Abstract
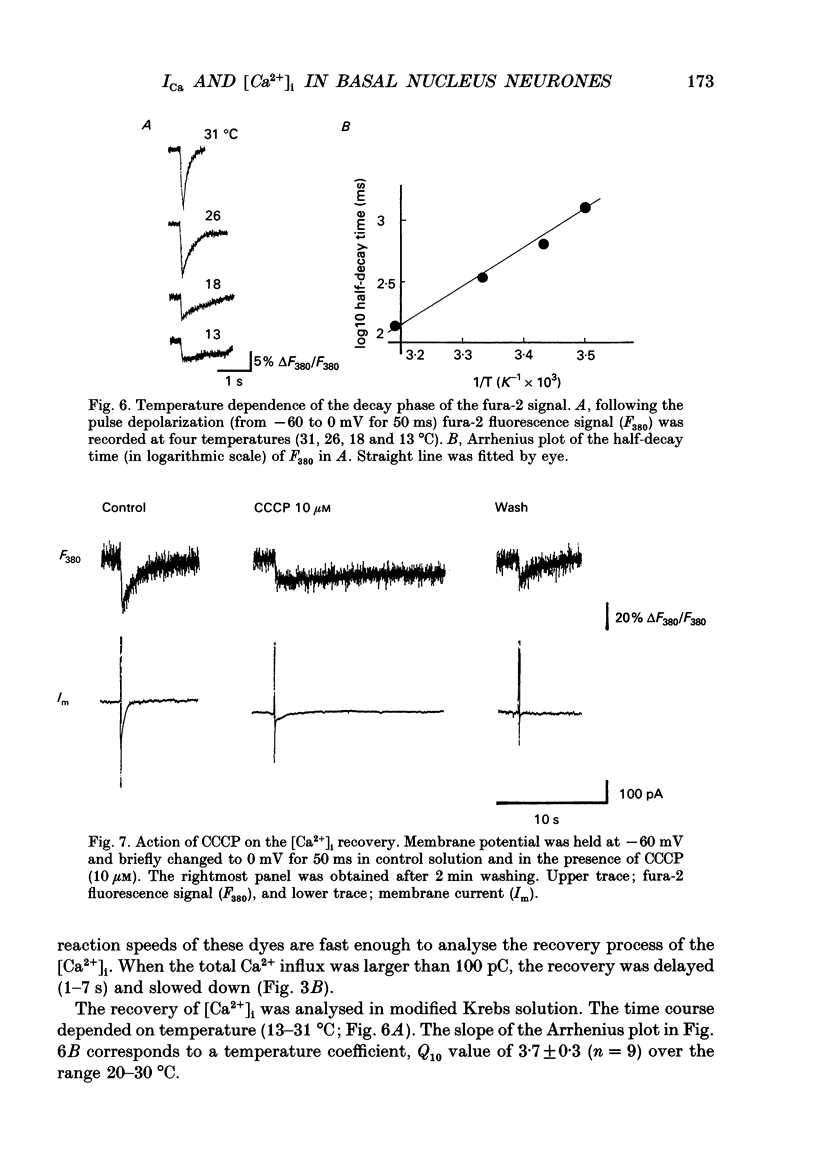
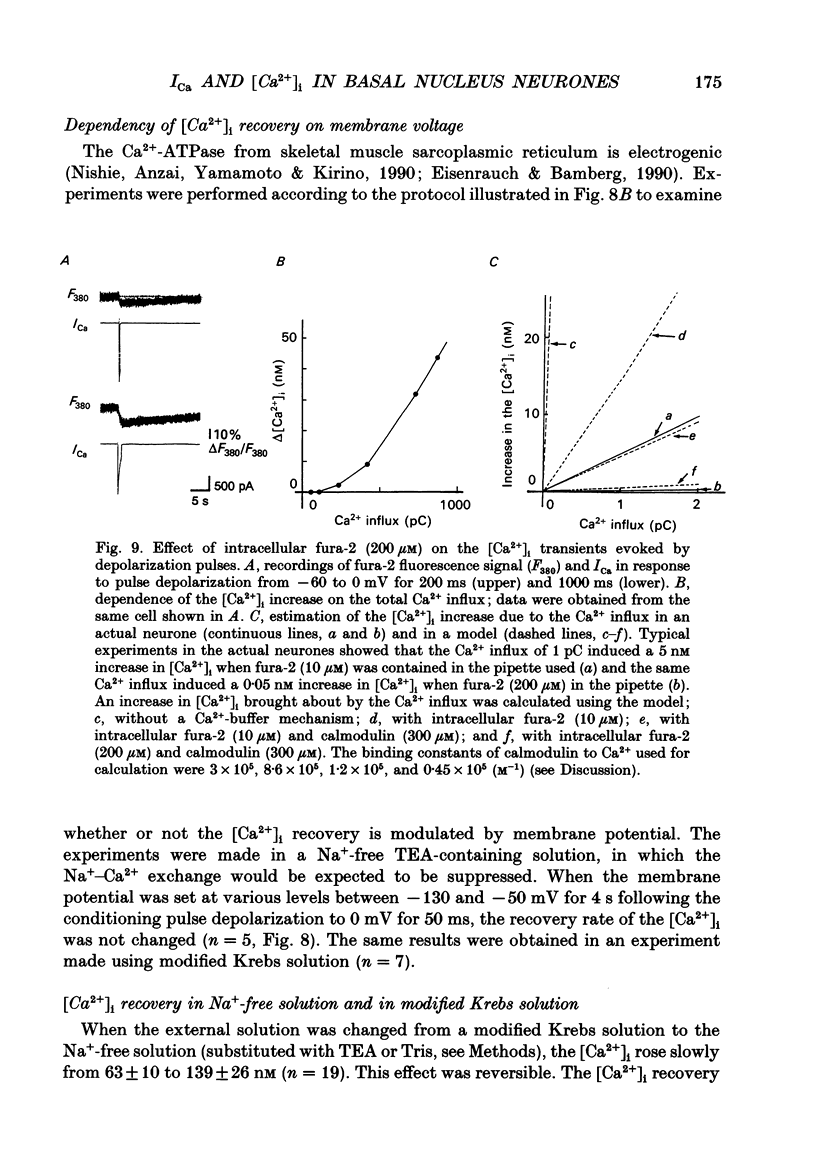
1. Neurones were acutely dissociated from the rat nucleus basalis. Whole-cell patch clamp recordings of calcium currents (ICa) and fura-2 microfluorimetric recordings of intracellular free Ca2+ concentration ([Ca2+]i) were made simultaneously. 2. Depolarization from -60 to 0 mV elicited ICa and a gradual increase in [Ca2+]i. After repolarization, ICa terminated in 0.7 ms, and [Ca2+]i recovered to control exponentially (1-5 s). 3. Both ICa and the transient [Ca2+]i increase in response to step depolarizations, were abolished in Ca2+ free extracellular solution and in Cd(2+)-containing solution. 4. Depolarizations from -90 mV to membrane potentials less negative than -40 mV induced ICa and an increase in [Ca2+]i. Depolarization to 0 mV elicited the maximum ICa, and produced the largest increase in [Ca2+]i. There was a parallel relationship between the [Ca2+]i increase and the magnitude of the ICa. 5. The [Ca2+]i increase was associated with an increase in total Ca2+ influx when the duration of the step depolarization was varied. The relationship between the total Ca2+ influx and the peak of [Ca2+]i transient reached an asymptote as total Ca2+ influx exceeded 200 pC. A similar finding was made when more than thirty action potentials were used in increasing [Ca2+]i. 6. The process of the [Ca2+]i recovery was slowed down by lowering the temperature, by an intracellular dialysis with vanadate, by extracellular application of a mitochondrial inhibitor, carbonyl cyanide m-chlorophenyl-hydrazone (CCCP), and by Na(+)-free external solution. It was unaffected by membrane potential (-50 to -130 mV). 7. When pipette solution contained a high concentration of fura-2 (200 microM), the [Ca2+]i increase per 1 pC of Ca2+ influx decreased, and the [Ca2+]i recovery was slowed. 8. The results indicate that the ICa through voltage-dependent Ca2+ channels elevates [Ca2+]i. The neurones possess a large capacity for Ca2+ buffering, and the recovery of [Ca2+]i requires both the Ca2+ pump and membrane Na(+)-Ca2+ exchange.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Calcium regulation by and buffer capacity of molluscan neurons during calcium transients. Cell Calcium. 1988 Apr;9(2):57–69. doi: 10.1016/0143-4160(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J., Parkes C. O. Calcium-binding protein distribution in the rat brain. Brain Res. 1982 May 13;239(2):519–525. doi: 10.1016/0006-8993(82)90526-1. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ Res. 1989 Aug;65(2):334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Eberhard M., Erne P. Kinetics of calcium binding to fluo-3 determined by stopped-flow fluorescence. Biochem Biophys Res Commun. 1989 Aug 30;163(1):309–314. doi: 10.1016/0006-291x(89)92136-0. [DOI] [PubMed] [Google Scholar]
- Eisenrauch A., Bamberg E. Voltage-dependent pump currents of the sarcoplasmic reticulum Ca2(+)-ATPase in planar lipid membranes. FEBS Lett. 1990 Jul 30;268(1):152–156. doi: 10.1016/0014-5793(90)80996-v. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallström A., Sato A., Sato Y., Ungerstedt U. Effect of stimulation of the nucleus basalis of Meynert on blood flow and extracellular lactate in the cerebral cortex with special reference to the effect of noxious stimulation of skin and hypoxia. Neurosci Lett. 1990 Aug 14;116(1-2):227–232. doi: 10.1016/0304-3940(90)90415-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Johnston M. V., McKinney M., Coyle J. T. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y., Takeda K., Yamazaki K. Quin2 protects against neuronal cell death due to Ca2+ overload. Brain Res. 1990 Sep 24;528(1):48–54. doi: 10.1016/0006-8993(90)90193-f. [DOI] [PubMed] [Google Scholar]
- Lamour Y., Dutar P., Rascol O., Jobert A. Basal forebrain neurons projecting to the rat frontoparietal cortex: electrophysiological and pharmacological properties. Brain Res. 1986 Jan 1;362(1):122–131. doi: 10.1016/0006-8993(86)91405-8. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Obata K., Carlson C. G., Yamaguchi K. Dissociated cell culture of cholinergic neurons from nucleus basalis of Meynert and other basal forebrain nuclei. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6325–6329. doi: 10.1073/pnas.82.18.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. A role for the mitochondrion in the protection of cells against calcium overload? Prog Brain Res. 1985;63:97–106. doi: 10.1016/S0079-6123(08)61978-0. [DOI] [PubMed] [Google Scholar]
- Niggli V., Sigel E., Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem. 1982 Mar 10;257(5):2350–2356. [PubMed] [Google Scholar]
- Nishie I., Anzai K., Yamamoto T., Kirino Y. Measurement of steady-state Ca2+ pump current caused by purified Ca2(+)-ATPase of sarcoplasmic reticulum incorporated into a planar bilayer lipid membrane. J Biol Chem. 1990 Feb 15;265(5):2488–2491. [PubMed] [Google Scholar]
- Nohmi M., Kuba K. Effects of Na+ gradient on the intracellular Ca2+ oscillation in the sympathetic ganglion cell: Na-Ca exchange in the neurone cell soma? Brain Res. 1984 Dec 17;324(1):171–174. doi: 10.1016/0006-8993(84)90638-3. [DOI] [PubMed] [Google Scholar]
- Oyanagi K., Takahashi H., Wakabayashi K., Ikuta F. Correlative decrease of large neurons in the neostriatum and basal nucleus of Meynert in Alzheimer's disease. Brain Res. 1989 Dec 18;504(2):354–357. doi: 10.1016/0006-8993(89)91384-x. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Surprenant A., Shen K. Z., North R. A., Tatsumi H. Inhibition of calcium currents by noradrenaline, somatostatin and opioids in guinea-pig submucosal neurones. J Physiol. 1990 Dec;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Hirai K., Katayama Y. Measurement of the intracellular calcium concentration in guinea-pig myenteric neurons by using fura-2. Brain Res. 1988 Jun 7;451(1-2):371–375. doi: 10.1016/0006-8993(88)90787-1. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. H., Hartley D. M., Koh J., Choi D. W. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science. 1990 Mar 23;247(4949 Pt 1):1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]


