Abstract
1. Several methods for evaluating light-evoked response latency and its variability in brisk-sustained (X) and brisk-transient (Y) retinal ganglion cells were tested. The most accurate procedure proved to be that described by Levick (1973), in which the time of the occurrence of the fourth impulse after stimulus onset is taken as an estimate of the latency.
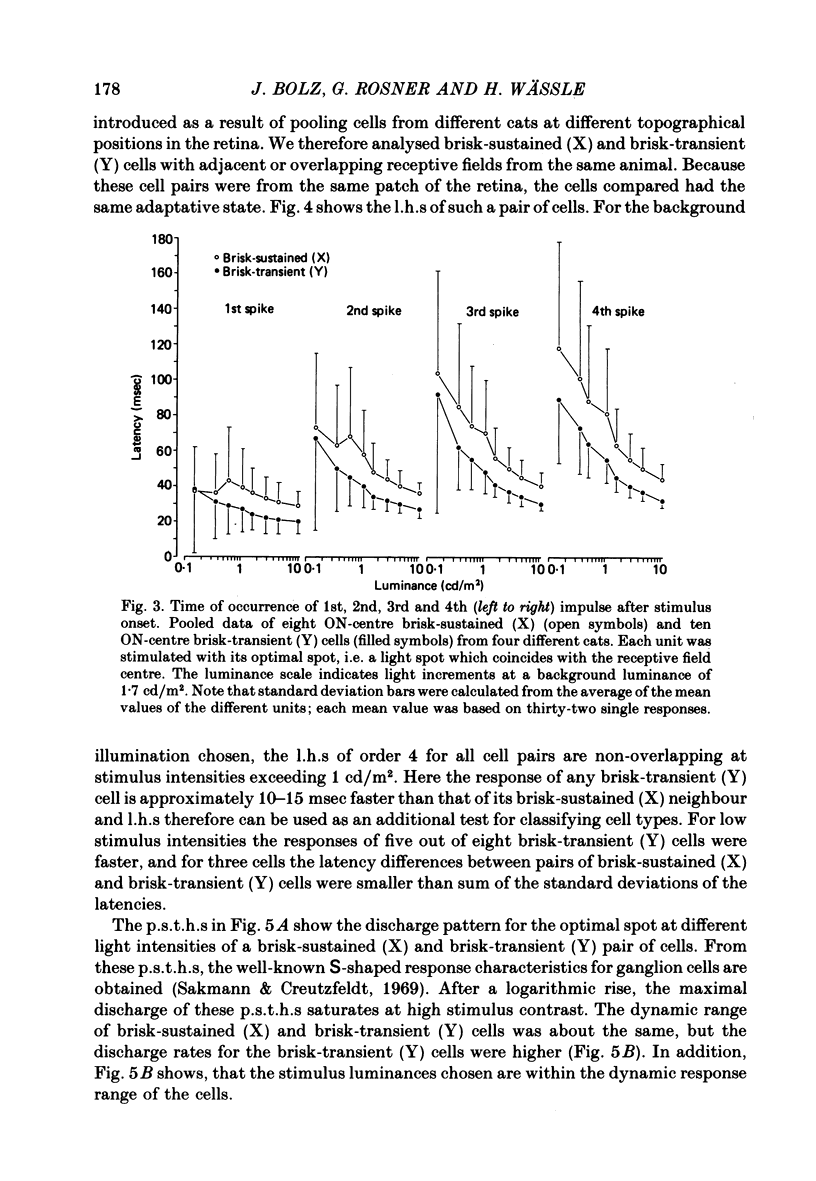
2. The shortest response latencies are obtained when the stimuli are the same size as the receptive field centre. At medium and high response amplitudes (> 150 impulses/sec) the response of brisk-transient (Y) cells to these optimal stimuli is 10-15 msec faster than that of adjacent brisk-sustained (X) cells.
3. The response latency of brisk-sustained (X) cells for stimuli larger than the receptive field centre increases, whereas that of brisk-transient (Y) cells remains constant. Brisk-sustained (X) cells respond faster than do brisk-transient (Y) cells to stimuli smaller than the receptive field centre.
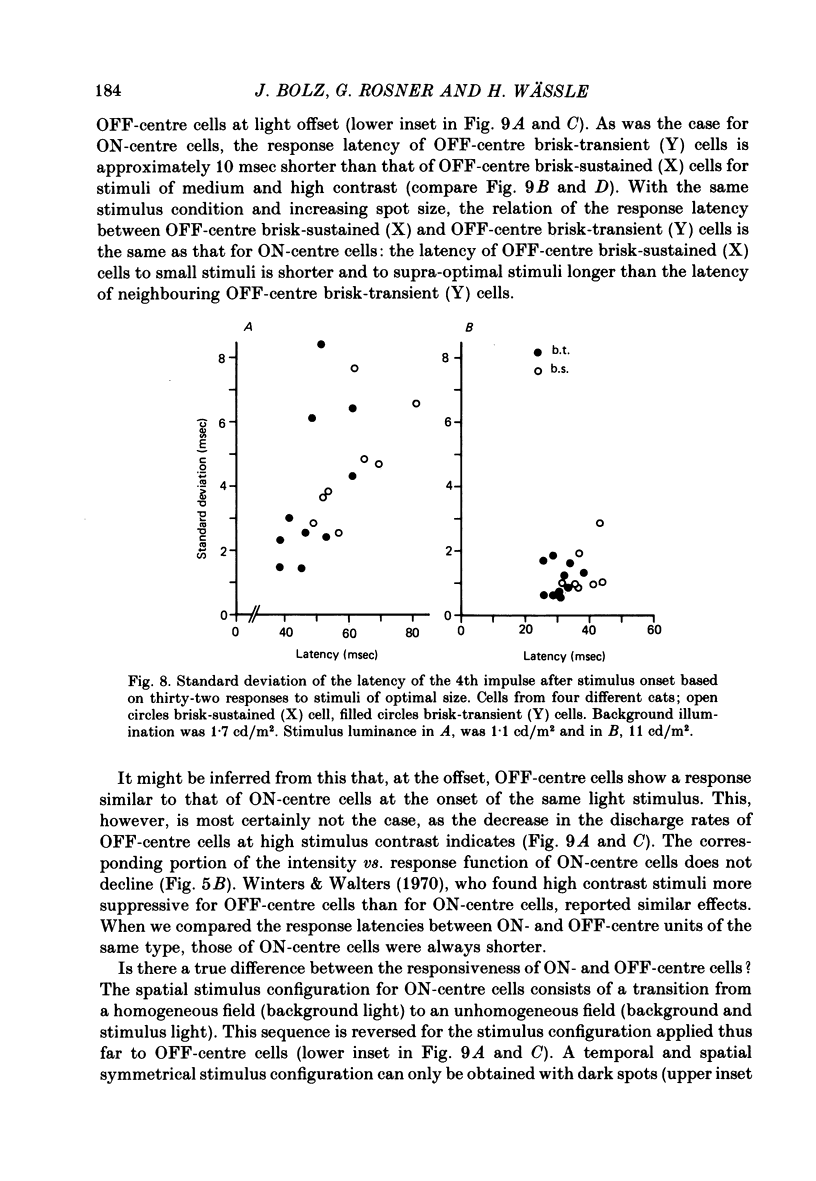
4. No systematic difference exists between brisk-sustained (X) and brisk-transient (Y) cells in regard to the temporal variability of the response. The standard deviation of the latency for stimuli of optimal size decreases from 2·0-8·0 msec at medium stimulus contrast to 0·6-2·0 msec at high stimulus contrast.
5. The response of OFF-centre cells to the disappearance of a light spot is always slower than that of an ON-centre cell of the same class to the onset of this stimulus. However, when OFF-centre cells are stimulated with dark spots, their response latency does not differ from that of ON-centre cells of the same class.
6. No simple relationship exists between the response latency and the response amplitude. At medium and high discharge rates, most brisk-transient (Y) cells respond faster than an adjacent brisk-sustained (X) cell with equal response. At the same response amplitude, the latencies become shorter as the background illumination is raised. The same discharge rate can be obtained with stimuli of sub-optimal and supra-optimal size, but the latency for the larger stimulus is shorter than that for the smaller one. Latency, therefore, is an additional parameter characterizing the light-evoked response.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B. Reconstructing the visual image in space and time. Nature. 1979 May 17;279(5710):189–190. doi: 10.1038/279189a0. [DOI] [PubMed] [Google Scholar]
- Barlow H. B. The Ferrier Lecture, 1980. Critical limiting factors in the design of the eye and visual cortex. Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):1–34. doi: 10.1098/rspb.1981.0022. [DOI] [PubMed] [Google Scholar]
- Breitmeyer B. G. Metacontrast with black and white stimuli: evidence for inhibition of on- and off-sustained activity by either on- or off-transient activity. Vision Res. 1978;18(10):1443–1448. doi: 10.1016/0042-6989(78)90241-9. [DOI] [PubMed] [Google Scholar]
- Breitmeyer B. G. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vision Res. 1975 Dec;15(12):1411–1412. doi: 10.1016/0042-6989(75)90200-x. [DOI] [PubMed] [Google Scholar]
- Burr D. C. Acuity for apparent vernier offset. Vision Res. 1979;19(7):835–837. doi: 10.1016/0042-6989(79)90162-7. [DOI] [PubMed] [Google Scholar]
- Burr D. C., Ross J. How does binocular delay give information about depth? Vision Res. 1979;19(5):523–532. doi: 10.1016/0042-6989(79)90137-8. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Morstyn R., Wagner H. G. Lateral geniculate relay of slowly conducting retinal afferents to cat visual cortex. J Physiol. 1976 Feb;255(1):299–320. doi: 10.1113/jphysiol.1976.sp011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Sakmann B., Scheich H., Korn A. Sensitivity distribution and spatial summation within receptive-field center of retinal on-center ganglion cells and transfer function of the retina. J Neurophysiol. 1970 Sep;33(5):654–671. doi: 10.1152/jn.1970.33.5.654. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Goldstick T. K., Linsenmeier R. A. The contrast sensitivity of cat retinal ganglion cells at reduced oxygen tensions. J Physiol. 1980 Jul;304:59–81. doi: 10.1113/jphysiol.1980.sp013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Hammond P. Contrasts in spatial organization of receptive fields at geniculate and retinal levels: centre, surround and outer surround. J Physiol. 1973 Jan;228(1):115–137. doi: 10.1113/jphysiol.1973.sp010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Inadequacy of nitrous oxide/oxygen mixtures for maintaining anaesthesia in cats: satisfactory alternatives. Pain. 1978 Aug;5(2):143–151. doi: 10.1016/0304-3959(78)90036-2. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Receptive field organization of 'sustained' and 'transient' retinal ganglion cells which subserve different function roles. J Physiol. 1972 Dec;227(3):769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. The outer disinhibitory surround of the retinal ganglion cell receptive field. J Physiol. 1972 Oct;226(2):511–544. doi: 10.1113/jphysiol.1972.sp009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith P. E., Kulikowski J. J. Pattern and flicker detection analysed by subthreshold summation. J Physiol. 1975 Aug;249(3):519–548. doi: 10.1113/jphysiol.1975.sp011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. L., Cleland B. G., Levick W. R. Axonal conduction latencies of cat retinal ganglion cells. J Neurophysiol. 1975 Nov;38(6):1395–1402. doi: 10.1152/jn.1975.38.6.1395. [DOI] [PubMed] [Google Scholar]
- Lennie P. Perceptual signs of parallel pathways. Philos Trans R Soc Lond B Biol Sci. 1980 Jul 8;290(1038):23–37. doi: 10.1098/rstb.1980.0080. [DOI] [PubMed] [Google Scholar]
- Lennie P. The physiological basis of variations in visual latency. Vision Res. 1981;21(6):815–824. doi: 10.1016/0042-6989(81)90180-2. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Variation in the response latency of cat retinal ganglion cells. Vision Res. 1973 Apr;13(4):837–853. doi: 10.1016/0042-6989(73)90047-3. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Zacks J. L. Responses of cat retinal ganglion cells to brief flashes of light. J Physiol. 1970 Mar;206(3):677–700. doi: 10.1113/jphysiol.1970.sp009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp U., Hauske G., Wolf W. Perceptual latencies to sinusoidal gratings. Vision Res. 1976;16(9):969–972. doi: 10.1016/0042-6989(76)90228-5. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- Morgan M. Stereoillusion based on visual persistence. Nature. 1975 Aug 21;256(5519):639–640. doi: 10.1038/256639a0. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979 Jun;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Ross J. Stereopsis by binocular delay. Nature. 1974 Mar 22;248(446):363–364. doi: 10.1038/248363a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffman K. P. Conduction velocity as a parameter in the organisation of the afferent relay in the cat's lateral geniculate nucleus. Brain Res. 1971 Sep 24;32(2):454–459. doi: 10.1016/0006-8993(71)90339-8. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J. Separate channels for the analysis of the shape and the movement of moving visual stimulus. J Physiol. 1973 Jun;231(3):385–402. doi: 10.1113/jphysiol.1973.sp010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A., Mitov D. Perception time and spatial frequency. Vision Res. 1976 Jan;16(1):89–82. doi: 10.1016/0042-6989(76)90081-x. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G., McKee S. P. Perception of temporal order in adjacent visual stimuli. Vision Res. 1977;17(8):887–892. doi: 10.1016/0042-6989(77)90062-1. [DOI] [PubMed] [Google Scholar]
- Winters R. W., Hamasaki D. I. Temporal characteristics of peripheral inhibition of sustained and transient ganglion cells in cat retina. Vision Res. 1976 Jan;16(1):37–45. doi: 10.1016/0042-6989(76)90074-2. [DOI] [PubMed] [Google Scholar]
- Winters R. W., Walters J. W. Transient and steady state stimulus-response relations for cat retinal ganglion cells. Vision Res. 1970 Jun;10(6):461–477. doi: 10.1016/0042-6989(70)90003-9. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]



