Abstract
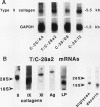
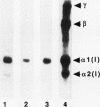
Immortalized human chondrocytes were established by transfection of primary cultures of juvenile costal chondrocytes with vectors encoding simian virus 40 large T antigen and selection in suspension culture over agarose. Stable cell lines were generated that exhibited chondrocyte morphology, continuous proliferative capacity (> 80 passages) in monolayer culture in serum-containing medium, and expression of mRNAs encoding chondrocyte-specific collagens II, IX, and XI and proteoglycans in an insulin-containing serum substitute. They did not express type X collagen or versican mRNA. These cells synthesized and secreted extracellular matrix molecules that were reactive with monoclonal antibodies against type II collagen, large proteoglycan (PG-H, aggrecan), and chondroitin-4- and chondroitin-6-sulfate. Interleukin-1 beta (IL-1 beta) decreased the levels of type II collagen mRNA and increased the levels of mRNAs for collagenase, stromelysin, and immediate early genes (egr-1, c-fos, c-jun, and jun-B). These cell lines also expressed reporter gene constructs containing regulatory sequences (-577/+3,428 bp) of the type II collagen gene (COL2A1) in transient transfection experiments, and IL-1 beta suppressed this expression by 50-80%. These results show that immortalized human chondrocytes displaying cartilage-specific modulation by IL-1 beta can be used as a model for studying normal and pathological repair mechanisms.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Pallante K. M., Niu Z., Leboy P. S., Golden E. B., Pacifici M. Rapid induction of type X collagen gene expression in cultured chick vertebral chondrocytes. Exp Cell Res. 1991 Mar;193(1):190–197. doi: 10.1016/0014-4827(91)90555-9. [DOI] [PubMed] [Google Scholar]
- Adolphe M., Froger B., Ronot X., Corvol M. T., Forest N. Cell multiplication and type II collagen production by rabbit articular chondrocytes cultivated in a defined medium. Exp Cell Res. 1984 Dec;155(2):527–536. doi: 10.1016/0014-4827(84)90212-x. [DOI] [PubMed] [Google Scholar]
- Alema S., Tato F., Boettiger D. myc and src oncogenes have complementary effects on cell proliferation and expression of specific extracellular matrix components in definitive chondroblasts. Mol Cell Biol. 1985 Mar;5(3):538–544. doi: 10.1128/mcb.5.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperley J. F., Luskey B. D., Williams D. A. Retroviral gene transfer of human adenosine deaminase in murine hematopoietic cells: effect of selectable marker sequences on long-term expression. Blood. 1991 Jul 15;78(2):310–317. [PubMed] [Google Scholar]
- Arbiser J. L., Arbiser Z. K., Majzoub J. A. Regulation of gene expression in choriocarcinoma by methotrexate and hydroxyurea. Endocrinology. 1991 Feb;128(2):972–978. doi: 10.1210/endo-128-2-972. [DOI] [PubMed] [Google Scholar]
- Asari A., Miyauchi S., Miyazaki K., Hamai A., Horie K., Takahashi T., Sekiguchi T., Machida A., Kohno K., Uchiyama Y. Intra- and extracellular localization of hyaluronic acid and proteoglycan constituents (chondroitin sulfate, keratan sulfate, and protein core) in articular cartilage of rabbit tibia. J Histochem Cytochem. 1992 Nov;40(11):1693–1704. doi: 10.1177/40.11.1431058. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bernier S. M., Goltzman D. Regulation of expression of the chondrocytic phenotype in a skeletal cell line (CFK2) in vitro. J Bone Miner Res. 1993 Apr;8(4):475–484. doi: 10.1002/jbmr.5650080412. [DOI] [PubMed] [Google Scholar]
- Block J. A., Inerot S. E., Gitelis S., Kimura J. H. Synthesis of chondrocytic keratan sulphate-containing proteoglycans by human chondrosarcoma cells in long-term cell culture. J Bone Joint Surg Am. 1991 Jun;73(5):647–658. [PubMed] [Google Scholar]
- Böhme K., Conscience-Egli M., Tschan T., Winterhalter K. H., Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol. 1992 Feb;116(4):1035–1042. doi: 10.1083/jcb.116.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrino D. A., Kujawa M. J., Lennon D. P., Caplan A. I. Altered cartilage proteoglycans synthesized by chick limb bud chondrocytes cultured in serum-free defined medium. Exp Cell Res. 1989 Jul;183(1):62–71. doi: 10.1016/0014-4827(89)90418-7. [DOI] [PubMed] [Google Scholar]
- Castagnola P., Moro G., Descalzi-Cancedda F., Cancedda R. Type X collagen synthesis during in vitro development of chick embryo tibial chondrocytes. J Cell Biol. 1986 Jun;102(6):2310–2317. doi: 10.1083/jcb.102.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Caubet J. F., Bernaudin J. F. Expression of the c-fos proto-oncogene in bone, cartilage and tooth forming tissues during mouse development. Biol Cell. 1988;64(1):101–104. doi: 10.1016/0248-4900(88)90100-1. [DOI] [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizdziel P. E., Hosoi J., Montgomery J. C., Wiseman R. W., Barrett J. C. Loss of a tumor suppressor gene function is correlated with downregulation of chondrocyte-specific collagen expression in Syrian hamster embryo cells. Mol Carcinog. 1991;4(1):14–24. doi: 10.1002/mc.2940040105. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. A., Ramis C. I., Fisher L. W., Gehron-Robey P., Termine J. D., Young M. F. Characterization of bone PG II cDNA and its relationship to PG II mRNA from other connective tissues. Nucleic Acids Res. 1986 Dec 22;14(24):9861–9876. doi: 10.1093/nar/14.24.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Fisher L. W., Heegaard A. M., Vetter U., Vogel W., Just W., Termine J. D., Young M. F. Human biglycan gene. Putative promoter, intron-exon junctions, and chromosomal localization. J Biol Chem. 1991 Aug 5;266(22):14371–14377. [PubMed] [Google Scholar]
- Gerstenfeld L. C., Kelly C. M., Von Deck M., Lian J. B. Comparative morphological and biochemical analysis of hypertrophic, non-hypertrophic and 1,25(OH)2D3 treated non-hypertrophic chondrocytes. Connect Tissue Res. 1990;24(1):29–39. doi: 10.3109/03008209009152420. [DOI] [PubMed] [Google Scholar]
- Gionti E., Pontarelli G., Cancedda R. Avian myelocytomatosis virus immortalizes differentiated quail chondrocytes. Proc Natl Acad Sci U S A. 1985 May;82(9):2756–2760. doi: 10.1073/pnas.82.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Properties of chick embryo chondrocytes grown in serum-free medium. J Biol Chem. 1984 Jun 10;259(11):6766–6772. [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Fukuo K., Birkhead J. R., Dudek E., Sandell L. J. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994 Jan;54(1):85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- Goldring M. B., Sandell L. J., Stephenson M. L., Krane S. M. Immune interferon suppresses levels of procollagen mRNA and type II collagen synthesis in cultured human articular and costal chondrocytes. J Biol Chem. 1986 Jul 5;261(19):9049–9055. [PubMed] [Google Scholar]
- Grigoriadis A. E., Heersche J. N., Aubin J. E. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1988 Jun;106(6):2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L. V., Hale J. E., Kemick M. L., Ishikawa Y., Wuthier R. E. Development of a new serum-free medium, USC-HC1, for growth and normal phenotype in postembryonic chicken growth plate chondrocytes. In Vitro Cell Dev Biol. 1986 Oct;22(10):597–603. doi: 10.1007/BF02623519. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Cleveland J., Rapp U., Nemuth G., Bolander M., Doege K., Yamada Y., Hassell J. R. An established rat cell line expressing chondrocyte properties. Exp Cell Res. 1988 Oct;178(2):457–468. doi: 10.1016/0014-4827(88)90414-4. [DOI] [PubMed] [Google Scholar]
- Ikebe T., Hirata M., Koga T. Effects of human recombinant tumor necrosis factor-alpha and interleukin 1 on the synthesis of glycosaminoglycan and DNA in cultured rat costal chondrocytes. J Immunol. 1988 Feb 1;140(3):827–831. [PubMed] [Google Scholar]
- Kato Y., Nakashima K., Iwamoto M., Murakami H., Hiranuma H., Koike T., Suzuki F., Fuchihata H., Ikehara Y., Noshiro M. Effects of interleukin-1 on syntheses of alkaline phosphatase, type X collagen, and 1,25-dihydroxyvitamin D3 receptor, and matrix calcification in rabbit chondrocyte cultures. J Clin Invest. 1993 Nov;92(5):2323–2330. doi: 10.1172/JCI116836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nasu N., Takase T., Daikuhara Y., Suzuki F. A serum-free medium supplemented with multiplication-stimulating activity (MSA) supports both proliferation and differentiation of chondrocytes in primary culture. Exp Cell Res. 1980 Jan;125(1):167–174. doi: 10.1016/0014-4827(80)90200-1. [DOI] [PubMed] [Google Scholar]
- Kimura T., Cheah K. S., Chan S. D., Lui V. C., Mattei M. G., van der Rest M., Ono K., Solomon E., Ninomiya Y., Olsen B. R. The human alpha 2(XI) collagen (COL11A2) chain. Molecular cloning of cDNA and genomic DNA reveals characteristics of a fibrillar collagen with differences in genomic organization. J Biol Chem. 1989 Aug 15;264(23):13910–13916. [PubMed] [Google Scholar]
- Kimura T., Mattei M. G., Stevens J. W., Goldring M. B., Ninomiya Y., Olsen B. R. Molecular cloning of rat and human type IX collagen cDNA and localization of the alpha 1(IX) gene on the human chromosome 6. Eur J Biochem. 1989 Jan 15;179(1):71–78. doi: 10.1111/j.1432-1033.1989.tb14522.x. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Mallein-Gerin F., Olsen B. R. Expression of simian virus 40 large T (tumor) oncogene in mouse chondrocytes induces cell proliferation without loss of the differentiated phenotype. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3289–3293. doi: 10.1073/pnas.90.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Champion J. E., McMahon J. A., Sukhatme V. P. Developmental expression of the putative transcription factor Egr-1 suggests that Egr-1 and c-fos are coregulated in some tissues. Development. 1990 Feb;108(2):281–287. doi: 10.1242/dev.108.2.281. [DOI] [PubMed] [Google Scholar]
- Medema R. H., Wubbolts R., Bos J. L. Two dominant inhibitory mutants of p21ras interfere with insulin-induced gene expression. Mol Cell Biol. 1991 Dec;11(12):5963–5967. doi: 10.1128/mcb.11.12.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P., Fuller L. F., Painter R. B. Establishment and characterization of a permanent pSV ori--transformed ataxia-telangiectasia cell line. Exp Cell Res. 1985 May;158(1):119–126. doi: 10.1016/0014-4827(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Niesor E. J., Wollheim C. B., Mintz D. H., Blondel B., Renold A. E., Weil R. Establishment of rat pancreatic endocrine cell lines by infection with simian virus 40. Biochem J. 1979 Mar 15;178(3):559–568. doi: 10.1042/bj1780559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D. F., Solursh M. Microtiter micromass cultures of limb-bud mesenchymal cells. In Vitro Cell Dev Biol. 1988 Feb;24(2):138–147. doi: 10.1007/BF02623891. [DOI] [PubMed] [Google Scholar]
- Rosselot G., Reginato A. M., Leach R. M. Development of a serum-free system to study the effect of growth hormone and insulinlike growth factor-I on cultured postembryonic growth plate chondrocytes. In Vitro Cell Dev Biol. 1992 Apr;28A(4):235–244. doi: 10.1007/BF02634239. [DOI] [PubMed] [Google Scholar]
- Ryan M. C., Sieraski M., Sandell L. J. The human type II procollagen gene: identification of an additional protein-coding domain and location of potential regulatory sequences in the promoter and first intron. Genomics. 1990 Sep;8(1):41–48. doi: 10.1016/0888-7543(90)90224-i. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. J., Morris N., Robbins J. R., Goldring M. B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991 Sep;114(6):1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Goldring M. B., Birkhead J. R., Krane S. M., Rahmsdorf H. J., Angel P. Stimulation of procollagenase synthesis parallels increases in cellular procollagenase mRNA in human articular chondrocytes exposed to recombinant interleukin 1 beta or phorbol ester. Biochem Biophys Res Commun. 1987 Apr 29;144(2):583–590. doi: 10.1016/s0006-291x(87)80006-2. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Nissley S. P., Kimura J. H., Rechler M. M., Caplan A. I., Hascall V. C. Effects of insulin and multiplication-stimulating activity on proteoglycan biosynthesis in chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Feb 25;256(4):2045–2052. [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Pan H. O., Kinoshita A., Tajima K., Takano Y. Establishment from a human chondrosarcoma of a new immortal cell line with high tumorigenicity in vivo, which is able to form proteoglycan-rich cartilage-like nodules and to respond to insulin in vitro. Int J Cancer. 1991 Jul 9;48(5):717–725. doi: 10.1002/ijc.2910480515. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Tajima K., Pan H. O., Enomoto M., Kinoshita A., Suzuki F., Takano Y., Mori Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989 Jul 15;49(14):3996–4002. [PubMed] [Google Scholar]
- Thenet S., Benya P. D., Demignot S., Feunteun J., Adolphe M. SV40-immortalization of rabbit articular chondrocytes: alteration of differentiated functions. J Cell Physiol. 1992 Jan;150(1):158–167. doi: 10.1002/jcp.1041500121. [DOI] [PubMed] [Google Scholar]
- Trojan J., Blossey B. K., Johnson T. R., Rudin S. D., Tykocinski M., Ilan J., Ilan J. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A., Benton H. P. Synthesis of type II collagen is decreased in cartilage cultured with interleukin 1 while the rate of intracellular degradation remains unchanged. Coll Relat Res. 1988 Sep;8(5):393–405. doi: 10.1016/s0174-173x(88)80013-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Ryseck R. P., Bravo R. Tissue-specific expression of c-jun and junB during organogenesis in the mouse. Development. 1989 Jul;106(3):465–471. doi: 10.1242/dev.106.3.465. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Rosenblatt M. F., Beier D. R., Cone R. D. Generation of murine stromal cell lines supporting hematopoietic stem cell proliferation by use of recombinant retrovirus vectors encoding simian virus 40 large T antigen. Mol Cell Biol. 1988 Sep;8(9):3864–3871. doi: 10.1128/mcb.8.9.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Bleiberg I., Yaron M. Some recombinant human cytokines stimulate glycosaminoglycan synthesis in human synovial fibroblast cultures and inhibit it in human articular cartilage cultures. Arthritis Rheum. 1989 Feb;32(2):173–180. doi: 10.1002/anr.1780320210. [DOI] [PubMed] [Google Scholar]