of interstitial cells associated with nerve fibres are present in the theca interna.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini D. F., Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974 Oct;63(1):234–250. doi: 10.1083/jcb.63.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. A., Spielman A. Permeability of the ovarian follicle of Aedes aegypti mosquitoes. J Cell Biol. 1971 Jul;50(1):201–221. doi: 10.1083/jcb.50.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLAIRS R. THE RELATIONSHIP BETWEEN OOCYTE AND FOLLICLE IN THE HEN'S OVARY AS SHOWN BY ELECTRON MICROSCOPY. J Embryol Exp Morphol. 1965 Apr;13:215–233. [PubMed] [Google Scholar]
- Bast R. E., Telfer W. H. Follicle cell protein synthesis and its contribution to the yolk of the Cecropia moth oocyte. Dev Biol. 1976 Aug;52(1):83–97. doi: 10.1016/0012-1606(76)90009-9. [DOI] [PubMed] [Google Scholar]
- Beams H. W., Kessel R. G. Synthesis and deposition of oocyte envelopes (vitelline membrane, chorion) and the uptake of yolk in the dragonfly (Odonata:Aeschnidae). J Cell Sci. 1969 Jan;4(1):241–264. doi: 10.1242/jcs.4.1.241. [DOI] [PubMed] [Google Scholar]
- Clementi F., Palade G. E. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969 Apr;41(1):33–58. doi: 10.1083/jcb.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E. Studies of the fine structure of ovarian interstitial tissue. 2. The ultrastructure of the thecal gland of the domestic fowl. Z Zellforsch Mikrosk Anat. 1970;109(2):195–211. doi: 10.1007/BF00365241. [DOI] [PubMed] [Google Scholar]
- Dahl E. Studies of the fine structure of ovarian interstitial tissue. 3. The innervation of the thecal gland of the domestic fowl. Z Zellforsch Mikrosk Anat. 1970;109(2):212–226. doi: 10.1007/BF00365242. [DOI] [PubMed] [Google Scholar]
- Dahl E. Studies of the fine structure of ovarian interstitial tissue. 5. Effects of gonadotropins on the thecal gland of the domestic fowl. Z Zellforsch Mikrosk Anat. 1971;113(1):133–156. doi: 10.1007/BF00331206. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A. B., Evans A. J., Perry M. M., Davidson M. H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J Reprod Fertil. 1977 May;50(1):179–181. doi: 10.1530/jrf.0.0500179. [DOI] [PubMed] [Google Scholar]
- Grau C. R., Wilson B. W. Avian oogenesis and yolk deposition. Experientia. 1964 Jan 15;20(1):26–26. doi: 10.1007/BF02146023. [DOI] [PubMed] [Google Scholar]
- Greenfield M. L. The oocyte of the domestic chicken shortly after hatching, studied by electron microscopy. J Embryol Exp Morphol. 1966 Jun;15(3):297–316. [PubMed] [Google Scholar]
- Guzsal E. Histological studies on the mature and post-ovulation ovarian follicle of fowl. Acta Vet Acad Sci Hung. 1966;16(1):37–44. [PubMed] [Google Scholar]
- Huxley H. E. Muscular contraction and cell motility. Nature. 1973 Jun 22;243(5408):445–449. doi: 10.1038/243445a0. [DOI] [PubMed] [Google Scholar]
- Kanaseki T., Kadota K. The "vesicle in a basket". A morphological study of the coated vesicle isolated from the nerve endings of the guinea pig brain, with special reference to the mechanism of membrane movements. J Cell Biol. 1969 Jul;42(1):202–220. doi: 10.1083/jcb.42.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. The chemistry and structure of basement membranes. Arthritis Rheum. 1969 Aug;12(4):427–443. doi: 10.1002/art.1780120411. [DOI] [PubMed] [Google Scholar]
- Mahowald A. P. Ultrastructural observations on oogenesis in Drosophila. J Morphol. 1972 May;137(1):29–48. doi: 10.1002/jmor.1051370103. [DOI] [PubMed] [Google Scholar]
- NALBANDOV A. V., JAMES M. F. The blood-vascular system of the chicken ovary. Am J Anat. 1949 Nov;85(3):347-77, incl 5 pl. doi: 10.1002/aja.1000850302. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Urakawan N., Iwata M. An electron microscopic study on 203 Hg transport in the ovarian tissue of laying Japanese quail. Nihon Juigaku Zasshi. 1976 Apr;38(2):83–92. doi: 10.1292/jvms1939.38.83. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D. A three-dimensional reconstruction of the polygonal pattern on placental coated-vesicle membranes. J Cell Sci. 1976 Jun;21(1):83–91. doi: 10.1242/jcs.21.1.83. [DOI] [PubMed] [Google Scholar]
- PRESS N. AN UNUSUAL ORGANELLE IN AVIAN OVARIES. J Ultrastruct Res. 1964 Jun;10:528–546. doi: 10.1016/s0022-5320(64)80027-7. [DOI] [PubMed] [Google Scholar]
- Paulson J., Rosenberg M. D. The function and transposition of lining bodies in developing avian oocytes. J Ultrastruct Res. 1972 Jul;40(1):25–43. doi: 10.1016/s0022-5320(72)80020-0. [DOI] [PubMed] [Google Scholar]
- Perdue J. F. The distribution, ultrastructure, and chemistry of microfilaments in cultured chick embryo fibroblasts. J Cell Biol. 1973 Aug;58(2):265–283. doi: 10.1083/jcb.58.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH T. F., PORTER K. R. YOLK PROTEIN UPTAKE IN THE OOCYTE OF THE MOSQUITO AEDES AEGYPTI. L. J Cell Biol. 1964 Feb;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol. 1973 Jul;58(1):189–211. doi: 10.1083/jcb.58.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell B., Solomon S. E. The ultrastructure of the follicle wall of the domestic fowl during the phase of rapid growth. Br Poult Sci. 1977 Sep;18(5):605–610. doi: 10.1080/00071667708416409. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjeide O. A., Galey F., Grellert E. A., I-San Lin R., De Vellis J., Mead J. F. Macromolecules in oocyte maturation. Biol Reprod. 1970 Jun;2(Suppl):14–43. doi: 10.1095/biolreprod2.supplement_2.14. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. Serum proteins and the livetins of hen's-egg yolk. Biochem J. 1962 May;83:346–355. doi: 10.1042/bj0830346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Dumont J. N. The induced synthesis and transport of yolk proteins and their accumulation by the oocyte in Xenopus laevis. J Cell Physiol. 1968 Oct;72(2 Suppl):73–89. doi: 10.1002/jcp.1040720407. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Ho T., Salter D. W., Jared D. W. Protein incorporation by isolated amphibian oocytes. IV. The role of follicle cells and calcium during protein uptake. Exp Cell Res. 1973 Dec;82(2):287–295. doi: 10.1016/0014-4827(73)90343-1. [DOI] [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- Wyburn G. M., Aitken R. N., Johnston H. S. The ultrastructure of the zona radiata of the ovarian follicle of the domestic fowl. J Anat. 1965 Jul;99(Pt 3):469–484. [PMC free article] [PubMed] [Google Scholar]
- Wyburn G. M., Johnston H. S., Aitken R. N. Fate of the granulosa cells in the hen's follicle. Z Zellforsch Mikrosk Anat. 1966;72(1):53–65. doi: 10.1007/BF00336897. [DOI] [PubMed] [Google Scholar]
- Wyburn G. M., Johnston H. S., Aitken R. N. Specialised plasma membranes in the preovulatory follicle of the fowl. Z Zellforsch Mikrosk Anat. 1965 Sep 24;68(1):70–79. doi: 10.1007/BF00332346. [DOI] [PubMed] [Google Scholar]