Abstract
Significant amounts of proteinase activity have been found in chondroitin ABC lyase (EC 4.2.2.4), chondroitin AC II lyase and endo-beta-D-galactosidase (keratanase) from commercial sources. It would appear, therefore, that certain earlier biochemical and histochemical studies, which employed these commercial enzyme preparations for their presumed ability to degrade only glycosaminoglycans, may require re-evaluation. A mixture of EDTA, N-ethylmaleimide, phenylmethanesulphonyl fluoride and pepstatin abolishes the effect of the contaminating proteinases on proteoglycan with less significant effect on the chondroitin lyase or keratanase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi T., Kunimoto S., Morishima H., Takeuchi T., Umezawa H. Effect of pepstatin on acid proteases. J Antibiot (Tokyo) 1971 Oct;24(10):687–694. doi: 10.7164/antibiotics.24.687. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Okada S. Action of chondroitinases. I. The mode of action of two chondroitinase-AC preparations of different origin. J Biochem. 1976 Dec;80(6):1201–1207. doi: 10.1093/oxfordjournals.jbchem.a131390. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Okada S. Crystallization and some properties of chondroitinase from Arthrobacter aurescens. J Biol Chem. 1975 Mar 10;250(5):1824–1828. [PubMed] [Google Scholar]
- Kimata K., Oike Y., Ito K., Karasawa K., Suzuki S. The occurrence of low buoyant density proteoglycans in embryonic chick cartilage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1431–1439. doi: 10.1016/0006-291x(78)91163-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Suzuki N., Suzuki S. Sequential degradation of keratan sulfate by bacterial enzymes and purification of a sulfatase in the enzymatic system. J Biol Chem. 1975 Feb 10;250(3):905–911. [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
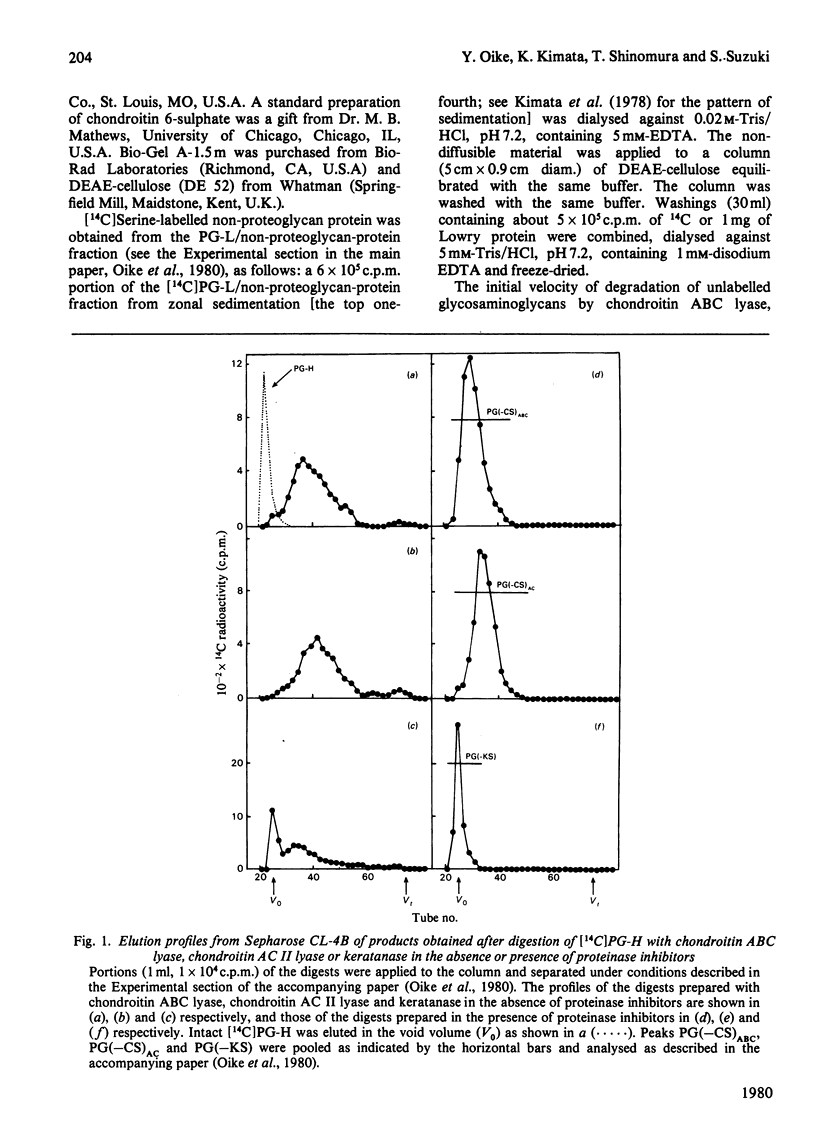
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Quintarelli G., Vocaturo A., Rodén L., Bellocci M., Vassallo L. M. Role of hyaluronic acid in the in vivo aggregation of cartilage proteoglycans. Connect Tissue Res. 1978;5(4):237–248. doi: 10.3109/03008207809152278. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


