Abstract
1. Neurotransmitter-receptors in the membrane of Xenopus oocytes have been studied using electrophysiological techniques. Neurotransmitters and related agents were applied while recording either membrane potential or membrane current. The majority of ovarian oocytes used were at stages IV and V.
2. Three types of oocytes were examined: inner ovarian epithelium covered (e.c.) oocytes; epithelium manually removed (e.r.) oocytes; and collagenase treated (c.t.) ooctyes.
3. Ovarian oocytes are sensitive to some cholinergic and catecholaminergic agents. Responses to serotonin were seldom observed and when present were much weaker than responses to other agents. No responses were observed to the amino acids: aspartate, glutamate, γ-aminobutyric acid, and glycine; or to octopamine and histamine.
4. Acetylcholine (ACh) usually depolarized the membrane, in a dose-dependent manner, with threshold concentrations as low as 10-9 m. The ACh-potential was due to an increase in Cl permeability and had a reversal potential around — 19 mV. The intracellular Cl ion activity, measured with a Cl-ion sensitive micro-electrode, was about 65 mm and the estimated Cl-ion equilibrium potential, ECl, agreed with the reversal potential of the ACh-potential.
5. Curare (10-4 m), tetrodotoxin (10-6 m), or α-bungarotoxin (10-6 g/ml.) did not block the response to 10-6 m-ACh; whereas atropine (10-7 m) blocked it. No response to nicotinic agents (e.g. nicotine, 1,1-dimethyl-4-phenylpiperazinium) was observed. These results suggest that the ACh receptors in the oocyte membrane are muscarinic in nature.
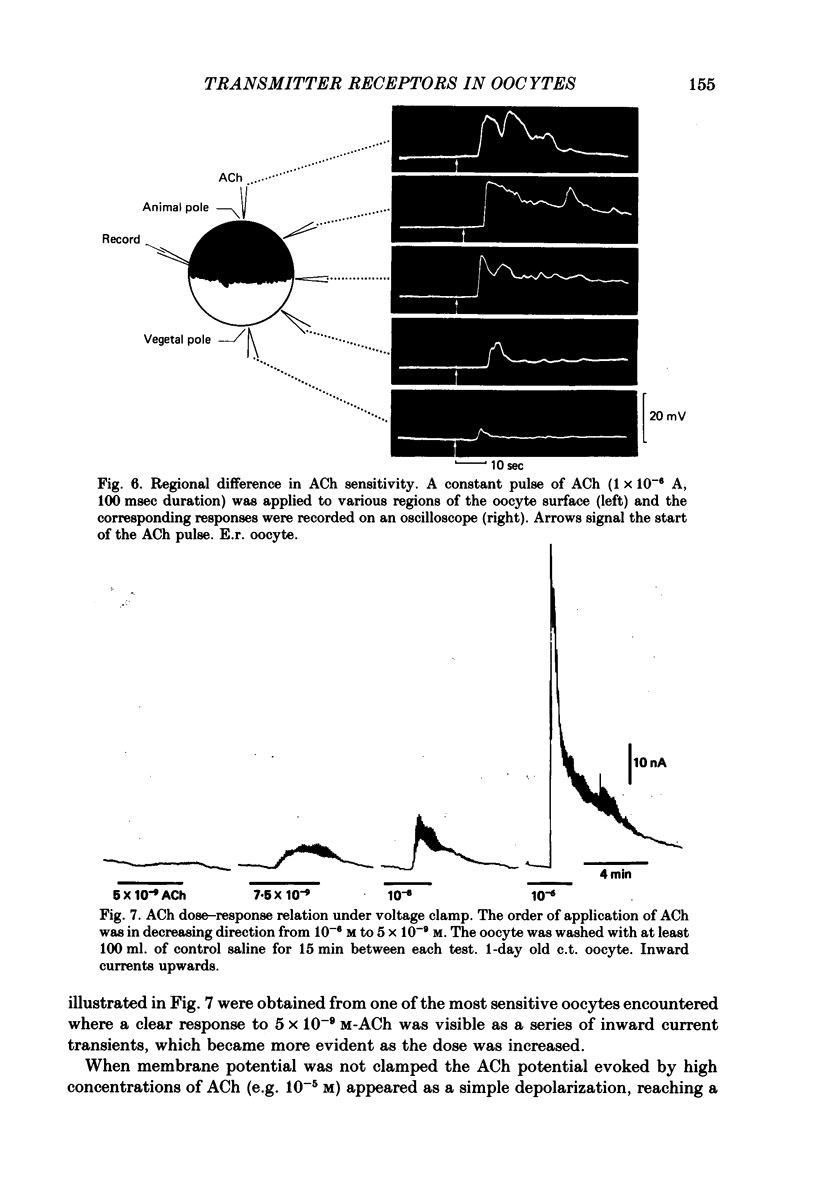
6. The apparent latency of the ACh potential, examined by ionophoretic application of ACh to e.r. oocytes and c.t. oocytes, ranged from 0·5 sec to over 20 sec. Intracellular injection of ACh was without effect.
7. Responses to catecholamines were observed mostly in e.c. oocytes; while in e.r. and c.t. oocytes they were rare and of very small amplitudes.
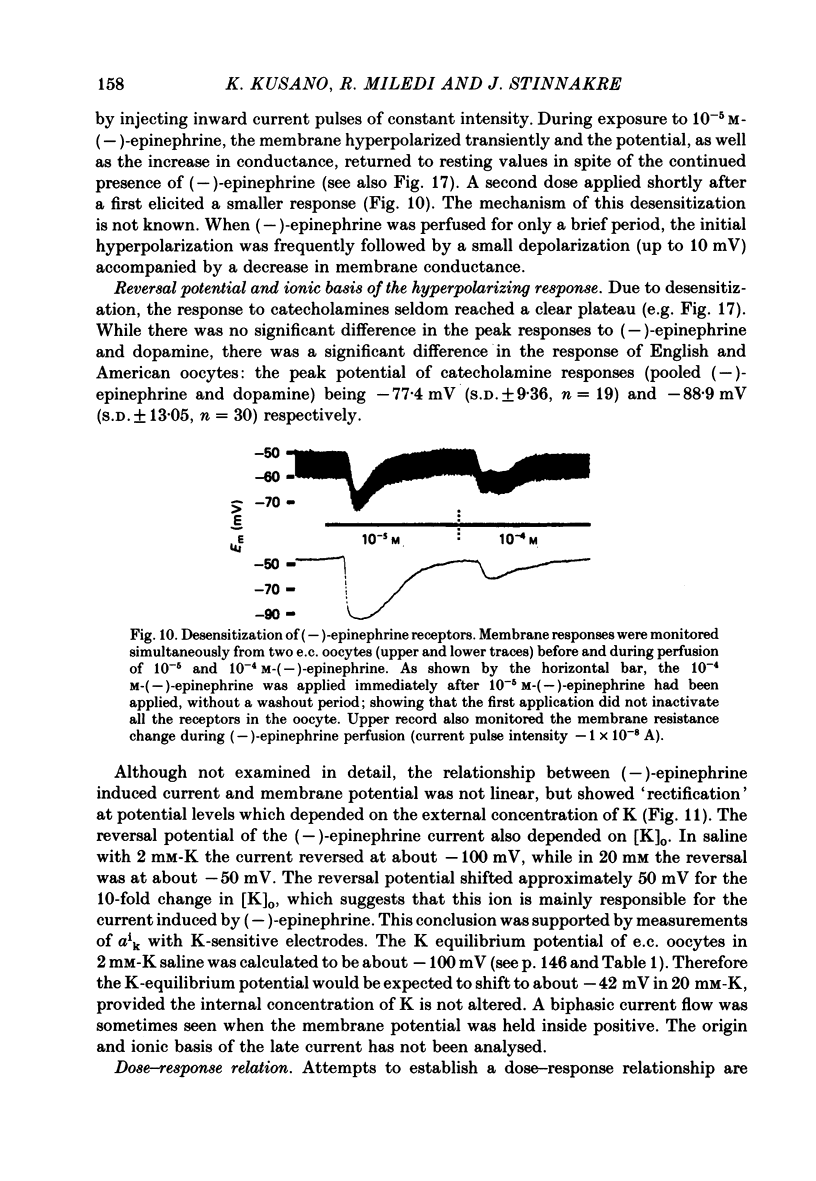
8. The usual response to both dopamine and (—)-epinephrine was a transient hyperpolarization manifested by an initial increase in K-permeability followed by a decrease. The latency of these responses ranged from 10 sec to over 30 sec and their reversal potential was nearly — 100 mV, which coincided with EK.
9. Oocytes responded to the β-adrenergic receptor agonist, isoproterenol, as well as (—)-epinephrine. Pre-treatment with the β-adrenergic receptor blocker, propranolol, abolished the response to both (—)-epinephrine and (—)-isoproterenol. The dopamine potential was also reduced considerably. Both the α-adrenergic receptor agonist, phenylephrine, and the α-adrenergic receptor blocker, phentolamine, were without effect.
10. Maturation of the oocytes, induced in vivo by gonadotropin or in vitro by progesterone, led to loss of responsiveness to both cholinergic and catecholaminergic agents.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Bennett D. Analogies between embryonic (T/t) antigens and adult major histocompatibility (H-2) antigens. Nature. 1975 Aug 14;256(5518):545–547. doi: 10.1038/256545a0. [DOI] [PubMed] [Google Scholar]
- Artzt K., Hamburger L., Jakob H., Jacob F. Embryonic surface antigens: a "quasi-endodermal" teratoma antigen. Dev Biol. 1976 Jul 1;51(1):152–157. doi: 10.1016/0012-1606(76)90130-5. [DOI] [PubMed] [Google Scholar]
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- BERGERS A. C., LI C. H. Amphibian ovulation in vitro induced by mammalian pituitary hormones and progesterone. Endocrinology. 1960 Feb;66:255–259. doi: 10.1210/endo-66-2-255. [DOI] [PubMed] [Google Scholar]
- Bishop M. R., Sastry B. V., Stavinoha W. B. Identification of acetylcholine and propionylcholine in bull spermatozoa by integrated pyrolysis, gas chromatography and mass spectrometry. Biochim Biophys Acta. 1977 Dec 22;500(2):440–444. doi: 10.1016/0304-4165(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the latency and form of the membrane responses of smooth muscle to the iontophoretic application of acetylcholine or carbachol. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):99–119. doi: 10.1098/rspb.1976.0068. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Brown H. M. Intracellular Na+, K+, and C1- activities in Balanus photoreceptors. J Gen Physiol. 1976 Sep;68(3):281–296. doi: 10.1085/jgp.68.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C. L., Wiley H. S., Dumont J. N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979 Jan 12;203(4376):182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- Buznikov G. A., Chudakova I. V., Berdysheva L. V., Vyazmina N. M. The role of neurohumors in early embryogenesis. II. Acetylcholine and catecholamine content in developing embryos of sea urchin. J Embryol Exp Morphol. 1968 Aug;20(1):119–128. [PubMed] [Google Scholar]
- Cornett L. E., Meizel S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4954–4958. doi: 10.1073/pnas.75.10.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Mangia F., Alfei L. Acetylcholine-elicited responses in primary and secondary mammalian oocytes disappear after fertilisation. Nature. 1979 Feb 22;277(5698):651–653. doi: 10.1038/277651a0. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Smith I., Purves R. D. Synaptic delay in the heart: an ionophoretic study. J Physiol. 1978 Jun;279:31–54. doi: 10.1113/jphysiol.1978.sp012329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Rogawski M. A., Miller R. J. Comparison of the effects of neuroleptic drugs on pre- and postsynaptic dopaminergic mechanisms in the rat striatum. Mol Pharmacol. 1976 Mar;12(2):251–262. [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Leonov B. V., Budantsev A. Iu. Obnaruzhenie serotonina v blastotsistakh krys metodom mikrospektrofluorimetrii. Dokl Akad Nauk SSSR. 1971 May 21;198(3):734–736. [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A. Water and electrolyte changes in amphibian eggs at ovulation. Exp Cell Res. 1965 Dec;40(3):664–667. doi: 10.1016/0014-4827(65)90245-4. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Watson D. E. Transmembrane electropotential changes in amphibian eggs at ovulation, activation and first cleavage. J Cell Physiol. 1966 Feb;67(1):85–92. doi: 10.1002/jcp.1040670110. [DOI] [PubMed] [Google Scholar]
- Nelson L. alpha-bungarotoxin binding by cell membranes. Blockage of sperm cell motility. Exp Cell Res. 1976 Sep;101(2):221–224. doi: 10.1016/0014-4827(76)90371-2. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Page S. Analysis of catecholamine effects in single atrial trabeculae of the frog heart. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):333–362. doi: 10.1098/rspb.1977.0074. [DOI] [PubMed] [Google Scholar]
- Robbins N., Molenaar P. C. Investigation of possible cholinergic mechanisms in fertilization of Xenopus eggs. Proc R Soc Lond B Biol Sci. 1981 Sep 17;213(1190):59–72. doi: 10.1098/rspb.1981.0053. [DOI] [PubMed] [Google Scholar]
- Robinson K. R. Electrical currents through full-grown and maturing Xenopus oocytes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):837–841. doi: 10.1073/pnas.76.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Steinhardt R. A. Maturation of Xenopus oocytes. II. Observations on membrane potential. Dev Biol. 1977 Jun;57(2):305–316. doi: 10.1016/0012-1606(77)90217-2. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., Buwalda R. J., Habets A. M. Intracellular ionic distribution, cell membrane permeability and membrane potential of the Xenopus egg during first cleavage. Exp Cell Res. 1974 Nov;89(1):1–14. doi: 10.1016/0014-4827(74)90180-3. [DOI] [PubMed] [Google Scholar]


