Abstract
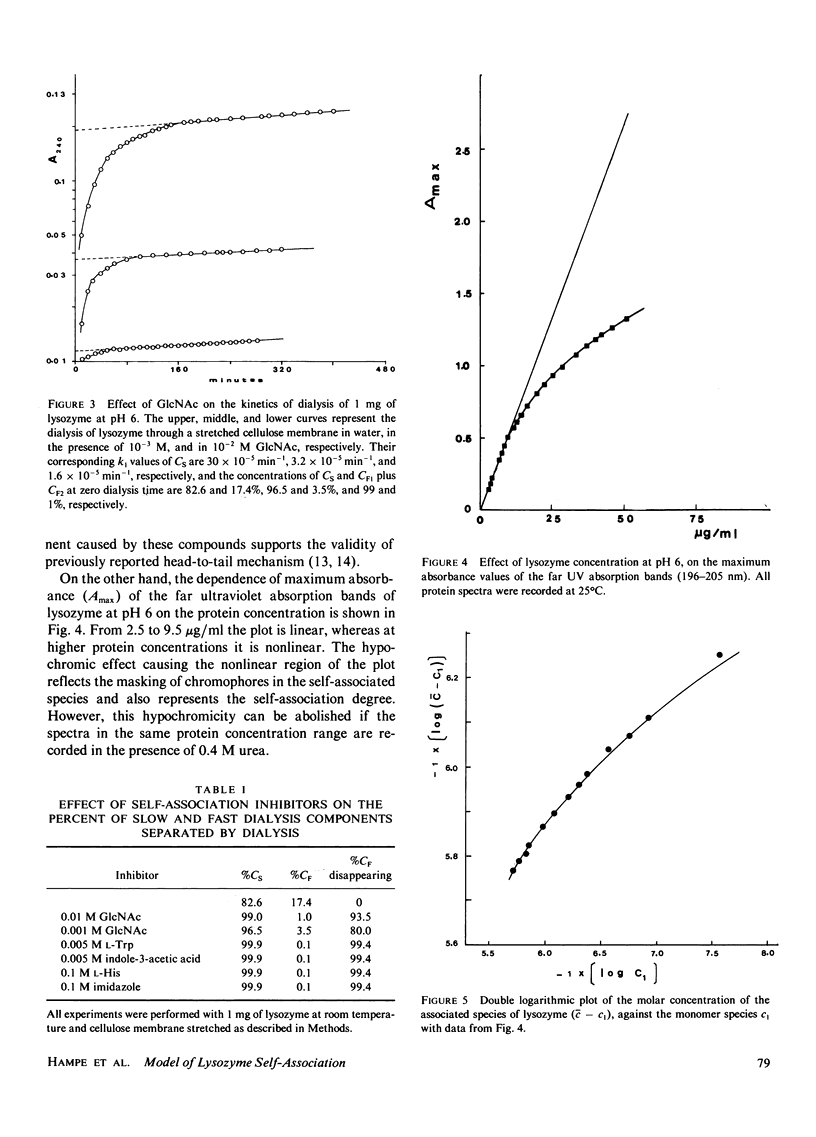
The concentration dependence of the self-association of hen egg-white lysozyme was studied spectrophotometrically at pH 6, 25 degrees C, and low ionic strength within a concentration range of 2.5-50 micrograms/ml. Of several possible mathematical models, an ideal or nearly ideal two-stage model representing an equilibrium between monomers and dimers and between dimers and trimers best describes the data. The dimerization and trimerization constants were found to be 2.5 x 10(-2) and 38 x 10(-2). Dialysis experiments confirmed that the mechanism involves three associating species. A "head-to-tail" contact between the associating sites was inferred from dialysis studies of the effect of indole and imidazole derivatives on lysozyme self-association.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. T., Jr, Filmer D. L. Sedimentation equilibrium in reacting systems. IV. Verification of the theory. Biochemistry. 1966 Sep;5(9):2971–2985. doi: 10.1021/bi00873a029. [DOI] [PubMed] [Google Scholar]
- Banerjee S. K., Pogolotti A., Jr, Rupley J. A. Self-association of lysozyme. Thermochemical measurements: effect of chemical modification of Trp-62, Trp-108, and Glu-35. J Biol Chem. 1975 Oct 25;250(20):8260–8266. [PubMed] [Google Scholar]
- Charlemagne D., Jollès P. Inhibition par des polymères de la Nacétylglucosamine de l'activité lysante de lysozymes vis-à-vis de Micrococcus luteus: influence du pH et de la force uonique. C R Acad Sci Hebd Seances Acad Sci D. 1974 Jul 15;279(3):299–302. [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Williams J. W. Self-association of muramidase (lysozyme) in solution at 25 degrees, pH 7.0, and I = 0.20. Biochemistry. 1970 Oct 27;9(22):4260–4267. doi: 10.1021/bi00824a004. [DOI] [PubMed] [Google Scholar]
- Derechin M. Analysis of associating systems using the multinomial theory. Biochemistry. 1971 Dec 21;10(26):4981–4986. doi: 10.1021/bi00802a023. [DOI] [PubMed] [Google Scholar]
- Ferreira A. T., Hampe O. G., Paiva A. C. The conformation of angiotensin II in aqueous solution. II. Dialysis and gel filtration behavior of [Asn 1-Val 5]-angiotensin II. Biochemistry. 1969 Aug;8(8):3483–3487. doi: 10.1021/bi00836a052. [DOI] [PubMed] [Google Scholar]
- Goldman R., Kedem O., Katchalski E. Kinetic behavior of alkaline phosphatase--collodion membranes. Biochemistry. 1971 Jan 5;10(1):165–172. doi: 10.1021/bi00777a024. [DOI] [PubMed] [Google Scholar]
- Goldman R., Kedem O., Silman I. H., Caplan S. R., Katchalski E. Papain-collodion membranes. I. Preparation and properties. Biochemistry. 1968 Feb;7(2):486–500. [PubMed] [Google Scholar]
- Goldman R., Lenhoff H. M. Glucose-6-phosphate dehydrogenase adsorbed on collodion membranes. Biochim Biophys Acta. 1971 Aug 20;242(2):514–518. doi: 10.1016/0005-2744(71)90246-4. [DOI] [PubMed] [Google Scholar]
- HAMAGUCHI K., KURONO A. STRUCTURE OF MURAMIDASE (LYSOZYME). I. THE EFFECT OF GUANIDINE HYDROCHLORIDE ON MURAMIDASE. J Biochem. 1963 Aug;54:111–122. [PubMed] [Google Scholar]
- Hampe O. G. Conformation of lysozyme in aqueous solution. Effect of ionic strength and protein concentration. Eur J Biochem. 1972 Nov 21;31(1):32–37. doi: 10.1111/j.1432-1033.1972.tb02496.x. [DOI] [PubMed] [Google Scholar]
- Mouton A., Jolles J. On the identity of human lysozymes isolated from normal and abnormal tissues or secretions. FEBS Lett. 1969 Aug;4(4):337–340. doi: 10.1016/0014-5793(69)80270-x. [DOI] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VAN HOLDE K. E. Evidence for dimerization of lysozyme in alkaline solution. J Biol Chem. 1961 Dec;236:PC82–PC83. [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VANHOLDE K. E. PHYSICAL STUDIES OF MURAMIDASE (LYSOZYME). II. PH-DEPENDENT DIMERIZATION. J Biol Chem. 1964 Aug;239:2516–2524. [PubMed] [Google Scholar]
- Shindo H., Cohen J. S., Rupley J. A. Self-association of hen egg-white lysozyme as studied by nuclear magnetic resonance. Biochemistry. 1977 Aug 23;16(17):3879–3882. doi: 10.1021/bi00636a025. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos A. J. Association sites of lysozyme in solution. I. The active site. J Biol Chem. 1969 Jun 25;244(12):3188–3193. [PubMed] [Google Scholar]
- Studebaker J. F., Sykes B. D., Wien R. A nuclear magnetic resonance study of lysozyme inhibition. Effects of dimerization and pH on saccharide binding. J Am Chem Soc. 1971 Sep 8;93(18):4579–4585. doi: 10.1021/ja00747a040. [DOI] [PubMed] [Google Scholar]
- Swan I. D. The inhibition of hen egg-white lysozyme by imidazole and indole derivatives. J Mol Biol. 1972 Mar 14;65(1):59–62. doi: 10.1016/0022-2836(72)90491-3. [DOI] [PubMed] [Google Scholar]
- WILCOX F. H., Jr, DANIEL L. J. Reduced lysis at high concentrations of lysozyme. Arch Biochem Biophys. 1954 Oct;52(2):305–312. doi: 10.1016/0003-9861(54)90128-9. [DOI] [PubMed] [Google Scholar]
- Wooten J. B., Cohen J. S. Protein mobility and self-association by deuterium nuclear magnetic resonance. Biochemistry. 1979 Sep 18;18(19):4188–4191. doi: 10.1021/bi00586a023. [DOI] [PubMed] [Google Scholar]
- Zehavi U., Lustig A. On the reversibility of substrate-induced dissociation of lysozyme aggregates. Native and reacted lysozyme. Biochim Biophys Acta. 1971 Apr 27;236(1):127–130. doi: 10.1016/0005-2795(71)90157-7. [DOI] [PubMed] [Google Scholar]
- Zehavi U., Lustig A. Substrate-induced dissociation of lysozyme dimers. Biochim Biophys Acta. 1969 Dec 23;194(2):532–539. doi: 10.1016/0005-2795(69)90115-9. [DOI] [PubMed] [Google Scholar]


