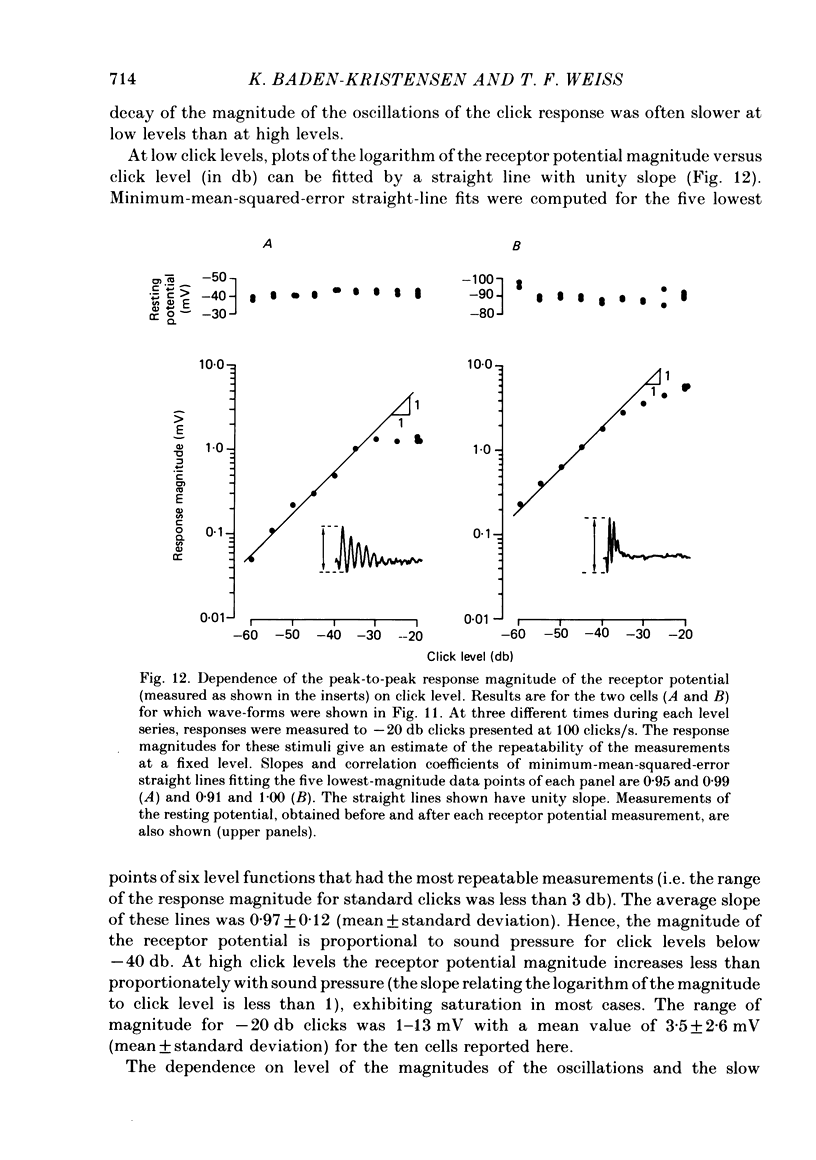
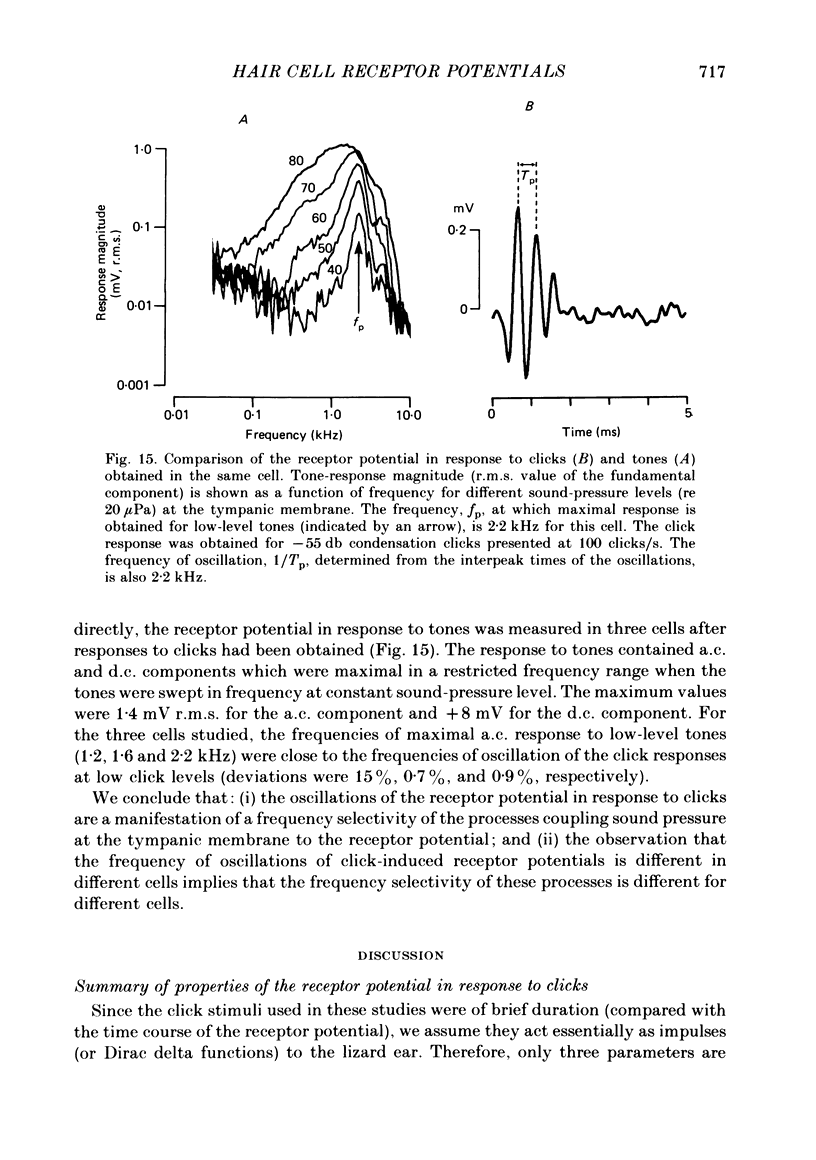
Abstract
Receptor potentials of single hair cells in the free-standing region of the basilar papilla of the anaesthetized alligator lizard were measured intracellularly with micropipettes. Stimuli were primarily acoustic pulses (clicks) delivered to the tympanic membrane. The receptor potential was independent of click repetition rate for the range 10-150 clicks/s. This property is presumed to be the basis of the rate independence of the extracellular cochlear microphonic potential. The receptor potential wave-form consisted of a fast oscillatory component (or oscillation) superimposed on a usually positive (depolarizing) slow component. Reversal of the stimulus polarity resulted in a reversal of the polarity of the oscillations; the polarity of the slow component remained unchanged. The relative magnitudes of the two components depended on click level. At the higher click levels the magnitudes of the slow and oscillatory components were comparable. The relation of the receptor potential to the stimulus was non-linear; the peak-to-peak magnitude of the receptor potential increased less than proportionately with increasing sound-pressure level, and reversal of the stimulus polarity did not result in a reversal of the receptor potential. The receptor-potential magnitude for high-level clicks ranged from 1-13 mV peak-to-peak with an average value of 3.5 mV. At the lower click levels the magnitude of the slow component was much smaller than that of the oscillatory component. The relation of the receptor potential to the acoustic stimulus approached that of a linear system, the magnitude of the receptor potential became approximately proportional to the sound-pressure level, and reversal of the stimulus polarity resulted in approximate reversal of the receptor potential. For low-level stimuli the frequency of the oscillations of the receptor potential in response to clicks was approximately equal to the frequency of maximal a.c. response to tones. Apparently, both phenomena reflect the frequency selectivity of the processes generating the receptor potential. The frequency of oscillations in the click response varied from one cell to another (range of 1.0-2.2 kHz in this study). The results are qualitatively consistent with a model (Weiss, Mulroy & Altmann, 1974) that contains a linear, band-pass filter followed by a rectifier followed by a low-pass filter.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeRosier D. J., Tilney L. G., Egelman E. Actin in the inner ear: the remarkable structure of the stereocilium. Nature. 1980 Sep 25;287(5780):291–296. doi: 10.1038/287291a0. [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Crawford A. C. The coding of sound pressure and frequency in cochlear hair cells of the terrapin. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):209–218. doi: 10.1098/rspb.1978.0101. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Matsuura S. Adaptive rundown of excitatory post-synaptic potentials at synapses between hair cells and eight nerve fibres in the goldfish. J Physiol. 1978 Mar;276:193–209. doi: 10.1113/jphysiol.1978.sp012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. G., Frishkopf L. S., Flock A. Receptor potentials from hair cells of the lateral line. Science. 1970 Jan 2;167(3914):76–79. doi: 10.1126/science.167.3914.76. [DOI] [PubMed] [Google Scholar]
- Holton T., Weiss T. F. Two-tone rate suppression in lizard cochlear nerve fibers, relation to receptor organ morphology. Brain Res. 1978 Dec 22;159(1):219–222. doi: 10.1016/0006-8993(78)90123-3. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIANG N. Y., PEAKE W. Components of electrical responses recorded from the cochlea. Ann Otol Rhinol Laryngol. 1960 Jun;69:448–458. doi: 10.1177/000348946006900213. [DOI] [PubMed] [Google Scholar]
- Miller M. R. Scanning electron microscope studies of some lizard basilar papillae. Am J Anat. 1973 Nov;138(3):301–329. doi: 10.1002/aja.1001380303. [DOI] [PubMed] [Google Scholar]
- Mulroy M. J., Altmann D. W., Weiss T. F., Peake W. T. Intracellular electric responses to sound in a vertebrate cochlea. Nature. 1974 May 31;249(456):482–485. doi: 10.1038/249482a0. [DOI] [PubMed] [Google Scholar]
- Mulroy M. J. Cochlear anatomy of the alligator lizard. Brain Behav Evol. 1974;10(1-3):69–87. doi: 10.1159/000124303. [DOI] [PubMed] [Google Scholar]
- Nadol J. B., Jr, Mulroy M. J., Goodenough D. A., Weiss T. F. Tight and gap junctions in a vertebrate inner ear. Am J Anat. 1976 Nov;147(3):281–301. doi: 10.1002/aja.1001470304. [DOI] [PubMed] [Google Scholar]
- Peake W. T., Ling A., Jr Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Am. 1980 May;67(5):1736–1745. doi: 10.1121/1.384300. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Tuning properties of cochlear hair cells. Nature. 1977 Jun 30;267(5614):858–860. doi: 10.1038/267858a0. [DOI] [PubMed] [Google Scholar]
- Sohmer H. S., Peake W. T., Weiss T. F. Intracochlear potential recorded with micropipets. I. Correlations with micropipet location. J Acoust Soc Am. 1971 Aug;50(2):572–586. doi: 10.1121/1.1912674. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Derosier D. J., Mulroy M. J. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol. 1980 Jul;86(1):244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vér I. L., Brown R. M., Kiang N. Y. Low-noise chambers for auditory research. J Acoust Soc Am. 1975 Aug;58(2):392–398. doi: 10.1121/1.380682. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Altmann D. W., Mulroy M. J. Endolymphatic and intracellular resting potential in the alligator lizard cochlea. Pflugers Arch. 1978 Jan 31;373(1):77–84. doi: 10.1007/BF00581152. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Mulroy M. J., Altmann D. W. Intracellular responses to acoustic clicks in the inner ear of the alligator lizard. J Acoust Soc Am. 1974 Mar;55(3):606–619. doi: 10.1121/1.1914571. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Mulroy M. J., Turner R. G., Pike C. L. Tuning of single fibers in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res. 1976 Oct 8;115(1):71–90. doi: 10.1016/0006-8993(76)90823-4. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Peake W. T. Cochlear potential response at the round-window membrane of the cat--a reply to the comment of G. R. Price. J Acoust Soc Am. 1972 Dec;52(6):1729–1734. doi: 10.1121/1.1913308. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Peake W. T., Sohmer H. S. Intracochlear potential recorded with micropipets. II. Responses in the cochlear scalae to tones. J Acoust Soc Am. 1971 Aug;50(2):587–601. doi: 10.1121/1.1912675. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Searle C. L., Baden-Kristensen K. Proportionality of intracellular resistance changes and receptor potentials in hair-cell models. Hear Res. 1981 Jul;4(3-4):243–250. doi: 10.1016/0378-5955(81)90009-5. [DOI] [PubMed] [Google Scholar]


