Abstract
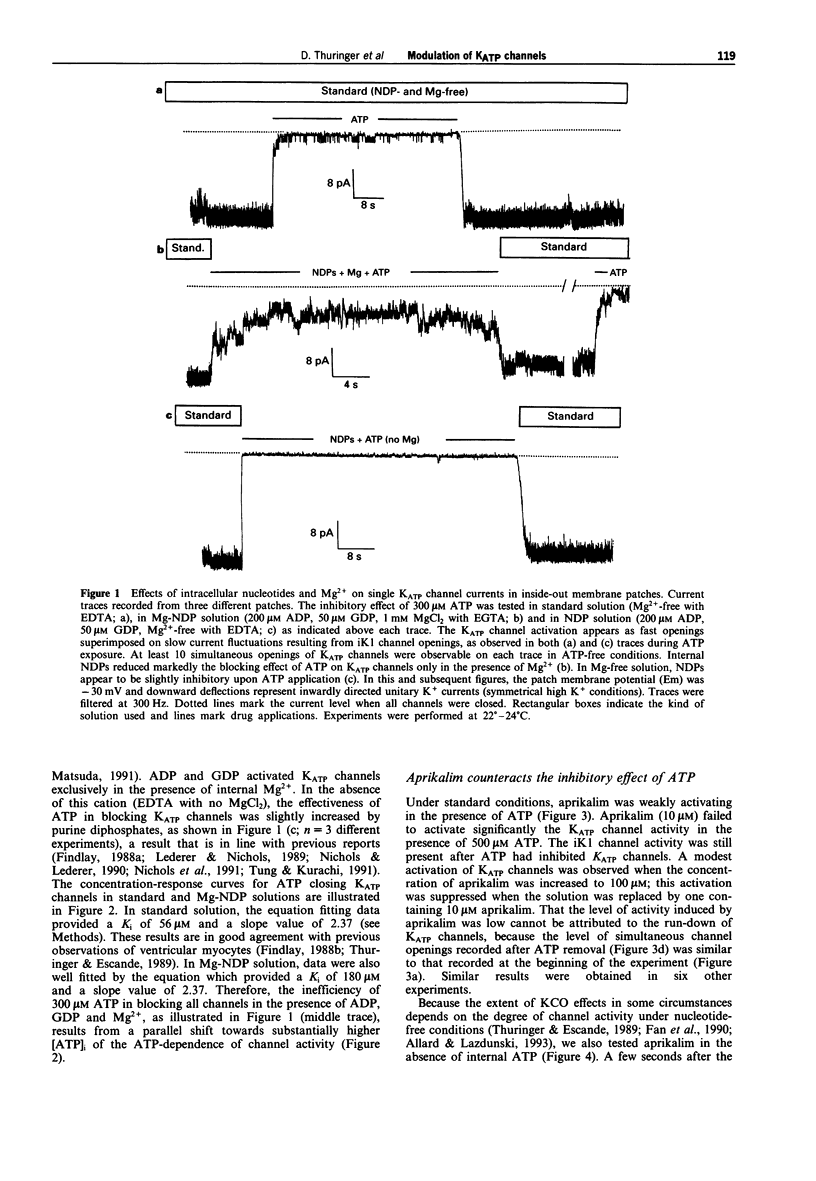
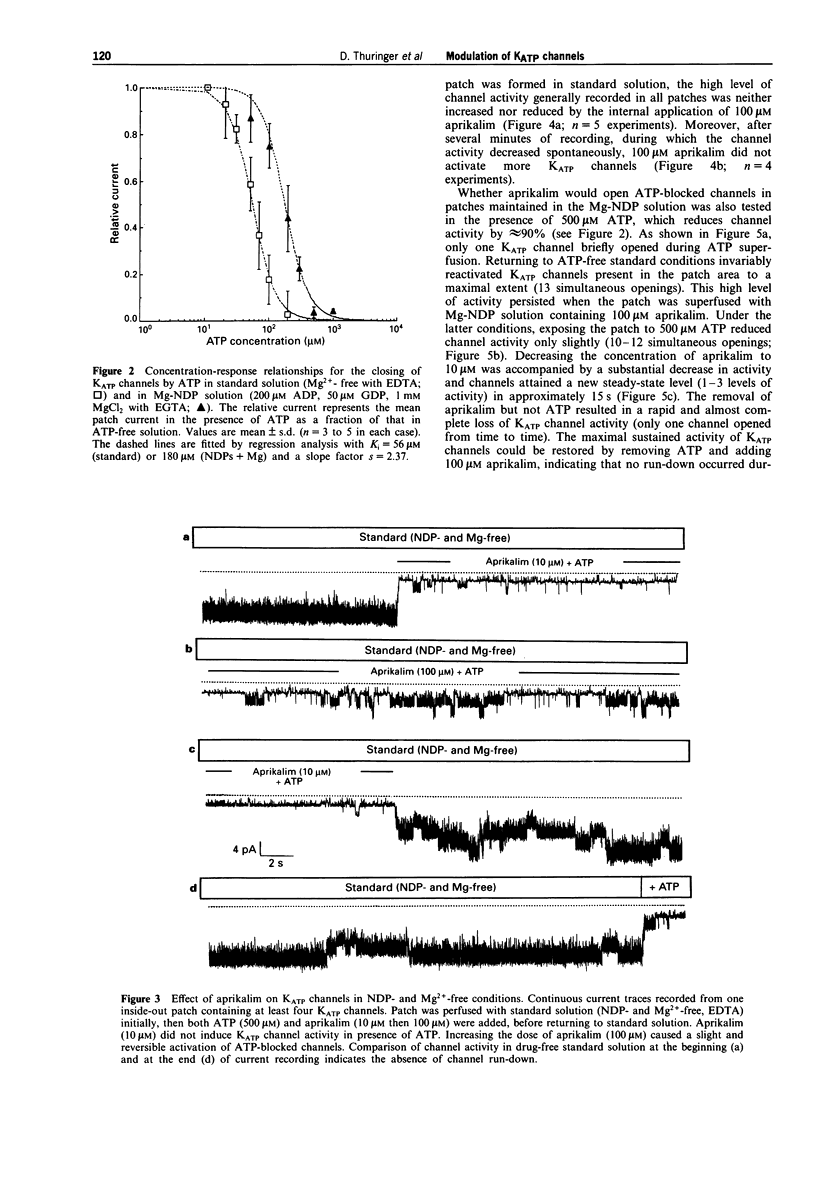
1. The effects of the potassium channel opener (KCO) aprikalim (RP 52891) on the nucleotide-induced modulation of ATP-sensitive K+ (KATP) channels in freshly dissociated ventricular myocytes of guinea-pig heart, were studied by use of the inside-out patch-clamp technique. The internal surface of the excised membrane patch was initially bathed with a standard solution (Mg(2+)-free with EDTA), then sequentially superfused with solutions containing nucleoside diphosphates (NDPs: 200 microM ADP and 50 microM GDP) and NDPs plus 1 mM MgCl2 (with EGTA; referred to as Mg-NDP solution). 2. The normalized concentration-response (channel closing) relationship to ATP was shifted to the right when the standard solution was replaced by the Mg-NDP solution. Hence, the internal concentration of ATP ([ATP]i) inhibiting the channel activity by half (Ki) increased from 56 microM to 180 microM, with an apparently constant slope factor (s = 2.37). NDPs in the absence of Mg2+ did not decrease the sensitivity of the channels to ATP. 3. In standard solution, aprikalim (100 microM) activated KATP channels in the presence of a maximally inhibitory [ATP]i (500 microM). This effect was strongly enhanced when aprikalim was applied to patches exposed to Mg-NDP solution, as demonstrated by the 9 fold increase in Ki for [ATP]i (from 180 microM to 1.5 mM and s = 2.37). 4. The ability of aprikalim to overcome the channel closing effects of ATP in Mg-NDP solution waned rapidly. Similarly, the NDP-induced activation of ATP-blocked channels was also time-dependent. Both activation processes disappeared before the channel run-down phenomenon appeared in ATP-free conditions. 5. In conclusion, aprikalim is much more potent in opening KATP channels in membrane patches bathed in Mg-NDP solution than in standard solution. However, under the former experimental conditions, the effect of aprikalim waned rapidly. It is proposed that the waning phenomenon results from changes in the intrinsic enzymatic activity of the KATP channel protein (possibly linked to the experimental conditions) which lead to the channel closure.
Full text
PDF

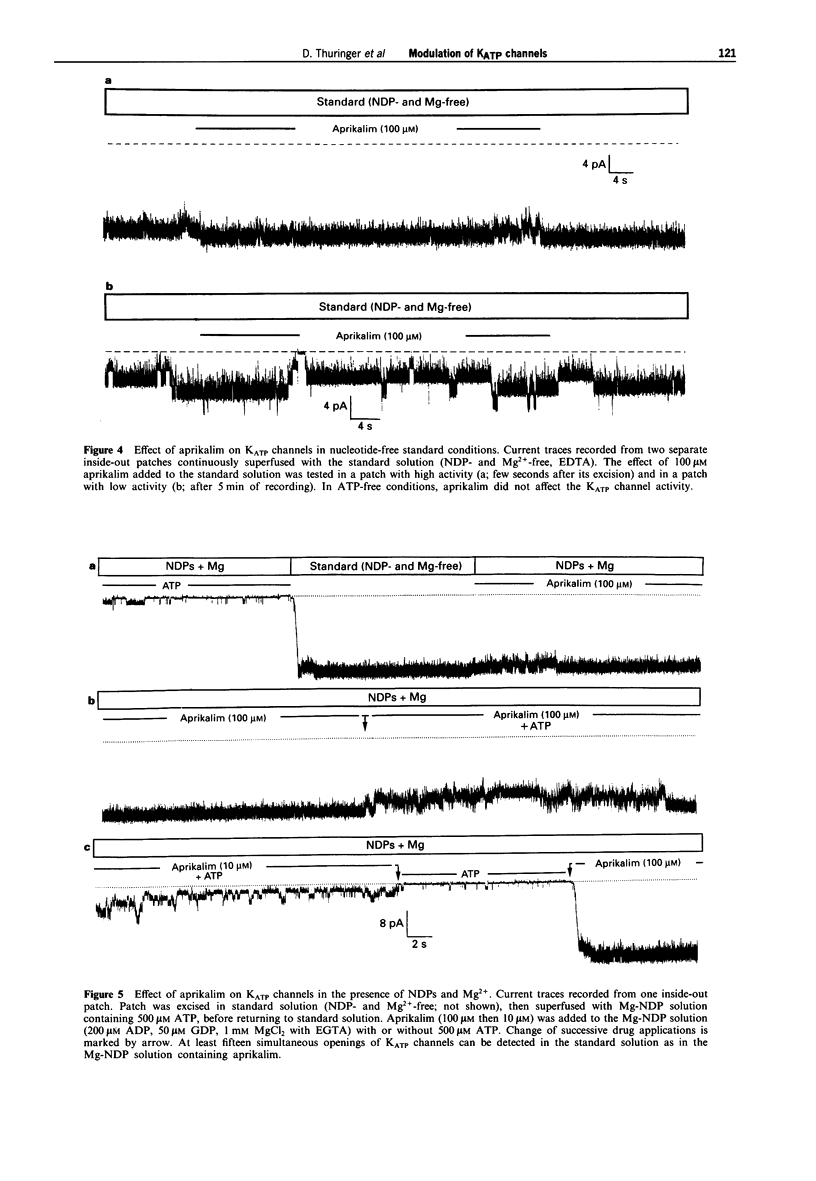






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard B., Lazdunski M. Pharmacological properties of ATP-sensitive K+ channels in mammalian skeletal muscle cells. Eur J Pharmacol. 1993 Jun 4;236(3):419–426. doi: 10.1016/0014-2999(93)90480-6. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K. E. Clinical pharmacology of potassium channel openers. Pharmacol Toxicol. 1992 Apr;70(4):244–254. doi: 10.1111/j.1600-0773.1992.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Auchampach J. A., Maruyama M., Cavero I., Gross G. J. The new K+ channel opener Aprikalim (RP 52891) reduces experimental infarct size in dogs in the absence of hemodynamic changes. J Pharmacol Exp Ther. 1991 Dec;259(3):961–967. [PubMed] [Google Scholar]
- Cavero I., Premmereur J. ATP sensitive potassium channel openers are of potential benefit in ischaemic heart disease. Cardiovasc Res. 1994 Jan;28(1):32–33. doi: 10.1093/cvr/28.1.32. [DOI] [PubMed] [Google Scholar]
- Deutsch N., Klitzner T. S., Lamp S. T., Weiss J. N. Activation of cardiac ATP-sensitive K+ current during hypoxia: correlation with tissue ATP levels. Am J Physiol. 1991 Sep;261(3 Pt 2):H671–H676. doi: 10.1152/ajpheart.1991.261.3.H671. [DOI] [PubMed] [Google Scholar]
- Deutsch N., Weiss J. N. ATP-sensitive K+ channel modification by metabolic inhibition in isolated guinea-pig ventricular myocytes. J Physiol. 1993 Jun;465:163–179. doi: 10.1113/jphysiol.1993.sp019671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Structure-activity relationships of K+ channel openers. Trends Pharmacol Sci. 1990 Oct;11(10):417–422. doi: 10.1016/0165-6147(90)90149-3. [DOI] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Escande D., Cavero I. K+ channel openers and 'natural' cardioprotection. Trends Pharmacol Sci. 1992 Jul;13(7):269–272. doi: 10.1016/0165-6147(92)90083-i. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Le Guern S., Courteix J., Laville M., Cavero I. Potassium channel openers act through an activation of ATP-sensitive K+ channels in guinea-pig cardiac myocytes. Pflugers Arch. 1989 Sep;414(6):669–675. doi: 10.1007/BF00582134. [DOI] [PubMed] [Google Scholar]
- Faivre J. F., Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim Biophys Acta. 1990 Nov 2;1029(1):167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- Fan Z., Makielski J. C. Intracellular H+ and Ca2+ modulation of trypsin-modified ATP-sensitive K+ channels in rabbit ventricular myocytes. Circ Res. 1993 Mar;72(3):715–722. doi: 10.1161/01.res.72.3.715. [DOI] [PubMed] [Google Scholar]
- Fan Z., Nakayama K., Hiraoka M. Multiple actions of pinacidil on adenosine triphosphate-sensitive potassium channels in guinea-pig ventricular myocytes. J Physiol. 1990 Nov;430:273–295. doi: 10.1113/jphysiol.1990.sp018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988 Jul;412(1-2):37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- Findlay I., Deroubaix E., Guiraudou P., Coraboeuf E. Effects of activation of ATP-sensitive K+ channels in mammalian ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1551–H1559. doi: 10.1152/ajpheart.1989.257.5.H1551. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J Membr Biol. 1988;101(1):83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Virág L., Furukawa N., Sawanobori T., Hiraoka M. Mechanism for reactivation of the ATP-sensitive K+ channel by MgATP complexes in guinea-pig ventricular myocytes. J Physiol. 1994 Aug 15;479(Pt 1):95–107. doi: 10.1113/jphysiol.1994.sp020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G. J., Auchampach J. A. Role of ATP dependent potassium channels in myocardial ischaemia. Cardiovasc Res. 1992 Nov;26(11):1011–1016. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
- Han J., So I., Kim E. Y., Earm Y. E. ATP-sensitive potassium channels are modulated by intracellular lactate in rabbit ventricular myocytes. Pflugers Arch. 1993 Dec;425(5-6):546–548. doi: 10.1007/BF00374883. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Wareham A. C., Head S. I. Mechanism of action of a K+ channel activator BRL 38227 on ATP-sensitive K+ channels in mouse skeletal muscle fibres. J Physiol. 1994 Aug 1;478(Pt 3):523–532. doi: 10.1113/jphysiol.1994.sp020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O., Ammälä C., Bokvist K., Fredholm B., Rorsman P. Stimulation of the KATP channel by ADP and diazoxide requires nucleotide hydrolysis in mouse pancreatic beta-cells. J Physiol. 1993 Apr;463:349–365. doi: 10.1113/jphysiol.1993.sp019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman S. D., Hamilton T. C. Potassium channel activator drugs: mechanism of action, pharmacological properties, and therapeutic potential. Med Res Rev. 1992 Mar;12(2):73–148. doi: 10.1002/med.2610120202. [DOI] [PubMed] [Google Scholar]
- Lynch J. J., Jr, Sanguinetti M. C., Kimura S., Bassett A. L. Therapeutic potential of modulating potassium currents in the diseased myocardium. FASEB J. 1992 Aug;6(11):2952–2960. doi: 10.1096/fasebj.6.11.1386585. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990 Apr;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H. Modulation of ischemia by regulation of the ATP-sensitive potassium channel. Cardiovasc Drugs Ther. 1993 Aug;7 (Suppl 3):507–513. doi: 10.1007/BF00877615. [DOI] [PubMed] [Google Scholar]
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci. 1993 Sep;14(9):332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Richer C., Pratz J., Mulder P., Mondot S., Giudicelli J. F., Cavero I. Cardiovascular and biological effects of K+ channel openers, a class of drugs with vasorelaxant and cardioprotective properties. Life Sci. 1990;47(19):1693–1705. doi: 10.1016/0024-3205(90)90342-o. [DOI] [PubMed] [Google Scholar]
- Ripoll C., Lederer W. J., Nichols C. G. Modulation of ATP-sensitive K+ channel activity and contractile behavior in mammalian ventricle by the potassium channel openers cromakalim and RP49356. J Pharmacol Exp Ther. 1990 Nov;255(2):429–435. [PubMed] [Google Scholar]
- Shen W. K., Tung R. T., Machulda M. M., Kurachi Y. Essential role of nucleotide diphosphates in nicorandil-mediated activation of cardiac ATP-sensitive K+ channel. A comparison with pinacidil and lemakalim. Circ Res. 1991 Oct;69(4):1152–1158. doi: 10.1161/01.res.69.4.1152. [DOI] [PubMed] [Google Scholar]
- Standen N. B. The G. L. Brown Lecture. Potassium channels, metabolism and muscle. Exp Physiol. 1992 Jan;77(1):1–25. doi: 10.1113/expphysiol.1992.sp003564. [DOI] [PubMed] [Google Scholar]
- Takano M., Noma A. Selective modulation of the ATP-sensitive K+ channel by nicorandil in guinea-pig cardiac cell membrane. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):592–597. doi: 10.1007/BF00169050. [DOI] [PubMed] [Google Scholar]
- Terzic A., Tung R. T., Inanobe A., Katada T., Kurachi Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP-dependent gating. Neuron. 1994 Apr;12(4):885–893. doi: 10.1016/0896-6273(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol Pharmacol. 1989 Dec;36(6):897–902. [PubMed] [Google Scholar]
- Tung R. T., Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J Physiol. 1991 Jun;437:239–256. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weik R., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: characterization of the ATP-binding site. J Membr Biol. 1989 Sep;110(3):217–226. doi: 10.1007/BF01869152. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Venkatesh N. Metabolic regulation of cardiac ATP-sensitive K+ channels. Cardiovasc Drugs Ther. 1993 Aug;7 (Suppl 3):499–505. doi: 10.1007/BF00877614. [DOI] [PubMed] [Google Scholar]


