Abstract
1. In order to investigate the mechanism of serosal pressure-induced inhibition of isosmotic fluid transport, the effect of 4·5 cm water serosal pressure on spontaneous water transfer (Jv) in rabbit gall-bladders was measured (in the presence of a supporting soft nylon net on the mucosal side) in a modified Ussing chamber. This allowed unidirectional Na+ fluxes ([Formula: see text] and [Formula: see text]), transepithelial potential difference and resistance (Rt) to be measured simultaneously. The effects of the serosal pressure were also investigated by light and electron microscopy.
2. During pressure application, Rt increased due to a covering effect of the mucosal support. The serosal pressure caused a parallel decrease in Jv and net Na+ transport ([Formula: see text]) across the free epithelial surface of 80-85%. About 85% of the decrease in [Formula: see text] was due to a decrease in [Formula: see text].
3. After inhibition of 93% of fluid absorption by serosal 10-3M-ouabain, pressure-induced change in Jv was only 8% of the spontaneous fluid transport rate.
4. Control Na+ flux ratio ([Formula: see text]) was 3·5. The pressure-induced increase in steady-state [Formula: see text] of 30-35% therefore contributed little to the decrease in [Formula: see text]. Further, this increase in [Formula: see text] was completely prevented by mucosal 10-3 M-amiloride.
5. All pressure-induced effects on transport and electrical parameters were reversible.
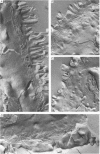
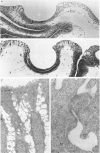
6. The light microscopical and scanning electron microscopical results showed that half of the epithelial surface was covered by the nylon net following serosal pressure application. Ruptures in the epithelium were not seen. Thin section and freeze fracture electron microscopy demonstrated continuous, well developed tight junctions both in control and experimental condition.
7. It is concluded that a serosal pressure of only 4·5 cm water causes inhibition of a cellular active Na+ and water transport with only minimal, if any, contribution from paracellular filtration. This would seem incompatible with the concept that an active ion transport mechanism localized in the basolateral cell membrane is responsible for transepithelial fluid transport. The possibility of a mechanical fluid transport mechanism via elements of a tubulo-cisternal endoplasmic reticulum is raised.
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulpaep E. L., Sackin H. Role of the paracellular pathway in isotonic fluid movement across the renal tubule. Yale J Biol Med. 1977 Mar-Apr;50(2):115–131. [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., MACINTOSH J. R. A model system for biological water transport. Nature. 1962 Jan 27;193:347–348. doi: 10.1038/193347a0. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Osmotic water flow in leaky epithelia. J Membr Biol. 1979 Dec 31;51(3-4):195–216. doi: 10.1007/BF01869084. [DOI] [PubMed] [Google Scholar]
- Frederiksen O. Functional distinction between two transport mechanisms in rabbit gall-bladder epithelium by use of ouabain, ethacrynic acid and metabolic inhibitors. J Physiol. 1978 Jul;280:373–387. doi: 10.1113/jphysiol.1978.sp012389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P. Effects of cytochalasin B and dimethylsulphoxide on isosmotic fluid transport by rabbit gall-bladder in vitro. J Physiol. 1977 Feb;265(1):103–118. doi: 10.1113/jphysiol.1977.sp011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P. Transcellular transport of isosmotic volumes by the rabbit gall-bladder in vitro. J Physiol. 1969 Mar;201(1):201–224. doi: 10.1113/jphysiol.1969.sp008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen O., Møllgård K., Rostgaard J. Lack of correlation between transepithelial transport capacity and paracellular pathway ultrastructure in Alcian blue-treated rabbit gallbladders. J Cell Biol. 1979 Nov;83(2 Pt 1):383–393. doi: 10.1083/jcb.83.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen O., Rostgaard J. Absence of dilated lateral intercellular spaces in fluid-transporting frog gallbladder epithelium. Direct microscopy observations. J Cell Biol. 1974 Jun;61(3):830–834. doi: 10.1083/jcb.61.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Dugas M. C., Schultz S. G. Sodium chloride transport by rabbit gallbladder. Direct evidence for a coupled NaCl influx process. J Gen Physiol. 1975 Jun;65(6):769–795. doi: 10.1085/jgp.65.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Rumrich G., Ullrich K. J. Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch. 1973 Oct 22;343(3):189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- HAKIM A., LESTER R. G., LIFSON N. Absorption by an in vitro preparation of dog intestinal mucosa. J Appl Physiol. 1963 Mar;18:409–413. doi: 10.1152/jappl.1963.18.2.409. [DOI] [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Hill A. E. Solute-solvent coupling in epithelia: contribution of the junctional pathway to fluid production. Proc R Soc Lond B Biol Sci. 1975 Dec 16;191(1105):537–547. doi: 10.1098/rspb.1975.0143. [DOI] [PubMed] [Google Scholar]
- Hill A. Salt-water coupling in leaky epithelia. J Membr Biol. 1980 Oct 31;56(3):177–182. doi: 10.1007/BF01869474. [DOI] [PubMed] [Google Scholar]
- Hill B. S., Hill A. E. Fluid transfer by Necturus gall bladder epithelium as a function of osmolarity. Proc R Soc Lond B Biol Sci. 1978 Feb 23;200(1139):151–162. doi: 10.1098/rspb.1978.0012. [DOI] [PubMed] [Google Scholar]
- Leyssac P. P., Bukhave K., Frederiksen O. Inhibitory effect of prostaglandins on isosmotic fluid transport by rabbit gall-bladder in vitro, and its modification by blocade of endogenous PGE-Biosynthesis with indomethacin. Acta Physiol Scand. 1974 Dec;92(4):496–507. doi: 10.1111/j.1748-1716.1974.tb05771.x. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B., Boulpaep E. L. Hydrostatic pressure changes related to paracellular shunt ultrastructure in proximal tubule. Kidney Int. 1980 Jun;17(6):732–748. doi: 10.1038/ki.1980.86. [DOI] [PubMed] [Google Scholar]
- Munck B. G., Rasmussen S. N. Paracellular permeability of extracellular space markers across rat jejunum in vitro. Indication of a transepithelial fluid circuit. J Physiol. 1977 Oct;271(2):473–488. doi: 10.1113/jphysiol.1977.sp012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møllgård K., Rostgaard J. Morphologic aspects of transepithelial transport with special reference to the endoplasmic reticulum. Soc Gen Physiol Ser. 1981;36:209–231. [PubMed] [Google Scholar]
- Møllgård K., Rostgaard J. Morphological aspects of some sodium transporting epithelia suggesting a transcellular pathway via elements of endoplasmic reticulum. J Membr Biol. 1978;40(Spec No):71–89. doi: 10.1007/BF02025999. [DOI] [PubMed] [Google Scholar]
- Onstad G. R., Schoenfield L. J., Higgins J. A. Fluid transfer in the everted human gallbladder. J Clin Invest. 1967 Apr;46(4):606–614. doi: 10.1172/JCI105562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Os C. H., Michels J. A., Slegers J. F. Effects of electrical gradients on volume flows across gall bladder epithelium. Biochim Biophys Acta. 1976 Sep 7;443(3):545–555. doi: 10.1016/0005-2736(76)90472-7. [DOI] [PubMed] [Google Scholar]
- Rostgaard J., Frederiksen O. Fluid transport and dimensions of epithelial cells and intercellular spaces in frog gallbladder. Studies in the living state, and during processing for electron microscopy. Cell Tissue Res. 1981;215(2):223–247. doi: 10.1007/BF00239111. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. The role of paracellular pathways in isotonic fluid transport. Yale J Biol Med. 1977 Mar-Apr;50(2):99–113. [PMC free article] [PubMed] [Google Scholar]
- Spring K. R., Hope A. Fluid transport and the dimensions of cells and interspaces of living Necturus gallbladder. J Gen Physiol. 1979 Mar;73(3):287–305. doi: 10.1085/jgp.73.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring K. R., Hope A. Size and shape of the lateral intercellular spaces in a living epithelium. Science. 1978 Apr 7;200(4337):54–58. doi: 10.1126/science.635571. [DOI] [PubMed] [Google Scholar]
- Spring K. R. Optical techniques for the evaluation of epithelial transport processes. Am J Physiol. 1979 Sep;237(3):F167–F174. doi: 10.1152/ajprenal.1979.237.3.F167. [DOI] [PubMed] [Google Scholar]
- Tormey J. M., Diamond J. M. The ultrastructural route of fluid transport in rabbit gall bladder. J Gen Physiol. 1967 Sep;50(8):2031–2060. doi: 10.1085/jgp.50.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITLOCK R. T., WHEELER H. O. COUPLED TRANSPORT OF SOLUTE AND WATER ACROSS RABBIT GALLBLADDER EPITHELIUM. J Clin Invest. 1964 Dec;43:2249–2265. doi: 10.1172/JCI105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H. A modified method for study of intestinal absorption in vitro. J Appl Physiol. 1956 Jul;9(1):137–140. doi: 10.1152/jappl.1956.9.1.137. [DOI] [PubMed] [Google Scholar]
- Whittembury G., de Martínez C. V., Linares H., Paz-Aliaga A. Solvent drag of large solutes indicates paracellular water flow in leaky epithelia. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):63–81. doi: 10.1098/rspb.1980.0158. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. Correlation between (Na + -K + )-activated ATPase activities and the rate of isotonic fluid transport of gallbladder epithelium. Biochim Biophys Acta. 1971 Jul 6;241(1):89–96. doi: 10.1016/0005-2736(71)90306-3. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Wiedner G., Wright E. M. Volume flows across gallbladder epithelium induced by small hydrostatic and osmotic gradients. J Membr Biol. 1979 Aug;49(1):1–20. doi: 10.1007/BF01871037. [DOI] [PubMed] [Google Scholar]