Abstract
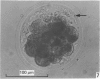
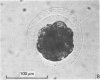
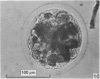
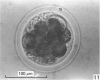
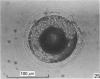
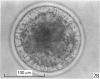
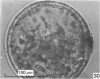
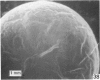
Forty nine embryos, twenty unfertilized eggs and five other fresh eggs of 'doubtful' status have been recovered from 58 pony mares in 122 flushes up to 22 days after ovulation. The fresh egg or embryo recovery rate was 78% with surgical methods (or at slaughter) and 40-60% with non-surgical methods of recovery. The fertilization rate was about 70%. It has been confirmed that horse embryos normally enter the uterus as blastocysts 5-6 days after ovulation. Three features of early embryo morphology have become clearer upon comparison with unfertilized eggs of similar ages; early embryos are often ellipsoidal in shape; dispersal of most of a thick gel coat seems to be hastened by fertilization; gradual disappearance of refractile granules from the perivitelline space is similar in fertilized and unfertilized eggs. A tense, transparent, acellular capsule (considered to be different from the zona pellucida) is acquired by the spherical blastocysts within the uterus and persists at least until a diameter of 34 mm is attained (at 21 days in the present series). The capsule seems to be analogous, in part, with the 'neozona' described in rabbit blastocyst before attachment, and trophoblastic cells appear to be involved in its formation. Cleavage stages of oviductal embryos and diameters of uterine blastocysts from this series have been described and illustrated and used to extend previous knowledge of early growth patterns in horse embryos.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. E. The reproductive status of female mink, Mustela vision, recorded as 'failed to mate'. J Reprod Fertil. 1973 Jun;33(3):527–529. doi: 10.1530/jrf.0.0330527. [DOI] [PubMed] [Google Scholar]
- Allen W. R., Stewart F., Trounson A. O., Tischner M., Bielański W. Viability of horse embryos after storage and long-distance transport in the rabbit. J Reprod Fertil. 1976 Jul;47(2):387–390. doi: 10.1530/jrf.0.0470387. [DOI] [PubMed] [Google Scholar]
- Betteridge K. J., Eaglesome M. D., Flood P. F. Embryo transport through the mare's oviduct depends upon cleavage and is independent of the ipsilateral corpus luteum. J Reprod Fertil Suppl. 1979;(27):387–394. [PubMed] [Google Scholar]
- Betteridge K. J., Mitchell D. A surgical technique applied to the study of tubal eggs in the mare. J Reprod Fertil Suppl. 1975 Oct;(23):519–524. [PubMed] [Google Scholar]
- Betteridge K. J., Mitchell D. Direct evidence of retention of unfertilized ova in the oviduct of the mare. J Reprod Fertil. 1974 Jul;39(1):145–148. doi: 10.1530/jrf.0.0390145. [DOI] [PubMed] [Google Scholar]
- Butterfield R. M., Matthews R. G. Ovulation and the movement of the conceptus in the first 35 days of pregnancy in thoroughbred mares. J Reprod Fertil Suppl. 1979;(27):447–452. [PubMed] [Google Scholar]
- Denker H. W., Gerdes H. J. The dynamic structure of rabbit blastocyst coverings. I. Transformation during regular preimplantation development. Anat Embryol (Berl) 1979;157(1):15–34. doi: 10.1007/BF00315639. [DOI] [PubMed] [Google Scholar]
- Gygax A. P., Ganjam V. K., Kenney R. M. Clinical, microbiological and histological changes associated with uterine involution in the mare. J Reprod Fertil Suppl. 1979;(27):571–578. [PubMed] [Google Scholar]
- Hamilton W. J. Cleavage stages of the ova of the horse, with notes on ovulation. J Anat. 1945 Jul;79(Pt 3):127–130.3. [PMC free article] [PubMed] [Google Scholar]
- Hughes J. P., Loy R. G. Artificial insemination in the equine. A comparison of natural breeding and artificial insemination of mares using semen from six stallions. Cornell Vet. 1970 Jul;60(3):463–475. [PubMed] [Google Scholar]
- Marrable A. W., Flood P. F. Embryological studies on the dartmoor pony during the first third of gestation. J Reprod Fertil Suppl. 1975 Oct;(23):499–502. [PubMed] [Google Scholar]
- Oguri N., Tsutsumi Y. Non-surgical egg transfer in mares. J Reprod Fertil. 1974 Dec;41(2):313–320. doi: 10.1530/jrf.0.0410313. [DOI] [PubMed] [Google Scholar]
- Oguri N., Tsutsumi Y. Non-surgical recovery of equine eggs, and an attempt at non-surgical egg transfer in horses. J Reprod Fertil. 1972 Nov;31(2):187–195. doi: 10.1530/jrf.0.0310187. [DOI] [PubMed] [Google Scholar]
- Steffenhagen W. P., Pineda M. H., Ginther O. J. Retention of unfertilized ova in uterine tubes of mares. Am J Vet Res. 1972 Dec;33(12):2391–2398. [PubMed] [Google Scholar]
- Webel S. K., Franklin V., Harland B., Dziuk P. J. Fertility, ovulation and maturation of eggs in mares injected with HCG. J Reprod Fertil. 1977 Nov;51(2):337–341. doi: 10.1530/jrf.0.0510337. [DOI] [PubMed] [Google Scholar]
- van Niekerk C. H., Allen W. R. Early embryonic development in the horse. J Reprod Fertil Suppl. 1975 Oct;(23):495–498. [PubMed] [Google Scholar]
- van Niekerk C. H., Gerneke W. H. Persistence and parthenogentic cleavage of tubal ova in the mare. Onderstepoort J Vet Res. 1966 Jun;33(1):195–232. [PubMed] [Google Scholar]