Abstract
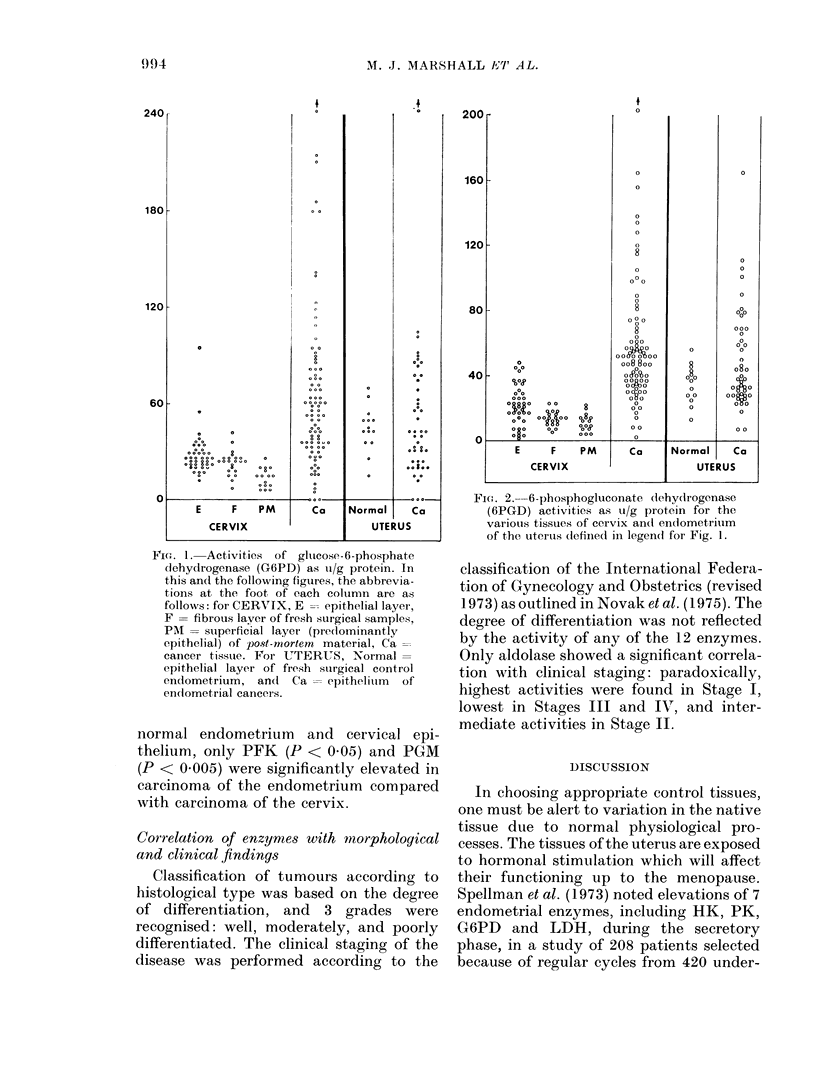
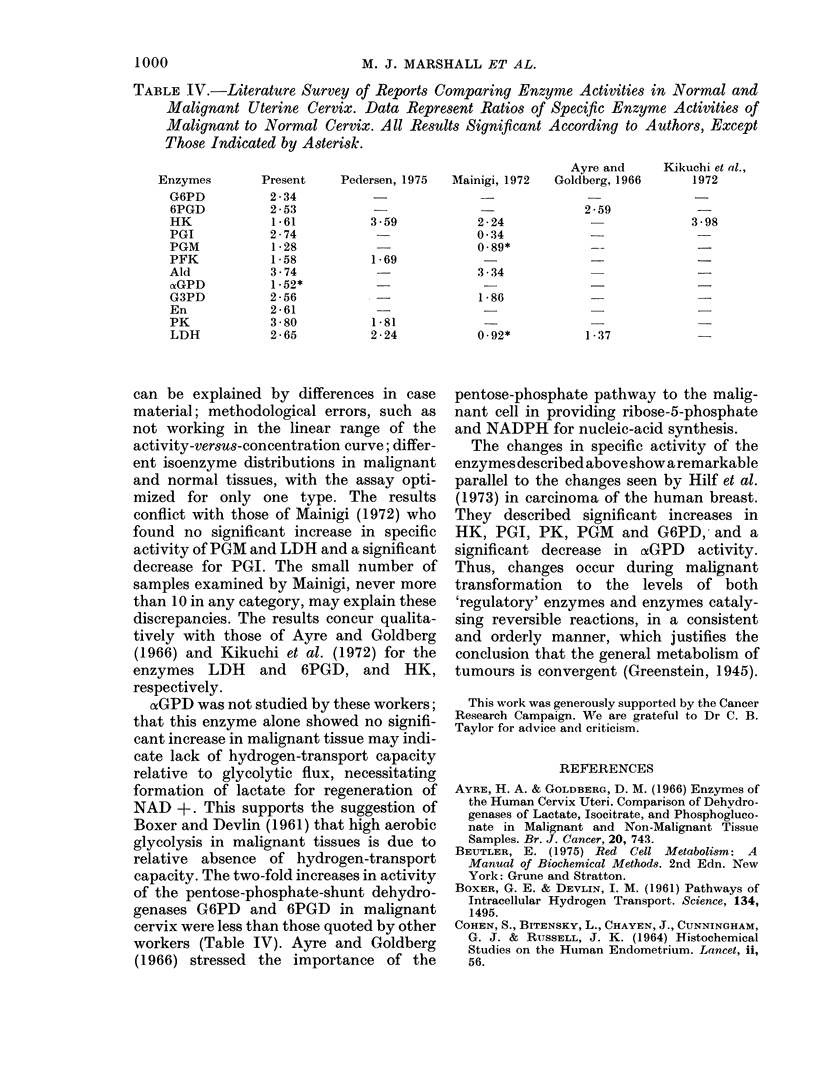
Twelve enzymes related to the direct oxidative and glycolytic pathways of glucose metabolism were assayed in 88 cancers of the cervix and 48 cancers of the endometrium of the human uterus, and the activities compared with those obtained from a group of control tissues. Significant increases for all but one of the enzymes studied (alpha-glycerolphosphate dehydrogenase) were found in cancer of the cervix, when compared with normal cervix epithelium. Hexokinase, phoshofructokinase, and aldolase appear to be rate-limiting in normal cervix epithelium; however, since the increase in activity of the first two in cancers was least of all the glycolytic enzymes, redundant enzyme synthesis probably occurs in the malignant cell for the enzymes catalysing reversible reactions. There was virtually no correlation between the activity of any enzyme measured in the cancer sample and histological assessments of the degree of malignancy of the tumour, or the clinical stage of the disease. All enzymes except pyruvate kinase had significantly higher activity in normal endometrium than in normal cervix epithelium, presumably reflecting the greater metabolic requirements of the former tissue. Only phosphoglucose isomerase and pyruvate kinase were significantly higher in endometrial cancer than in normal endometrium, and there were few significant differences between cancers of the cervix and of the endometrium, despite the marked differences in their tissues of origin. These results suggest the changes occur during malignant transformation to the activities of both regulatory enzymes and those catalysing reversible reactions, in a manner justifying the conclusion that the general metabolism of tumours is convergent.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayre H. A., Goldberg D. M. Enzymes of the human cervix uteri. Comparison of dehydrogenases of lactate, isocitrate, and phosphogluconate in malignant and non-malignant tissue samples. Br J Cancer. 1966 Dec;20(4):743–750. doi: 10.1038/bjc.1966.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., BITENSKY L., CHAYEN J., CUNNINGHAM G. J., RUSSELL J. K. HISTOCHEMICAL STUDIES ON THE HUMAN ENDOMETRIUM. Lancet. 1964 Jul 11;2(7350):56–58. doi: 10.1016/s0140-6736(64)90064-9. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss W. E. A review of isozymes in cancer. Cancer Res. 1971 Nov;31(11):1523–1542. [PubMed] [Google Scholar]
- Fottrell P. F., Spellman C. M., O'Dwyer E. Lactate dehydrogenase isoenzymes in human endometrium. Clin Chim Acta. 1969 Dec;26(3):584–585. doi: 10.1016/0009-8981(69)90093-x. [DOI] [PubMed] [Google Scholar]
- Hilf R., Wittliff J. L., Rector W. D., Savlov E. D., Hall T. C., Orlando R. A. Studies on certain cytoplasmic enzymes and specific estrogen receptors in human breast cancer and in nonmalignant diseases of the breast. Cancer Res. 1973 Sep;33(9):2054–2062. [PubMed] [Google Scholar]
- Howard B. V., Morris H. P., Bailey J. M. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972 Jul;32(7):1533–1538. [PubMed] [Google Scholar]
- Kikuchi Y., Sato S., Sugimura T. Hexokinase isozyme patterns of human uterine tumors. Cancer. 1972 Aug;30(2):444–447. doi: 10.1002/1097-0142(197208)30:2<444::aid-cncr2820300222>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langvad E., Pedersen S. N. LHD-isoenzyme patterns in the nonmalignant and malignant uterine cervix. Cancer. 1969 May;23(5):1171–1175. doi: 10.1002/1097-0142(196905)23:5<1171::aid-cncr2820230524>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Mainigi K. D. Activities of certain enzymes of glycolytic pathway in normal, chronic cervicitis and malignant human cervix uteri. Oncology. 1972;26(5):427–437. doi: 10.1159/000224695. [DOI] [PubMed] [Google Scholar]
- Pedersen S. N. Respiration and glycolysis in malignant and non-malignant tissue from the human cervix uteri. Acta Obstet Gynecol Scand. 1968;47(4):469–481. doi: 10.3109/00016346809157500. [DOI] [PubMed] [Google Scholar]
- Pedersen S. N. The glycolytic enzyme activity of the human cervix uteri. Cancer. 1975 Feb;35(2):469–474. doi: 10.1002/1097-0142(197502)35:2<469::aid-cncr2820350226>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- ROSA C. G. Oxidative enzymes in human vaginal smears. Observations of the succinic dehydrogenase and diphosphopyridine nucleotide-diaphorase systems. Obstet Gynecol. 1960 Sep;16:354–359. [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- Singhal R. L., Lafreniere R. Induction of uterine phosphofructokinase by cyclic 3',5'-adenosine monophosphate. Endocrinology. 1970 Nov;87(5):1099–1104. doi: 10.1210/endo-87-5-1099. [DOI] [PubMed] [Google Scholar]
- Spellman C. M., Fottrell P. F., Baynes S., O'Dwyer E. M., Clinch J. D. A study of some enzymes and isoenzymes of carbohydrate metabolism in human endometrium during the menstrual cycle. Clin Chim Acta. 1973 Oct 30;48(3):259–268. doi: 10.1016/0009-8981(73)90194-0. [DOI] [PubMed] [Google Scholar]
- Yochim J. M., Pepe G. J. Effect of ovarian steroids on nucleic acids, protein, and glucose-6-phosphate dehydrogenase activity in endometrium of the rat; a metabolic role for progesterone in "progestational differentiation". Biol Reprod. 1971 Oct;5(2):172–182. doi: 10.1093/biolreprod/5.2.172. [DOI] [PubMed] [Google Scholar]


